Chapter 31
Cerebellum
The cerebellum is a softball-sized structure located at the base of the skull. Grab the back of your head just above where it meets your neck: your hand is now cupped around your cerebellum. As with most brain systems, much of what we know about the cerebellum stems from symptoms of damage or pathology and from its connectivity with the rest of the brain. From such evidence it has long been clear that the cerebellum is an important component of the motor system. Severe abnormalities of movement are produced by pathologies of the cerebellum. Cerebellar output influences systems that are unambiguously motor, and inputs to the cerebellum convey information known to be essential for movement such as joint angles and loads on muscles. Recently it has become equally clear that the role of the cerebellum is not limited to movement. This is indicated by its interconnections with nonmotor structures; by the more subtle, nonmotor deficits seen with cerebellar lesions; and by functional imaging studies where regions of the cerebellum show activation during nonmotor tasks. A deeper understanding of the cerebellum will require identification of the common aspects or computational demands that these motor and nonmotor functions share.
Research on the anatomy, physiology, and function of the cerebellum is complemented by computational analyses of the rules for input/output transformations and the means by which the cerebellar circuit implements such transformations. This interdisciplinary approach was made possible by the seminal work of Eccles, Ito, and Szentágothai, who in 1967 published The Cerebellum as a Neuronal Machine, which described most of the essential aspects of the cellular and synaptic organization of the cerebellum. Two years later, David Marr published a remarkable paper in which he inferred some basic computational properties of the cerebellum solely on the basis of the wiring diagram that Eccles and associates had elucidated. The relative clarity of the cerebellar circuit and the close interconnections between cerebellar activity and motor output have enabled rigorous experimental and computational analyses of the relationship between brain and behavior, and even between circuit plasticity and learning.
Cerebellar Anatomy and Circuit
The anatomical, circuit, and physiological characteristics of the cerebellum suggest that it carries out a different type of computation than the cerebral cortex. One indication of this distinction is that Purkinje cells, which provide the only output of the cerebellar cortex, are inhibitory (GABAergic) and fire about 50 action potentials every second, even in the absence of sensory input. Their downstream target neurons in the vestibular and cerebellar nuclei, which comprise the sole output of the entire cerebellum, are also spontaneously active. High tonic firing rates enable these cerebellar cell types to respond rapidly, precisely, and bidirectionally to input signals. As a result, the cerebellum can exert tight temporal control over its diverse downstream target regions. This chapter first discusses cerebellar anatomy, circuit, and development, and then examines the cellular and synaptic specializations that underlie cerebellar function. The last section demonstrates the relationship between circuit and function during behavior and learning.
Phylogenetic Development
The cerebellum is present in all vertebrates. In agnathans (lampreys and hagfish), it is a rudimentary structure that assists the functions of the well-developed vestibulo-ocular, vestibulospinal, and reticulospinal systems. The cerebellum is somewhat larger in fishes, where, on the input side, it processes sensory information from the vestibular, lateral line, and to a lesser extent proprioceptive and somatosensory systems. On the output side it is connected to the vestibular and reticular nuclei. In amphibians the region of the cerebellum that receives proprioceptive and other sensory information is expanded, and it increases still further in reptiles, birds, and most mammals, where it constitutes the largest portion of the cerebellum. A diverse range of brain regions send inputs to or receive outputs from the cerebellum. In primates and dolphins, the hugely expanded lateral hemispheres are connected with the enlarged cerebral cortex via the thalamus and pons.
Gross Anatomy
The cerebellum consists of a three-layered cortex folded repeatedly, accordion-style, around the three pairs of cerebellar nuclei buried in the middle. Each fold of the cerebellar cortex is called a folium (leaf), and in most species the folia run roughly transverse to the long axis of the body, from ear to ear. The folia are grouped into lobules, numbered I to X; lobulation is fairly consistent across individuals of the same species and even across species, despite great variation in cerebellar development. The lobules in turn are grouped into three lobes. The flocculonodular lobe (also known as the vestibulocerebellum) is the phylogenetically oldest portion and is located on the inferior surface. Above the posterolateral fissure sit the anterior and posterior lobes, separated from each other by the primary fissure (Fig. 31.1). If the entire human cerebellar cortex were unfolded and flattened, it would have about the same area as a sheet of paper, but arranged in a long thin strip.
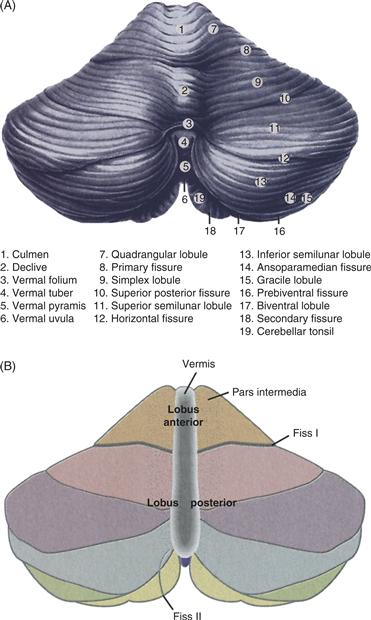
Figure 31.1 Cerebellar structure and functional subdivisions. (A) Dorsal view of the human cerebellum. (B) Functional subdivisions.
Adapted from Nieuwenhuys, Voogd, and van Huijzen (1979).
The cerebellar cortex influences the rest of the brain exclusively via projections to neurons in the vestibular and cerebellar nuclei (also termed the “deep cerebellar nuclei”). From medial to lateral, the cerebellar nuclei comprise the fastigius, the interpositus (further divided into the globose and emboliform in humans), and the dentate. The cerebellar cortex, excluding the flocculonodular lobe, can be divided into three longitudinal (parasagittal) zones based on the projection of the cortex onto the three cerebellar nuclei (Fig. 31.2). The medial zone receives information from a wide range of sensory systems, including vestibular, visual, somatosensory, and auditory. The medial zone also processes signals relating to autonomic, visceral, cardiovascular, and immune function. It projects to the fastigial nuclei, which in turn send outputs to the vestibular and reticulospinal systems, as well as the thalamus and superior colliculus. The intermediate zone of the cerebellar cortex receives proprioceptive and somatosensory information from the spinal cord, as well as information from the motor cortex via the pontocerebellar nuclei. Its axons target the interpositus nuclei, which in turn have a major projection to the red nucleus, with some axons continuing to the contralateral thalamus (VL). There are also a sparser number of collaterals projecting to the spinal cord and pons. The lateral zone receives diverse types of information from the pontine nuclei, which carry signals from the widespread regions of the cerebral cortex. The lateral zone projects to the dentate nuclei, whose predominant output is to the thalamus (VL) and reticular formation, with lesser outputs to the red nucleus and spinal cord (Fig. 31.2). Finally, the flocculonodular lobe receives vestibular, visual, and motor information, and sends its output exclusively to the brainstem vestibular nuclei. In addition to the premotor outputs shown in Figure 31.2, each ouput nucleus also sends inhibitory outputs to subregions of the inferior olive: vestibular and fastigial nuclei to the dorsal cap of Kooy, the beta nucleus, and weakly to the medial accessory olive; interpositus to the dorsal and medial accessory olive, with a weaker projection to the cap of Kooy; and the dentate to the principal olive.
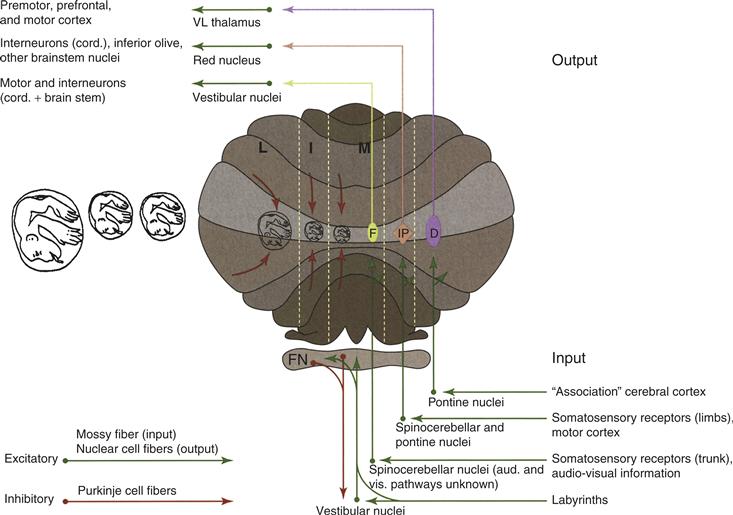
Figure 31.2 Cerebellar inputs and outputs. The cerebellum is shown schematically with the flocculonodular (FN) lobe unfolded and represented at the inferior surface. Green arrows indicate excitatory projections into and out of the cerebellum; red arrows indicate the inhibitory projections from cerebellar cortex to the deep cerebellar nuclei and the vestibular nuclei.
Adapted from Thach, Fig. 31-3. In Mountcastle, J. B. (1980). Medical Physiology, Volume I. 14 Ed. C. V. Mosby Co., St. Louis.
Mapping studies reveal that the cerebellum is not organized purely somatotopically like the sensory cerebral cortices. Part of the difficulty in defining cerebellar organizing principles is that the cerebellum combines diverse sensory inputs to create and refine motor commands. Thus, neither sensory nor motor mapping individually may square exactly with cerebellar organization. Sensory mapping studies reveal a “fractured somatotopy,” in which the regions of cerebellum that respond to touch of paw sites, for example, are located close to each other, but sensation for forelimb is represented elsewhere. The cerebellum can also be partitioned into parasagittal zones that may represent distinct motor control modules (see Box 31.1).
Two different schema have been proposed to account for cerebellar organization. In the first, each major subdivision of the cerebellum controls all parts of the body, but during different behavioral requirements: the flocculonodular lobe and medial cerebellum modulate vestibular-driven behaviors and eye movements; the intermediate zone controls somatic reflexes; and the lateral zone concerns itself with movements under voluntary control. In a second schema, the medial zone primarily controls musculature of the trunk, the intermediate zone that of the proximal limbs, and the lateral zone the distal limbs. Both of these organizing principles are supported by experimental and clinical studies in which the different cerebellar nuclei are lesioned or inactivated (Fig. 31.3). Neither accounts for the role of the cerebellum in regulating autonomic, emotional, or cognitive functions. The inputs and outputs of the cerebellum are diverse, and the outputs are relatively poorly characterized to date. A unifying view of cerebellar function that draws on analyses of neuronal activity, circuits, and behavior remains to be established.
Box 31.1 Parasagittal Stripes
The Basis of Cerebellar Microcircuits?
One of the greatest unknowns about the cerebellar circuit is whether the prototypical olivocerebellar loop, from olive → Purkinje cells → cerebellar nuclei → back to olive, is actually organized so that an individual olivary neuron receives inhibitory feedback from the same inhibitory nucleus neurons that it influences indirectly via Purkinje cells. In other words, does the microcircuit (at the level of individual neurons) reflect the macrocircuit (at the level of entire structures)? The most suggestive work on this question comes from anatomical studies on the parasagittal zones of the cerebellum. Histological experiments have revealed that many proteins, including aldolase C, acetylcholinesterase, calbindin, and parvalbumin, are expressed differentially in parasagittal stripes that run the length of each longitudinal zone. These stripes, which have been given names (1+, 1−, 2+, 2−, etc.), are constant in their presence and location within a species and to some extent across species (Pakan, Graham, Gutiérrez-Ibáñez, & Wylie, 2011).
Recent work has highlighted the potential significance of these stripes in organization of microcircuits in the cerebellum. Each olivary neuron gives rise to 7 to 10 climbing fibers in the cortex; all of the Purkinje cells that receive input from one olivary neuron are located within a stripe defined by aldolase C staining. Even in cases where the olivary axon diverges to different cerebellar lobules, the climbing fibers in both lobules obey the pattern established by aldolase C stripes: either all branches are in positive stripes or all in negative stripes. Furthermore, a group of Purkinje cells that are innervated by a small cluster of olivary neurons typically exhibit converging axon arbors in the cerebellar nuclei as well, within a subregion that is also defined by aldolase C staining (Sugihara, 2011). This convergence holds true even when the Purkinje cells are located in separate lobules. The axon collaterals of olivary neurons, which extend into the cerebellar nuclei, also form small clusters of terminals suggestive of microcircuit targeting. In turn, a cluster of nuclear neurons appears to target the same olivary region from which it receives input. Even cortical interneurons may respect the boundaries defined by aldolase stripes (Sillitoe & Joyner, 2007). Together these data are strongly suggestive of a closed loop olivo-cortical-nuclear microcircuit.
These findings are exclusively anatomical, but some experiments have extended these concepts to physiology and function as well. For example, muscle injection of a rabies virus tracer, which can travel retrogradely across many synapses, reveals one or more longitudinal stripes of Purkinje cells bounded by aldolase C compartments. Physiologically, Purkinje cells in aldolase positive and negative stripes express different complements of glutamate transporters, some of which are more effective than others. As a result, it is easier to trigger metabotropic glutamate receptor activity, and the consequent cascade leading to long-term depression of the parallel fiber → Purkinje cell synapse, in aldolase-negative than in aldolase-positive compartments (Wadiche & Jahr, 2001). In sum, the parasagittally organized stripes of the cerebellum are highly significant for both circuit and function.
Martha Bagnall
References
1. Pakan JM, Graham DJ, Gutiérrez-Ibáñez C, Wylie DR. Organization of the cerebellum: correlating zebrin immunohistochemistry with optic flow zones in the pigeon flocculus. Visual Neuroscience. 2011;28:163–173.
2. Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annual Review of Cell and Developmental Biology. 2007;23:549–577.
3. Sugihara I. Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum. 2011;10:449–463.
4. Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313.
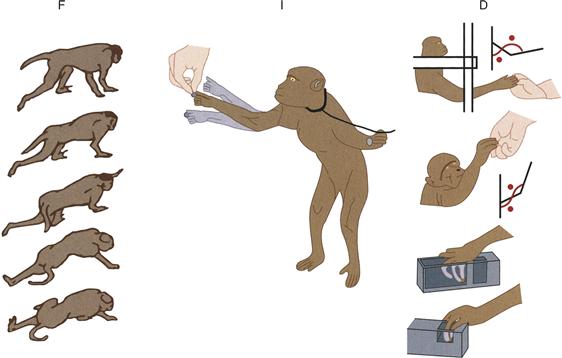
Figure 31.3 Major deficits produced by microinjection of muscimol and kainic acid into different regions of the cerebellum. F indicates the standing and walking deficit seen after muscimol injections into the fastigius. I indicates arm position (tremor) during reaching after muscimol injection into the interpositus. D indicates the deficits in reaching and pinching after muscimol injection into the dentate.
Adapted from Thach et al. (1980).
Basic Cerebellar Circuit
The structure of the cerebellar circuit is highly stereotyped (Fig. 31.4; though see Box 31.2). Mossy fiber inputs, which are excitatory, arise from a wide variety of pontine, medullary, and spinal areas (Fig. 31.2). Mossy fibers diverge to send axon collaterals to the cerebellar nuclei before ascending to the inner, granular layer of the cerebellar cortex; they typically synapse bilaterally but in some cases are restricted ipsilaterally. In each folium of the cortex, a mossy fiber makes up to 50 large glomerular synapses with a relatively large presynaptic specialization known as a rosette, which excites one dendrite from each of ~20–30 granule cells. Granule cells typically have 3 to 5 short dendrites, each of which terminates in a glomerulus; thus, each granule cell receives input from 3 to 5 mossy fibers. Granule cells are the most common cell type in the entire brain (~50 billion in humans), providing an unparalleled capacity for combinatorial and sparse coding of inputs. The axons of granule cells ascend radially, making a few synaptic contacts on Purkinje cells on their way to the outer molecular layer, where they bifurcate into a T shape and extend long, unmyelinated branches termed the parallel fibers (so named because they run transversely, parallel to the folia). Each parallel fiber reaches up to 1 cm through the cortex and releases glutamate onto the dendritic spines of ~75 Purkinje cells. The parallel fibers in any one region of the cortex may arise from granule cells in the medial, intermediate, and lateral zones of the cerebellum. Thus, each Purkinje cell may receive information about sensory conditions, internal states, and the plans of the organism. It is notable that while the synapse from mossy fiber to granule cell is robust, each parallel fiber synapse on Purkinje cells is quite small (1 nS measured at soma). Therefore, it is assumed that the convergent activity of many parallel fibers is required to modulate the simple spike firing rate of Purkinje cells. Approximately 150,000 parallel fibers contact a given Purkinje cell.
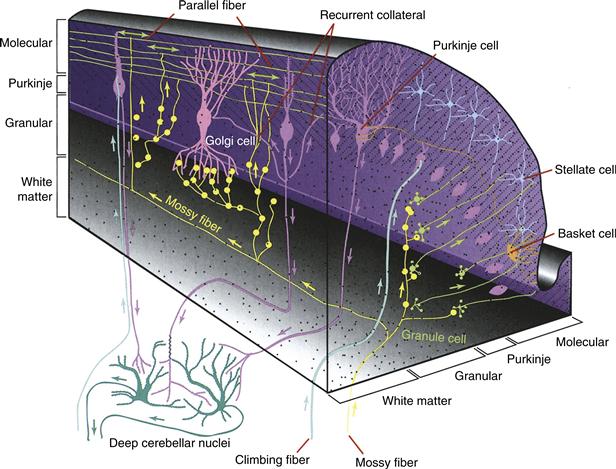
Figure 31.4 Schematic view of cerebellar cortex. This section shows the three-dimensional relationship between the different cell types— specifically, the medullary layer or white matter, granular layer, basket cells, stellate cells, Purkinje cells, Golgi cells, granular cells, parallel fibers, climbing fibers, mossy fibers, deep cerebellar nuclei, and recurrent collaterals.
Adapted from Fox, C. A. (1962). The structure of the cerebellar cortex. In Correlative Anatomy of the Nervous System. New York, MacMillan. [Fig. 16.10A].
Box 31.2 Differences between the Vestibular and Nonvestibular Portions of the Cerebellum
Phylogenetically, the vestibular and fastigial (medial) cerebellar nuclei predate the interpositus and dentate. Perhaps as a result, the vestibular and fastigial cerebellar circuits exhibit some distinctive properties compared to their relatively younger neighbors:
1. Unipolar brush cells are present in highest densities in the granule cell layer of the flocculonodular lobe. These excitatory neurons receive mossy fiber input, like granule cells, but synapse locally onto granule cells. Golgi cells feed back to unipolar brush cells with mixed glycinergic and GABAergic synapses, in contrast to the purely GABAergic feedback to granule cells (Dugue, Dumoulin, Triller, & Dieudonne, 2005). The role of these neurons in the vestibular circuit is unclear.
2. Olivary neurons in the dorsal cap of Kooy, to which both the vestibular and fastigial nuclei project, have different intrinsic electrical properties than those in the principal and accessory olive. Neurons in the dorsal cap exhibit an approximately linear relationship between injected current and resulting firing rate, tonically firing at up to 35 Hz during a current step. In contrast, neurons in the rest of the olive exhibit significant subthreshold oscillations, usually only fire a couple of spikes at a maximum of 10 Hz in response to depolarization, and are relatively insensitive to depolarizing current injection (Urbano, Simpson, & Llinas, 2006).
3. In a similar vein, the GABAergic feedback from the vestibular cerebellum to the dorsal cap of Kooy is phasic and time-locked to presynaptic firing. The comparable feedback from nonvestibular cerebellum to the principal and accessory olive is slow, aphasic, and appears to be mediated by asynchronous release of GABA (Best & Regehr, 2009).
4. The vestibular and fastigial nuclei contain two major types of premotor projection neurons: glutamatergic neurons that project to contralateral targets, and glycinergic neurons that project to ipsilateral targets. Premotor projection neurons in the interpositus and dentate are exclusively contralateral-projecting and glutamatergic (although the dentate nucleus contains in addition some nucleocortical glycinergic neurons of unknown role) (Bagnall et al., 2009; Shin et al., 2011; Uusisaari & Knopfel, 2010).
5. Some projection neurons in the vestibular nuclei synapse directly onto motor neurons, whereas projection neurons in the nonvestibular cerebellum influence behavior less directly via synapses onto thalamus, pons, medulla, and colliculi.
As a result of these differences, it is not known whether computations carried out by one portion of the cerebellum are necessarily the same as those carried out by the other. The generally homologous structures of the vestibular and nonvestibular parts of the cerebellum may mask significant underlying dissimilarities in the nature of their information processing. This distinction may be related to the need for high-speed processing in the vestibular system, or perhaps to the relatively tight coupling between vestibular signaling and motor output.
Martha Bagnall and Michael Mauk
The second major input to the cerebellum is the climbing fiber, which originates from inferior olivary neurons in the contralateral brainstem. Climbing fibers branch in the sagittal plane and make contact with 7 to 10 Purkinje cells, often in more than one lobule. After a developmental pruning period, each Purkinje cell receives input from only one climbing fiber, which makes about 300 synaptic contacts onto its soma and dendrites. The simultaneous release of multiple vesicles of glutamate from each of these contacts results in a powerful postsynaptic current that drives an unusual action potential termed a “complex spike.” The complex spike consists of an action potential followed by several spikelets riding on the back of a larger plateau depolarization (Fig. 31.5). Two to four of these spikelets are sufficiently large to drive axonal action potentials, so that a downstream neuron in the cerebellar or vestibular nuclei is thought to see a short, high-frequency burst of Purkinje input after each complex spike. Olivary neurons fire at about 1 Hz and increase firing as an animal encounters novel conditions or tries to adapt its movement. Most climbing fiber axons also emit collaterals in the cerebellar nuclei, but these synaptic connections have only been studied to date at the anatomical, not physiological, level.
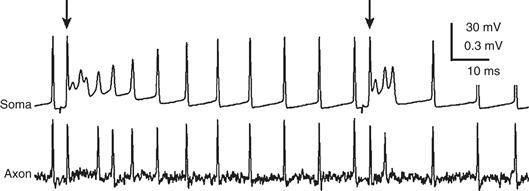
Figure 31.5 Whole-cell recording of a Purkinje cell at the soma (top) and cell-attached recording from its axon (bottom). The Purkinje cell fires “simple spikes” spontaneously, all of which are transmitted down the axon. At the times indicated by arrows, a complex spike is elicited by climbing fiber stimulation. Two to three of the spikes associated with the complex spike drive axonal spiking.
Adapted from Khaliq and Raman (2005).
Purkinje cell output is inhibitory and targets exclusively the neurons of the cerebellar and vestibular nuclei. The axon of a Purkinje cell projects ipsilaterally to one of these nuclei, where it diverges onto three main types of neurons: (1) GABAergic neurons that project back to the inferior olive, closing an inhibitory feedback loop; (2) large projection neurons, most of which are excitatory; and (3) short-axon inhibitory neurons of unknown function. The large projection neurons serve as the sole output of the cerebellar circuit and send their axons to regions of the brain as diverse as the thalamus, superior colliculus, and pons. A small subset project back to the cerebellar cortex, with unknown functional consequences (Gould & Graybiel, 1976; Uusisaari & Knopfel, 2010). Surprisingly, given their role as sole output of the large and computationally intense cerebellum, cerebellar and vestibular nucleus neurons are few in number, approximately 1/30 as many as Purkinje cells (Palkovits, Mezey, Hamori, & Szentagothai, 1977). Similar to Purkinje cells, they fire spontaneously at high rates in vivo and in vitro (~30 Hz, with significant variation), with their firing rates modulated during various types of behaviors.
Complete Cerebellar Circuit
Five other cell types make up the complete cerebellar circuit (Fig. 31.4). Inhibitory Golgi cells reside in the upper portion of the granule cell layer. Their ascending dendrites extend to the outer molecular layer, where they receive excitatory inputs from parallel fibers; their basal dendrites remain in the granule cell layer, where they receive excitatory inputs from mossy fibers. Each Golgi cell synapses onto several thousand granule cells, enabling it to provide both feedforward and feedback inhibition, depending on whether its activity is driven more by mossy fibers or parallel fibers, respectively. Golgi cells also appear to make direct inhibitory synapses on each other (Hull & Regehr, 2012).
Also in the granule cell layer are Lugaro cells, located just below the Purkinje cell layer. Lugaro cells form inhibitory synaptic contacts onto Golgi cells as well as basket and stellate cells in the outer molecular layer (see below). A distinguishing characteristic is that Lugaro cells, unlike Golgi cells, are sensitive to serotonin. Serotonergic activation of Lugaro cells may indirectly activate other circuit elements by inhibiting inhibitory Lugaro cells (Geurts, De Schutter, & Dieudonne, 2003).
The third identified member of the granule cell layer is the glutamatergic unipolar brush cell (Mugnaini, Sekerková, & Martina, 2011), which is found in highest densities in vestibular regions (the nodulus and flocculus) of the cerebellum. Like granule cells, they receive excitatory inputs from mossy fibers, but their axons branch locally within the granule cell layer and form excitatory synapses onto granule cells. They are inhibited by Golgi cells at glycinergic or mixed glycinergic/GABAergic synapses. In vitro studies have shown that unipolar brush cells respond to brief bursts of excitatory input with prolonged bursts of output that far outlast the stimulus. The influence of this cell type on network computation is far from understood.
Two types of GABAergic interneurons, basket and stellate cells, reside in the outer molecular layer. Both are excited by parallel fibers and inhibit Purkinje cells; basket cells target the soma and perisomatic region of the Purkinje cell, whereas stellate cells contact Purkinje dendrites. Thus, both basket and stellate cells provide feedforward inhibition from granule cells to Purkinje cells. Both cell types also receive weaker, nonsynaptic excitation from climbing fibers. Basket cells, but not stellate cells, are inhibited by Purkinje cell axon collaterals that ramify near the Purkinje cell layer, creating an inhibitory loop. Because the basket cell axons traverse along parasagittal strips, this inhibitory loop may provide a way for each Purkinje cell in a parasagittal strip to entrain the firing of its sister Purkinje cells via disinhibition (inhibition of inhibition). Classically, these outer molecular layer interneurons are thought to provide a type of “off-beam” inhibition, wherein the parallel fibers excite Purkinje cells directly in their path, but Purkinje cells located to either side are inhibited by basket and stellate cells. Thus, the net effect of activity in a small cluster of granule cells would be an increase in firing in the transverse strip of Purkinje cells directly above and a decrease in firing in the strips of Purkinje cells on either side of the “on-beam” activity. Whether this occurs outside of the context of experiments stimulating beams of parallel fibers and whether basket and stellate cells contribute equally to this phenomenon are both unclear.
Finally, in addition to the mossy fiber and climbing fiber inputs to the cerebellum, there is a third type of modulatory input with a widespread, nonlaminar distribution. These afferents originate in the locus ceruleus, raphe nuclei, and other brainstem and hypothalamic nuclei. They innervate the cerebellar and vestibular nuclei and all cortical layers, with some preference from the molecular layer. Modulatory inputs synapse onto Purkinje cells as well. Afferents from the locus ceruleus release norepinephrine, those from the raphe release serotonin, those from the brainstem release acetylcholine, and those from the hypothalamus release histamine. Synapses of nonlaminar afferents have been difficult to define at the electron microscopic level because of their sparseness and lack of distinctive structural characteristics. As a result, little is known about the specifics of their connectivity or their functional roles in modulating cerebellar activity (Ito, 2009).
Development
Cerebellar cell types develop at different times and at different places. First to be formed are the neurons of the cerebellar nuclei. They are followed soon after by Purkinje cells, which originate in the ventricular epithelium and migrate to their ultimate location in the cortex. Golgi cells, basket cells, stellate cells, astrocytes, and Bergmann glia also originate at the ventricular epithelium and migrate to their final positions after the migration of the Purkinje cells. The Bergmann glia later guide the descent of the granule cells from the external granule cell layer.
Once the Purkinje, Golgi, basket, and stellate cells have formed, climbing fibers enter the cerebellum from the inferior olive and begin to innervate the Purkinje cells. Much later, after the Purkinje cells have begun to receive synapses from parallel fibers, most of the climbing fiber contacts with Purkinje cells are eliminated. Mossy fibers also enter the cerebellum and grow to the level just below the Purkinje cell layer. They will ultimately synapse on granule cells, which have yet to arrive.
The granule cells first develop at a very distant site, posterior and lateral in the anlagen at the rhombic lip. They then crawl over the Purkinje cells to form an external granule cell layer. Some granule cells may cross the midline to the other side of the cerebellum as they make this migration. The granule cells extend their axons, which branch to form parallel fibers that run as coronal beams through the dendrites of the Purkinje cells. Only then do the granule cells descend, guided by the Bergmann glia, to form the internal granule cell layer. Each granule cell is connected by the proximal portion of its axon to the branch point of its parallel fibers, which remain in the external granule cell layer. In the internal granule cell layer, each granule cell dendrite is contacted by the terminals of the mossy fibers and gradually forms synaptic connections with them. Synapses are also made between granule cells and the intrinsic cortical inhibitory neurons—the Golgi, stellate, and basket cells. Notably, the output side of the cerebellar circuit (vestibular and nuclear neurons and Purkinje cells) forms first, the input side (mossy fibers) then arrives, and the “matrix” that connects the two (the granule cells and intrinsic inhibitory neurons) is the last to develop (Ray & Dymecki, 2009; Sillitoe & Joyner, 2007).
In humans, the first cerebellar structures develop at approximately 32 days after fertilization, and development is not completed until after birth. The cerebellar cortex of the early embryo has six distinct layers, but this number is ultimately reduced to three. The cortex begins to differentiate slightly earlier in the vermis, flocculus, and median sections of the hemispheres than in the lateral hemispheres. By 7 months after fertilization, the cerebellar nuclei have attained the shape and location they will have in the adult. At birth, the cerebellar cortex consists of four uneven layers, and the Purkinje cells and basket cells are weakly developed. The fourth layer (the external granule cell layer) disappears within the first postnatal year. In humans, full myelination of cerebellar connections is not complete until the second year of life (Brody, Kinney, Kloman, & Gilles, 1987; Wang and Zoghbi, 2001).
Summary
The cerebellum is a prominent structure in the nervous systems of most vertebrates. The cerebellum receives inputs from many parts of the nervous system but predominantly influences motor circuits and the cerebral cortex. This suggests that many different kinds of information are brought together to coordinate and adaptively control movement and certain cognitive functions. The intrinsic structure is relatively simple and very stereotyped throughout the cerebellum, very different from that of the cerebrum. The ontogenetic development is conspicuously late: cell migrations and fiber connections continue to occur after birth and the development of the rest of the motor nervous system.
Cellular and Synaptic Performance of the Cerebellar Circuit
Distinctive Properties of Cerebellar Neurons
The continuous high firing rates of both Purkinje cells and vestibular/nuclear neurons are unusual in comparison to most other brain regions. These cerebellar neurons are not only active during behaviors, but they also play critical roles in the induction and expression of long-term memories. These constraints pose a distinct set of challenges for the underlying cellular and synaptic physiology. This section examines the adaptations and performance of several aspects of the cerebellar circuit: high spontaneous firing rates, four distinctive synaptic connections, and multiple forms of synaptic and intrinsic plasticity.
Spontaneous, Continuous High Firing Rates
The advantage of the high firing rates of Purkinje and nuclear/vestibular neurons is thought to be the resulting rapid, bidirectional modulation of firing rate that enables tight temporal control over movement and planning. However, high firing rates incur a significant metabolic cost. Each time a neuron fires an action potential, sodium and calcium enter the neuron and potassium exits. These ions must be pumped back across the membrane with ATP-driven ion exchangers to regain their balance. Purkinje and cerebellar/vestibular nucleus cells express a specific complement of ion channels that help minimize this cost and maintain the cell’s spontaneous rate. They are enriched for Kv3 potassium channel subtypes, which have rapid kinetics that speed cell repolarization. As a result, action potentials are brief in duration, limiting the window for sodium and calcium entry, so that fewer of these ions will have to be pumped back outside. Rapid repolarization together with specialized sodium channels (Bant & Raman, 2010; Raman & Bean, 1991) minimizes the inactivation of sodium channels that typically occurs during each action potential (Carter & Bean, 2011; Gittis et al., 2010). These biophysical properties are critical for enabling cerebellar neurons to rapidly and precisely modify their downstream targets.
Mossy Fiber Glomerulus
The mossy fiber axon terminates in glomerular structures in the granule cell layer, each of which can contain a dendritic terminus from each of ~20 to 30 granule cells and one Golgi cell basal dendrite. Each mossy fiber forms 15 to 50 such glomeruli per folium, so that it directly influences as many as 1000 granule cells and 50 Golgi cells in one folium. Mossy fiber firing patterns vary across the cerebellum, depending on the source of the fiber, and can range from prolonged firing at tens of Hz to short bursts at instantaneous frequencies upwards of 200 Hz. The mossy fiber to granule cell synapse achieves reliable transmission of high bursts by rapid recycling and reloading of a large pool of available vesicles (Saviane & Silver, 2006). It is thought that the conjunctive activity of several mossy fiber inputs is required to trigger granule cell firing in vivo, but it is possible that just one is sufficient. The small size and close quarters of granule cells make it technically challenging to record the spiking of individual neurons, leaving this question open to further study.
Climbing Fiber Synapse
The climbing fiber that arises in the inferior olive makes an intricate, ivy-like connection with each of its Purkinje cell targets, synapsing along the entire dendritic arbor at ~2–3 µm intervals. Ascending axonal branches also have contact points with molecular layer interneurons, although the dominant mode of transmission appears to be through spillover of glutamate from nearby release sites (Szapiro & Barbour, 2009). While the climbing fiber ascending synapse onto Purkinje cells is the best studied, the axon also makes a number of transverse branches of unknown function, with few conventional synapses (Nishiyama, Fukaya, Watanabe, & Linden, 2007).
Each synaptic contact between the climbing fiber and Purkinje cell is ensheathed by glial processes, deriving from Bergmann glia, that reduce the spillover of glutamate from the synaptic cleft to other sites. This specialization may be due to the large amount of glutamate released by the climbing fiber: not only does each axon make hundreds of contacts with one Purkinje cell, each contact is able to release multiple vesicles of glutamate at a time (Wadiche & Jahr, 2001). Furthermore, inferior olive neurons can fire complex spikes as well, in which a high-frequency burst (3–4 spikes at 100–200 Hz) is successfully transmitted down the axon, leading to an even larger glutamate release (Mathy et al., 2009). The result of all this glutamate release is the Purkinje cell complex spike. Each complex spike causes ~2 to 3 spikes to propagate down the Purkinje cell axon, at instantaneous rates of up to ~400 Hz (Fig. 31.5). However, the specific waveform of the complex spike, and thus the number of action potentials that propagate down the Purkinje cell axon, is determined by the characteristics of climbing fiber synaptic transmission and is subject to change. Interestingly, the ~50 Hz simple spike activity in a Purkinje cell is often suppressed for tens of ms after the complex spike, so that the net effect of this synapse is a burst-pause. The flood of calcium triggered by climbing fiber activity is a critical signal for parallel fiber plasticity (see below).
Purkinje Cell to Cerebellar Nuclear Neuron Synapse
Purkinje cells fire simple spikes spontaneously at ~50 Hz, modulate their firing up to 100–200 Hz, and can achieve instantaneous frequencies of up to 400 Hz in response to climbing fiber input. How do the output neurons of the cerebellar circuit respond? The Purkinje cell synapse onto cerebellar nucleus neurons is robust. Each axon terminal is large (~3–4 µm diameter) and contains around 10 individual release sites (Telgkamp, Padgett, Ledoux, Woolley, & Raman, 2004). Furthermore, each Purkinje cell axon makes several synaptic contacts onto a single cerebellar nucleus neuron, providing a total of 100 to 200 release sites at a unitary connection. Although the probability of transmitter release is low at each site, the sheer number of release sites guarantees that a given Purkinje cell can reliably release transmitter to a cerebellar nucleus neuron even at high rates of presynaptic firing.
The high spontaneous rates of cerebellar nucleus neuron firing enable Purkinje cell inhibition to sculpt postsynaptic responses in several different ways. Dozens of Purkinje cell axons converge onto individual nuclear neurons. Synchrony in the firing of converging Purkinje cells enables inhibition to produce precise delays in postsynaptic firing with little effect on overall rate; conversely, asynchronous firing of Purkinje cells results in a reduction in nuclear neuron firing rates (Person & Raman, 2012). Most nuclear neurons respond to the offset of hyperpolarization with a transient increase in firing rate, termed rebound firing, before settling into a new, elevated rate relative to the inhibited state. Whether Purkinje cell inhibition is sufficiently strong to engage rebound firing remains unclear. One working hypothesis is that the relief of Purkinje cell inhibition, evoked by a synchronized pause in Purkinje cell firing, serves as a precisely timed signal to increase cerebellar nuclear neuron firing and modulate downstream behavior. In this scenario, the timing of the Purkinje cell pause would be controlled by temporal coding provided by granule cell inputs so that learned Purkinje cell responses can be temporally specific.
Cerebellar Nuclear to Olivary Input
GABAergic neurons in the cerebellar and vestibular nuclei give rise to thin axons that project to the inferior olive, closing the feedback loop of the cerebellum. Little is known about the firing rates of these GABAergic neurons with respect to behavior, because of the difficulty in identifying these neurons during in vivo recordings. However, the connection is clearly required for extinction of learned reflexes (as described below). Nuclear and vestibular neurons make synapses on olivary dendrites. In vivo studies have shown that stimulation of the nucleo-olivary tract causes a slow, long-lasting inhibition of olivary firing. Recent work has revealed that the sluggishness of this response derives from asynchronous release of GABA, yielding a barrage of miniature synaptic events. The resulting synaptic current is small in amplitude but lasts tens of milliseconds (Best & Regehr, 2009). Interestingly, inputs from vestibular nuclei to the olive do not share this characteristic: they are phasic and rapid in time course (Box 31.2). Nuclear synapses onto olivary dendrites occur preferentially close to the gap junctions that interconnect olivary neurons (De Zeeuw et al., 1998). This localization suggests that nuclear input might selectively weaken olivary coupling (Lang, Sugihara, & Llinas, 1996).
Long-Term Depression and Potentiation at the Parallel Fiber to Purkinje Cell Synapse
The cerebellum is the site of certain types of memory formation and storage. Activity-dependent plasticity has been found in synapses and neurons throughout the cerebellar circuit (Carey, 2011). The following section describes two forms of cerebellar plasticity that have been long hypothesized to mediate cerebellar-dependent learning.
Marr first proposed the existence of plasticity at the parallel fiber to Purkinje synapses whose induction is controlled by the climbing fiber input (Marr, 1969). Later, Masao Ito and colleagues demonstrated climbing fiber-controlled induction of long-term depression (LTD) at these synapses (Ito, Sakurai, & Tongroach, 1982; Ito, 1996). This plasticity and the related implication that the climbing fibers provide a “teaching” input to the cerebellum are at the core of theories of cerebellum function (Ito, 2001).
The strength of the granule cell to Purkinje cell synapse can be studied over the period of several hours in slice preparation (or more rarely, in vivo). Classic early studies showed that when a set of parallel fibers is stimulated repeatedly and in conjunction with the other major input to a Purkinje cell, the climbing fiber, the amplitude of the parallel fiber synapse is reduced. This depression is maintained for the duration of recording and thus is termed parallel fiber long-term depression (Fig. 31.6A). Somatic depolarization of the Purkinje cell can substitute for climbing fiber activity. Subsequent studies revealed more about the relative timing required between parallel fiber and climbing fiber activation to induce parallel fiber LTD: best induction of LTD requires parallel fiber activation 100–250 ms prior to the climbing fiber input. This agrees well with in vivo studies suggesting that climbing fiber inputs modify synapses onto Purkinje cells that were active 100–200 ms prior (Raymond & Lisberger, 1996). In contrast, repeated stimulation of the parallel fiber pathway by itself, in the absence of climbing fiber activity, leads to potentiation of the stimulated inputs (long-term potentiation) (Fig. 31.6B) (Coesmans, Weber, De Zeeuw, & Hansel, 2004). Mechanistic analyses reveal that paired parallel and climbing fiber input produces an mGluR-driven signaling cascade in Purkinje cells (Linden et al., 1991), leading to the removal of AMPA-type glutamate receptors from the parallel fiber synapse (Wang & Linden, 2000). However, the requirement of parallel fiber LTD for cerebellar motor learning is unclear. Using genetically modified mice in which the mechanisms responsible for the expression of LTD are specifically impaired, some authors have found impairments in motor learning while others see no changes (De Zeeuw et al., 1998; Schonewille et al., 2011).
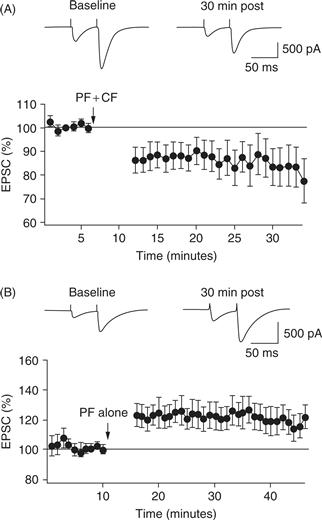
Figure 31.6 Whole-cell recordings from Purkinje cells showing the synaptic result of parallel fiber stimulation over time. (A) After a baseline period, parallel fiber stimulation is paired with conjunctive climbing fiber stimulation every 1 s for 5 min (arrow). Afterwards, the parallel fiber synaptic amplitude is depressed (LTD). (B) The parallel fiber is stimulated as in A, but in the absence of climbing fiber input potentiated (LTP).
Adapted from Coesmans et al. (2004).
Synaptic and Intrinsic Forms of Plasticity in Vestibular and Cerebellar Nucleus Neurons
Miles and Lisberger proposed the hypothesis that cerebellar learning is mediated by plasticity downstream of Purkinje cells, at synapses onto cerebellar and vestibular nucleus neurons (Miles & Lisberger, 1981). They proposed that an “error signal” is calculated in the cerebellar cortex and the result of that calculation is conveyed by the Purkinje cells to their target neurons, where it serves as a teaching input to control the induction of plasticity. In vivo recording studies provided strong evidence for this hypothesis during adaptation of the VOR and during eyelid conditioning.
Recent work has found two major forms of plasticity in Purkinje cell target neurons. The first is changes in intrinsic excitability. Target neurons in the vestibular and cerebellar nuclei can modify their intrinsic input-output functions, so that a synaptic input of a given size that once elicited a modest response (as evaluated by postsynaptic spiking) now elicits a strong one, or vice versa (Shadmehr, Smith, & Krakauer, 2010; Zhang & Linden, 2003). This type of plasticity might be useful for generalized changes of behavioral gains—the magnitude of the response to a given stimulus.
The second type of plasticity is synaptic. The strength of synapses from excitatory mossy fibers onto their postsynaptic targets in the cerebellar or vestibular nuclei can be bidirectionally modified. The learning rules, inspired by ideas from Lisberger and Miles applied to eyelid conditioning (Mauk & Donegan, 1997), are distinctive. Long-term potentiation (LTP) and LTD of the mossy fiber to nuclear neuron synapse are controlled by Purkinje cell inhibition in conjunction with afferent synaptic stimulation. Afferent input combined with release from hyperpolarization is required to trigger an increase in synaptic strength, whereas afferent input without release from hyperpolarization triggers decreases in synaptic strength (McElvain, Bagnall, Sakatos, & du Lac, 2010; Pugh & Raman, 2009; Zhang & Linden, 2003). Increases and decreases in Purkinje cell firing rate are the most obvious potential sources of cerebellar nuclear hyperpolarization and release, respectively. Each of these forms of plasticity is a candidate substrate of cerebellar learning, and their relative roles are under active debate and investigation.
Cerebellar Function
The stereotyped circuitry and cellular mechanisms of signaling and plasticity within the cerebellar cortex and nuclei suggest a common role for diverse regions of the cerebellum. But what exactly is that role? Dysfunction of the cerebellum leads to a variety of clinical manifestations (see Box 31.3). As described in the following sections, the cerebellum is thought to adaptively modify downstream circuits, optimizing performance in the face of changes in the external and internal environments. To optimize circuit function and behaviors effectively, the cerebellum requires information from sensory, motor, and cortical systems. Each region of the cerebellum exerts its influence via cerebellar or vestibular nucleus neurons that themselves influence a variety of downstream circuits and behaviors.
Box 31.3 Clinical Testing Can Reveal Cerebellar Damage
In the early 1900s, Gordon Holmes carefully described the movement deficits associated with discrete cerebellar lesions caused by gunshot wounds (Holmes, 1939). Lesions caused by cerebellar infarct also provide information about the role of portions of the cerebellum, although interpretation of these data can be difficult because of the frequent spread of damage to extracerebellar regions. The common theme through these studies is that cerebellar damage does not impair sensory perception, but instead causes deficits in motor coordination and adaptation. These deficits are consistent with a role for the cerebellum in producing a forward model of movement, which predicts the expected sensory result of a movement and updates the forward model if errors occur (Shadmehr & Krakauer, 2008). Several types of cerebellar deficits are seen in human patients:
Ataxia is a condition that involves lack of coordination between movements of body parts. The term often is used in reference to gait or movement of a specific body part, as in “ataxic arm movements.” Ataxia of posture, stance, and rhythm can derive from damage to vestibular and fastigial nuclei and vestibulocerebellum, while the intermediate and dentate nuclei and associated cortex appear more responsible for limb placement and multisegmental coordination. Asynergia is an inability of cerebellar patients to correct their movements for the interaction torques between multiple joints, leading to inaccurate reaching or stepping movements.
Dysmetria is an inability to make a movement of the appropriate distance. Hypometria is undershooting a target, and hypermetria is overshooting a target. Patients with cerebellar damage tend to make hypermetric movements when they move rapidly and hypometric movements when they move more slowly and wish to be accurate.
Dysdiadochokinesia is an inability to make rapidly alternating movements of a limb. It appears to reflect abnormal agonist-antagonist control. It is thought to be due to damage to the interpositus and/or dentate nuclei and resulting perturbation of muscle stretch reflexes.
Action tremor, or intention tremor, is an involuntary oscillation that occurs during limb movement and disappears when the limb is at rest. Cerebellar action tremor is generally of high amplitude and low frequency (3–5 Hz). Titubation is a tremor of the entire trunk during stance and gait. Lesions of cerebellar target structures (e.g., the red nucleus and the thalamus) often result in cerebellar outflow tremor, or postural tremor. Most prominent when a limb is actively held in a static posture, postural tremor attenuates during limb movement and disappears when the limb is at rest. Like dysdiadochokinesia, these tremors are also thought to be caused by deficient stretch reflexes.
Hypotonia is an abnormally decreased muscle tone. It is manifest as a decreased resistance to passive movement, so that a limb swings freely upon external perturbation. Hypotonia often is limited to the acute phase of cerebellar disease.
Nystagmus is an involuntary and rhythmic eye movement that usually consists of a slow and a fast phase. In a unilateral cerebellar lesion, the fast phase of nystagmus is toward the side of the lesion.
Patients with cerebellar damage are impaired both in normal motion and during motor adaptation. For example, a normal subject can adapt to wearing prism eyeglasses that shift the world to one side. After the prisms are removed, the subject shows a slow return to normal behavior, indicating that learning took place. In contrast, a cerebellar patient shows neither adaptation to the prisms, nor any sign of learning afterwards. Saccade learning is similarly impaired in cerebellar patients.
There is some promise for rehabilitation of cerebellar patients. Recent work suggests that these patients can learn to correct for small errors, either by using remaining functional circuits or through other brain circuits entirely. Cumulative learning of small adjustments can produce an overall large change in the resulting behavior, suggesting that compensation may be possible for certain types of deficit (Bastian, 2011).
Martha Bagnall and Tom Thach
Mossy Fibers and Climbing Fibers Convey Distinct Types of Information to Cerebellar Circuits
Precerebellar mossy fiber neurons distributed throughout the midbrain (red nucleus), pons, medulla, and spinal cord convey signals that inform the cerebellum about the sensory world, movements, and cortical plans for action (including, perhaps, cognitive plans that do not result in overt movements). Each type of mossy fiber neuron conveys a specific type of information to the cerebellum (Kolkman, McElvain, & du Lac, 2011). Neurons in the vestibular and external cuneate nucleus and in Clarke’s column in the spinal cord transmit sensory signals to the cerebellum (vestibular, somatosensory, and proprioceptive). Sensory information about internal state and autonomic function are handled by neurons in the lateral reticular nucleus. “Efference copy” signals (which inform the brain about commands for movement sent to motor neurons) are conveyed to the cerebellum by neurons in the nucleus prepositus and pontine reticular formation. Neurons in the pons mediate the connections between the cerebral cortex and the cerebellum. Some mossy fiber neurons fire high-frequency bursts of action potentials, while others fire tonically and modulate their firing rates in response to inputs, thus providing the cerebellum with continuous streams of information.
In contrast, neurons in the inferior olive fire rarely and convey discrete signals to the cerebellum. Each of several subdivisions of the inferior olive comprises neurons that encode unexpected signals, or errors, in a particular domain. For example, neurons in the caudal portion of the dorsal cap of the olive increase their firing rates (from 1 to 2 Hz) under only one condition: when images move horizontally across the retina. Neighboring neurons in the rostral dorsal cap fire when images move vertically. Some olivary neurons are activated in response to head motion and somatosensory, proprioceptive, nociceptive, or viscerosensory signals, while others appear to be activated when movements are planned or initiated by the superior colliculus or cerebral cortex.
Cerebellar and Vestibular Nuclei Influence Several Neural Circuits and Behaviors
Vestibular Nucleus
The vestibular nuclei comprise several subdivisions (see Chapter 29) and are important for several different behaviors. The vestibulo-ocular reflex (VOR) is the simplest and most extensively studied behavior under cerebellar control. The VOR enables clear vision during head movements by producing compensatory eye movements (see Chapter 32). A simple form of motor learning—adaptation—occurs whenever images move persistently during head movement. Adaptation can either increase or decrease the gain of reflexive eye movements. The floccular lobe of the cerebellum is required to induce adaptive changes in the VOR. Remarkably, although the VOR is mediated by as few as two muscles, Purkinje cells in the flocculus synapse onto at least five different cell types in the vestibular nucleus (Shin et al., 2011). The relative contribution of plasticity in the flocculus versus these vestibular nucleus neurons to motor learning in the VOR remains to be elucidated.
Cerebellar inputs to lateral vestibular nucleus are critical for coordinating and calibrating postural movements (see Chapter 29). Caudal vestibular nucleus neurons, which convey information about head tilt and gravity to autonomic brainstem regions (Holstein et al., 2011), are responsible for increasing blood pressure rapidly during the transition from lying down to standing up.
Fastigius (Medial Cerebellar Nucleus)
The fastigius controls all musculature involved in stance and gait. It receives input from the vermal cortex, vestibular complex, lateral reticular nucleus, and (indirectly) spinocerebellar pathways. Ablation of the fastigius dramatically impairs movements requiring control of equilibrium, such as unsupported sitting, stance, and gait. Single-unit recordings in the fastigius and vermal cortex of decerebrate cats, but not the interpositus or dentate, have shown neural discharge that is correlated with both walking and scratching movements. Rostral fastigial neurons are critical for the regulation of blood pressure and other autonomic functions. The caudal portion of the fastigius contains neurons that project to midline nuclei of the thalamus (Steriade, 1995), as well as neurons responsible for maintaining the accuracy of saccadic eye movements.
Interpositus (Intermediate Cerebellar Nucleus)
Inactivation of both the interpositus and the dentate with cooling probes elicits tremor that depends on proprioceptive feedback but is also influenced by vision. Inactivation of the interpositus causes a large-amplitude, 3- to 5-Hz action tremor as animals reach for food but has minimal effects on gait. Neurons in the interpositus fire when the holding position of a limb is perturbed. Their activity appears to control antagonist muscles that check the reflex movement of the limb to its position. Interpositus neurons also modulate their activity in relation to sensory feedback, including that from tremor accompanying movement, consistent with its role in controlling the antagonist muscles to dampen the tremor. Other evidence suggests that the interpositus is important in determining whether the pattern of activity in muscles acting at a joint represents reciprocal activation or co-contraction. During behaviors that involve co-contraction, interpositus neurons fire as if activating both agonist and antagonist muscles, and Purkinje cells are silent. In behaviors where agonists and antagonists are reciprocally active, both interpositus cells and Purkinje cells fire in similar patterns. One interpretation of these results is that alternating firing in Purkinje cells creates (through inhibition) a similar pattern of activity in the interpositus cells, which in turn produces the alternation between agonist and antagonist muscles. Other work suggests that the interpositus contributes to stretch reflex excitability by controlling the discharge of gamma motor neurons (Chapter 28). The interpositus also houses neurons required for classical conditioning of the eyelid response, as described below.
Dentate (Lateral Cerebellar Nucleus)
Neuronal activity in the dentate precedes the onset of movement and may also precede firing in the motor cortex. Dentate neurons fire preferentially at the onset of movements that are triggered by mental associations. Single-unit recordings in the motor cortex, dentate, and interpositus can be correlated with electromyograms as monkeys make wrist movements in response to stimuli. In tasks where movements are triggered by light, the order of activity is dentate, motor cortex, interpositus, and muscles. When a transient force perturbs the wrist, however, the firing order is muscles, interpositus, motor cortex, and dentate. These results suggest that the dentate influences movements triggered by stimuli that are mentally associated with the movement, whereas the interpositus is more involved in compensatory or corrective movements initiated via feedback from the movement itself. In other studies, the dentate responded strongly when movements were triggered by either visual or auditory signals but not proprioceptive signals. Firing in both dentate and interpositus neurons is thought to relate more closely to movements involving multiple joints than to those involving single joints. Neither nucleus codes for any specific parameter (e.g., velocity, amplitude, or duration) during single-jointed movements. In sum, the dentate plays a role in initiating movements that require a mental interpretation of the visual or auditory signal, and both the dentate and the interpositus are increasingly active during multijointed movements.
Theories of Cerebellar Function
With the knowledge available about cerebellar anatomy, cellular and synaptic physiology, interconnectedness with the rest of the brain, and effects of cerebellar damage on behavior, research has been strongly influenced by a variety of computationally oriented ideas about cerebellar function. Pronounced deficits in muscle tone following cerebellar damage led to early ideas that the cerebellum could serve to reinforce tone. Other theories emphasized the importance of the cerebellum in motor coordination. The organization of parallel fibers and their relatively slow conduction times were incorporated into an influential model, proposed by Braitenberg and colleagues, which postulated that the cerebellar circuit produces precise timing. Behavioral experiments in patients with cerebellar damage have lent support to the notion that the cerebellum is critical for specifying motor timing (Spencer, Zelaznik, Diedrichsen, & Ivry, 2003). The 10-Hz tremor produced by damaging inferior olive neurons with the drug harmaline and observations that olivary neurons can emit 8- to 10-Hz bursts of firing led Llinas, Welsh, and others to suggest that the cerebellum provides clocklike timing signals to the rest of the brain.
The wealth of sensory and cortical information conveyed to the cerebellum, the indirect influence of cerebellar output nuclei on motor neurons, and the extensive interconnection to nonmotor regions of the cerebral cortex (Strick, Dum, & Fiez, 2009) suggest that the primary role of the cerebellum may not be motor per se but instead may be to evaluate sensory or cortical information in the context of ongoing movements or plans. The hypothesis that the cerebellum predicts the consequences of movements is supported by behavioral studies in patients with cerebellar damage (Bastian, 2011; Shadmehr, Smith, & Krakauer, 2010). Cerebellar involvement in certain aspects of cognitive and emotional processes is consistent with a prominent role for prediction in cerebellar function (Box 31.4).
Box 31.4 Cognition, Emotion, and the Cerebellum
It no longer is viable to consider the function of the cerebellum as being confined to the control of voluntary movement, speech, and equilibrium. Considerable evidence suggests that the cerebellum is critical also for cognition and emotion. Anatomical studies demonstrate that the cerebellum is an important part of the distributed neural circuitry that subserves cognitive processing. The frontal, association, and paralimbic cerebral cortices known to subserve higher-order functions are linked with the cerebellum in a precisely organized system of feedforward and feedback loops, and physiological studies indicate that these pathways are functionally relevant (Strick et al., 2009). Cerebellar ablation and stimulation experiments in animals have demonstrated cerebellar influences on many nonmotor functions, including classical conditioning, navigational skills, cognitive flexibility, sham range, predatory attack, and aggression. Functional neuroimaging investigations of the morphologic correlates of cognitive processing using PET and fMRI in humans have revealed sites of activation in the cerebellum in a number of cognitive tasks. These include linguistic processing, verbal working memory, shifting attention, mental imagery, classical conditioning, motor learning, sensory processing, and modulation of emotion. Furthermore, there appears to be a topographic organization of the sites within the cerebellum activated by these different cognitive processes.
Clinical investigations of adults and children with diseases confined to the cerebellum have defined a cerebellar cognitive affective syndrome characterized by impairments of executive processing, working memory, visual spatial reasoning, language disturbances (ranging from mutism to agrammatism), and a flattened or inappropriate affect. The net effect of these deficits is a lowering of overall intellectual ability. The posterior lobe of the cerebellum appears particularly important in the generation of this syndrome, and the vermis is consistently involved when the affective component is pronounced. Elements of this clinical syndrome have also been noted in patients with developmental cerebellar anomalies, cerebellar degeneration, autism, and fragile X syndrome. In addition, cerebellar abnormalities, particularly in the vermis, have been observed in patients with schizophrenia.
The relationship between the cerebellum and nonmotor function has been conceptualized as follows.
1. The cerebellum is able to subserve cognitive and emotional functions because it is anatomically interconnected with the associative and paralimbic cortices.
2. The convergence of inputs from multiple regions of the cerebral cortex to adjacent areas within the cerebellum facilitates cerebellar regulation of supramodal functions.
3. The cerebellar contribution to cognitive and emotional processes is one of modulation rather than generation.
4. The cerebellum performs computations for cognitive functions similar to those for the sensorimotor system—but the nature of the information being modulated is different.
5. The disruption of the cerebellar influences on higher functions can lead to dysmetria of thought or impairment of mental agility.
6. The cerebellum may play an important role in the development of cognitive and emotional function.
The potential for further discovery in this field places the cerebellum, previously thought of as a motor control device, in the forefront of current behavioral neuroscience research.
Sascha du Lac and Jeremy D. Schmahmann
The most influential theory, which implicated the cerebellum in motor learning, was proposed more than four decades ago by Marr (1969). At that time, the basic cerebellar circuit had been recently identified; from it, Marr inferred the essential learning attributes of the cerebellum including plasticity at the granule cell to Purkinje cell synapses and the role of climbing fibers as a teaching input to control the induction of this plasticity. These ideas inspired Masao Ito’s demonstration, in the context of the VOR, of climbing fiber controlled LTD (and much later the demonstration of the induction of LTP when granule to Purkinje synapses are active without a climbing fiber input; Fig. 31.6). An early link between circuit and behavior came from experiments showing changes in Purkinje cell simple and complex spikes during the course of motor learning (Fig. 31.7). The importance of learning in the cerebellum and how it may contribute to cerebellar computation and function remain actively investigated issues.

Figure 31.7 Simple and complex spikes recorded during motor learning task.
Adapted from Ito; original reference Gibert & Thach (1977).
Computation as a Unifying Theme
What computations does the cerebellum perform on its inputs? Like all brain systems, the essence of what the cerebellum does is this: it receives inputs from other parts of the brain, acts on these inputs with rules determined by its connectivity and synaptic and cellular properties, and produces output to other brain systems. Given that the synaptic organization of the cerebellum is relatively uniform, it is likely that the same computational principles apply qualitatively for cerebellar regions involved in motor, autonomic, and cognitive functions.
Analyses of what (and how) the cerebellum computes have been facilitated greatly by the advantages provided by a limited number of experimental preparations amenable to quantitative behavioral analyses. These include adaptation of the vestibulo-ocular reflex, saccadic eye movements, and reaching, as well as motor learning in smooth pursuit eye movements. Insights from these behaviors have been complemented by the analysis of classical associative (Pavlovian) conditioning of eyelid responses.
Cerebellum and Eyelid Conditioning
The procedures of eyelid conditioning are relatively straightforward: an animal is presented with training trials that involve a relatively neutral stimulus such as a tone paired in time with a reinforcing “unconditioned stimulus” such as a puff of air or mild electrical stimulation near the eye; we will use “puff” for convenience. In an untrained subject the puff elicits a reflex eye blink response, but the tone elicits no response. After a sufficient number of training trials, the tone comes to elicit learned eyelid responses (Fig. 31.8A; sample eyelid sweeps). Remarkably, the timing of these learned eyelid responses precisely anticipates the timing of the puff onset: animals can be trained using a range of temporal intervals between tone and air puff onsets and are capable of learning responses appropriate for each interval: the learned responses peak just before the air puff occurs (Fig. 31.8B). This learning is bidirectional: presenting the tone without the air puff to an already trained animals results in extinction of the learned eyelid responses (Fig. 31.8C).

Figure 31.8 Eyelid conditioning. (A) Top, schematic of the training procedure. A tone is presented for a brief duration (usually ~500 ms), coterminating with a brief air puff to the eye. Bottom, traces of eyelid movement during training. Each line represents one trial; the period in blue is the tone presentation. On the first day of training, animals only blink in response to the air puff; by the second day, the eyelid begins to move before the end of the tone, eventually reaching peak movement that is well timed to block the air puff. (B) Animals can learn a range of tone durations, with appropriately timed blinks regardless of the interval between tone onset and air puff. (C) Extinction of prior learning. A well-trained animal blinks at the appropriate time even when the air puff is not presented, but after repeated trials with no air puff, the animal ceases to blink in response to the tone alone.
Support for the conclusion that eyelid conditioning is mediated by cerebellar learning comes from lesion studies and from experiments employing electrical stimulation and reversible pharmacological inactivation. Lesions of either the cerebellar cortex or the cerebellar nucleus (anterior interpositus) prevent naive animals from acquiring learned eyelid responses (Garcia, Steele, & Mauk, 1999). Robust eyelid conditioning can be induced by direct electrical stimulation of mossy fibers, which can substitute for the tone, combined with stimulation of climbing fibers, which can substitute for the air puff. Reversible inactivation studies provide complementary evidence for a critical role of the cerebellum: pharmacological inactivation of the red nucleus (which receives inputs from the interpositus cerebellar nucleus) during training precludes expression of responses, but learned responses are present in the first trials after removal of the block. This indicates that the plasticity responsible for learning happens upstream of the red nucleus. Such studies demonstrate that eyelid conditioning is mediated by neuronal plasticity within the cerebellar cortex, the cerebellar nuclei, or both (Fig. 31.9).
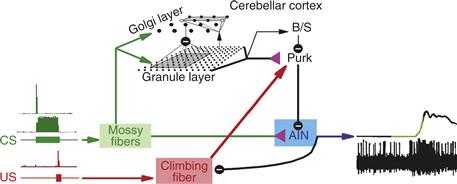
Figure 31.9 Circuit underlying eyelid conditioning. The conditioned (CS; tone) and unconditioned stimuli (US; air puff) activate mossy and climbing fibers, respectively. Some mossy fibers fire throughout the duration of the tone, while others fire only at the onset (green). Mossy fibers diverge to granule and Golgi cells in the granule layer, and to neurons in the anterior interpositus nucleus (AIN). The granule cells influence basket/stellate (B/S) and Purkinje cells, which in turn modulate AIN firing. Plasticity at the granule cell to Purkinje synapse, as well as the mossy fiber to nucleus synapse, appears to occur during learning. As a result, AIN neurons are thought to see a powerful barrage of Purkinje inhibition during the beginning of the tone. The relief of inhibition combined with mossy fiber excitation at the appropriate time triggers AIN firing, which (via the red nucleus; not shown) causes motor neuron firing to close the eyelid.
Behavioral analyses suggest differential roles for the cerebellar cortex and nuclei in eyelid conditioning. Lesions to the cerebellar cortex disrupt the learned timing of the conditioned responses. In intact animals, learned responses peak at the time that the air puff was presented. In contrast, animals with cerebellar cortex lesions are unable to learn appropriately timed responses and instead respond at a short, fixed latency. Subsequent work using stimulation and reversible inactivation studies has shown that these short-latency responses are mediated by plasticity in the cerebellar nucleus. Together, these findings suggest (1) cerebellar learning is mediated by plasticity in both the cerebellar cortex and the cerebellar nuclei, (2) the cerebellar cortex is necessary for the learned and adaptive timing of the conditioned responses, and (3) consistent with the Miles and Lisberger theory, the cerebellar cortex provides the teaching signal for the induction of plasticity in the cerebellar nuclei.
Eyelid conditioning studies have revealed a role for the inhibitory feedback from cerebellar nuclei to the inferior olive (Medina, Nores, & Mauk, 2002). This portion of the cerebellar circuit has been particularly difficult to examine because olivary-projecting neurons are intermingled with other cerebellar nucleus neurons and because the olive itself is a tiny structure located deep in the brainstem. Pharmacological blockade of synaptic receptors implicates the inferior olive in memory storage. Learned eyelid responses are extinguished (a form of forgetting) if the tone conditioned stimulus is presented repeatedly by itself, without the air puff. Eventually, animals cease to emit appropriately timed eyelid responses to the tone. In previously trained animals, infusion of GABAergic synaptic blockers into the olive during tone-alone training completely prevents extinction. Conversely, infusion of excitatory synaptic antagonists causes extinction, even when the animals are being presented with the normal training stimulus of tone together with the air puff.
Feedforward Prediction Contributes to Cerebellar Coordination, Timing, and Learning
Analyses of eyelid conditioning and other behaviors suggest a common computational framework that ties together many of the functions proposed for the cerebellum. In all behaviors, sensory information can be used to coordinate, guide, and adjust movements. But most sensory systems are too slow to provide signals that can be used effectively to coordinate and control fast movements. The solution provided by the cerebellum is elegant: instead of using sensory signals to adjust ongoing movements, the cerebellum learns how to optimize subsequent movements. The computation performed by the cerebellum is thus a form of feedforward prediction.
In principle there are two ways to use sensory input to guide appropriate output: feedback and feedforward. Feedback is familiar and simple—for example, a thermostat. The measured temperature (sensory input) is compared to a target, and differences are translated into turning on the heater or cooler. Feedback systems of this sort can be accurate and reliable, but they cannot be fast. Attempts to make a feedback system fast result in oscillations as, in the thermostat example, small deviations from the target produce alternating activations of the heater and cooler as each output causes overshoot of the target temperature (Fig. 31.10). Feedforward control can be faster because it involves anticipation of errors from previous experience. A hypothetical feedforward thermostat would be outfitted with a variety of sensory devices for temperature, humidity, number of people in the room, position of the sun, number of windows open, and so on. When a rapid change in room temperature is required, this hypothetical feedforward thermostat would predict, from previous experience, the burst of hot or cold air required to produce the rapid change.
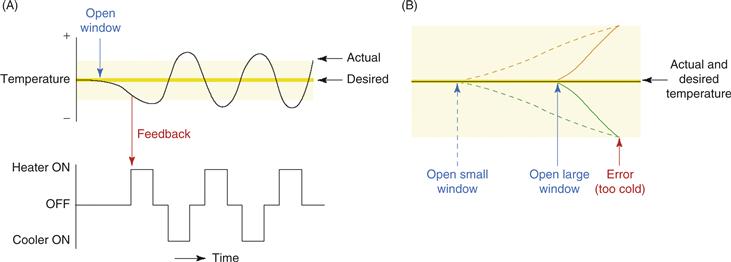
Figure 31.10 Feedback and feedforward control. (A) An example of a feedback-regulated thermostat. Though simple and accurate, feedback regulation tends to be slow, and attempts to speed it up cause oscillations. In this example, an attempted correction by a powerful heating system (in response to a decrease in temperature caused by opening a window) overshoots the mark, forcing another attempted adjustment by a powerful cooling system that also overshoots the mark, and so on. The upper and lower boundaries of the yellow area indicate the thresholds for detecting temperature change. (B) An example of a feedforward thermostat, which counteracts (orange lines) the changes in temperature predicted by the opening of windows (green lines). Though much quicker than feedback control, feedforward control is more complicated, requiring the ability to detect error-predicting stimuli (windows opening) and to delay anticipatory responses appropriately. Here, the opening of a large window (unbroken green line) predicts a faster decrease in temperature than does the opening of a small window (broken green line). The anticipatory response (increasing room temperature; broken or unbroken orange lines) must therefore be appropriately delayed in response to the predictive stimulus (opening a large or small window). This type of calibration requires associative learning that is temporally specific.
Modified from Ohyama et al. (2003).
If the thermostat’s response yields the wrong outcome, then a feedforward thermostat should learn from this mistake so that subsequent predictions in similar circumstances are better. The properties required of this learning, as instantiated in the cerebellum, are fairly specific. Learning should produce changes in cerebellar signals that are timed to occur just before the error occurred (since that was the errant output). Cerebellar learning is thus expected to be associative: it should only modify the future output of the system for inputs that are similar to the ones that were present just before the error. In addition, learning should show temporal flexibility. The learned changes in output should not be time locked to the onset of any given mossy fiber input, but should be delayed after their onset so that the changed responses fix the errant prediction—namely, the one that occurred just before the arrival of the error signal. In other words, the error signal should correct the output that just occurred.
Eyelid conditioning studies reveal precisely these properties in cerebellar learning (Ohyama, Nores, Murphy, & Mauk, 2003). It is associative in that it takes mossy fiber inputs predicting climbing fiber inputs to occur. Moreover, the timing of the responses is precisely as described above. Pairing a mossy fiber input with a climbing fiber input produces a change in cerebellar output that is not time locked to the onset of the mossy fiber input but is instead timed to peak just before the input from the climbing fiber. This shows that cerebellar learning can develop predictions, through trial and error learning with climbing fiber error signals, that improve the prediction about the proper cerebellar output for a given mossy fiber input.
These considerations may tie together many key ideas about cerebellar function. Motor coordination is improved by feedforward predictions, which must be learned. This learning requires associative learning with quite specific temporal properties. It requires motor learning with timing. An important challenge for the future will be to determine how feedforward prediction contributes to the various motor, cognitive, and autonomic behaviors that engage the cerebellum.
Summary
Ideas about cerebellar function have been driven by analyzing the effects of lesions or dysfunction of the cerebellum, by considering the nature of cerebellar inputs and outputs, and by examining the properties of cells and synapses within the cerebellar circuit. Analyses of cerebellar function that incorporate behavioral analyses, neuronal recordings, and computational perspectives are providing a framework for unifying a wide range of experimental results. The cerebellum appears to optimize downstream circuits by learning to predict the consequences of intended actions.
References
1. Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, du Lac S. Glycinergic projection neurons of the cerebellum. Journal of Neurosciences. 2009;29:10104–10110.
2. Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12357–12362.
3. Bastian AJ. Moving, sensing and learning with cerebellar damage. Current Opinion in Neurobiology. 2011;21:596–601.
4. Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron. 2009;62:555–565.
5. Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy I An autopsy study of myelination. Journal of Neuropathology and Experimental Neurology. 1987;46:283–301.
6. Carey MR. Synaptic mechanisms of sensorimotor learning in the cerebellum. Current Opinion in Neurobiology. 2011;21:609–615.
7. Carter BC, Bean BP. Incomplete inactivation and rapid recovery of voltage-dependent sodium channels during high frequency firing in cerebellar Purkinje neurons. Journal of Neurophysiology. 2011;105:860–871.
8. Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700.
9. De Zeeuw CI, Hansel C, Bian F, et al. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508.
10. De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SK, Ruigrok TJ. Microcircuitry and function of the inferior olive. Trends in Neurosciences. 1998;21:391–400.
11. Dugue GP, Dumoulin A, Triller A, Dieudonne S. Target-dependent use of co-released inhibitory transmitters at central synapses. Journal of Neuroscience. 2005;25:6490–6498.
12. Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. Journal of Neuroscience. 1999;19:10940–10947.
13. Geurts FJ, De Schutter E, Dieudonne S. Unraveling the cerebellar cortex: Cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum. 2003;2:290–299.
14. Gittis AH, Moghadam SH, du Lac S. Mechanisms of sustained high firing rates in two classes of vestibular nucleus neurons: Differential contributions of resurgent Na, Kv3, and BK currents. Journal of Neurophysiology. 2010;104:1625–1634.
15. Gould BB, Graybiel AM. Afferents to the cerebellar cortex in the cat: Evidence for an intrinsic pathway leading from the deep nuclei to the cortex. Brain Research. 1976;110:601–611.
16. Holmes G. The cerebellum of man The Hughlings Jackson memorial lecture. Brain. 1939;62:1–30.
17. Holstein GR, Friedrich Jr VL, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience. 2011;175:104–117.
18. Hull C, Regehr WG. Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity. Neuron. 2012;73:149–158.
19. Ito M. Cerebellar long-term depression. Trends in Neurosciences. 1996;19:11–12.
20. Ito M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiological Reviews. 2001;81:1143–1195.
21. Ito M. Functional roles of neuropeptides in cerebellar circuits. Neuroscience. 2009;162:666–672.
22. Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. Journal of Physiology. 1982;324:113–134.
23. Jorntell H, Hansel C. Synaptic memories upside down: Bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238.
24. Khaliq ZM, Raman IM. Axonal propagation of simple and complex spikes in cerebellar Purkinje neurons. Journal of Neuroscience. 2005;25:454–463.
25. Kolkman KE, McElvain LE, du Lac S. Diverse precerebellar neurons share similar intrinsic excitability. Journal of Neuroscience. 2011;31 16675–16674.
26. Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. Journal of Neurophysiology. 1996;76:255–275.
27. Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–89.
28. Marr D. A theory of cerebellar cortex. Journal of Physiology. 1969;202:437–470.
29. Mathy A, Ho SS, Davie JT, Duguid IC, Clark BA, Hausser M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–399.
30. Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory. 1997;4:130–158.
31. Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–333.
32. McElvain LE, Bagnall MW, Sakatos A, du Lac S. Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron. 2010;68:763–775.
33. Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: A new hypothesis. Annual Review of Neuroscience. 1981;4:273–299.
34. Mugnaini E, Sekerková G, Martina M. The unipolar brush cell: A remarkable neuron finally receiving deserved attention. Brain Research Reviews. 2011;66:220–245.
35. Nishiyama H, Fukaya M, Watanabe M, Linden DJ. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron. 2007;56:472–487.
36. Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends in Neurosciences. 2003;26:222–227.
37. Palkovits M, Mezey E, Hamori J, Szentagothai J. Quantitative histological analysis of the cerebellar nuclei in the cat I Numerical data on cells and on synapses. Experimental Brain Research. 1977;28:189–209.
38. Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–505.
39. Pugh JR, Raman IM. Nothing can be coincidence: Synaptic inhibition and plasticity in the cerebellar nuclei. Trends in Neurosciences. 2009;32:170–177.
40. Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. Journal of Neuroscience. 1999;19:1663–1674.
41. Ray RS, Dymecki SM. Rautenlippe redux—toward a unified view of the precerebellar rhombic lip. Current Opinion in Cell Biology. 2009;21:741–747.
42. Raymond JL, Lisberger SG. Behavioral analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. Journal of Neuroscience. 1996;16:7791–7802.
43. Saviane C, Silver RA. Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature. 2006;439:983–987.
44. Schonewille M, Gao Z, Boele HJ, et al. Reevaluating the role of cerebellar LTD in motor learning. Neuron. 2011;70:43–50.
45. Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Experimental Brain Research. 2008;185:359–381.
46. Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annual Review Neuroscience. 2010;33:89–108.
47. Shin M, Moghadam SH, Sekirnjak C, et al. Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. Journal of Neurosciences. 2011;31:10776–10786.
48. Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439.
49. Steriade M. Two channels in the cerebello-thalamo-cortical system. Journal of Comparative Neurology. 1995;354:57–70.
50. Strick PL, Dum RP, Fiez JA. The cerebellum and nonmotor function. Annual Review Neuroscience. 2009;32:413–434.
51. Szapiro G, Barbour B. Parasynaptic signalling by fast neurotransmitters: The cerebellar cortex. Neuroscience. 2009;162:644–655.
52. Telgkamp P, Padgett DE, Ledoux VA, Woolley CS, Raman IM. Maintenance of high-frequency transmission at purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron. 2004;41:113–126.
53. Urbano FJ, Simpson JI, Llinas RR. Somatomotor and oculomotor inferior olivary neurons have distinct electrophysiological phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16550–16555.
54. Uusisaari M, Knopfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2010;9:42–55.
55. Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nature Neuroscience. 2001;8:1329–1334.
56. Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nature Reviews Neuroscience. 2001;2:484–491.
57. Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647.
58. Zhang W, Linden DJ. The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nature Reviews Neuroscience. 2003;4:885–900.