Chapter 15
Neurogenesis and Migration
Introduction
The epithelium lining the brain vesicles in the neural tube, which is termed the ventricular zone (VZ), is the site of primary neurogenesis in the CNS. The idea that CNS neurogenesis occurs in the thin epithelium of the embryonic brain vesicles became apparent when Fred and Mary Sauer showed that cells in the neuroepithelium contain different amounts of DNA, with diploid cells located on the ventricular surface (Sauer & Chittenden, 1959). Clear images of the pseudo-columnar character of the neuroepithelium of embryonic rat brain were achieved in 3H-thymidine labeling studies of the neocortex (Berry & Rogers, 1965) and in serial scanning and electron micrographs, which showed movements of the nuclei during the principal phases of the cell cycle (Hinds & Ruffett, 1971). Cells in interphase extend processes across the thin wall of the epithelium, one cell deep at this stage, and divide at the ventricular surface. During the cell cycle, the nucleus undergoes interkinetic migration, moving from the apical surface of the ventricular zone (VZ) during G1, entering S phase as the nucleus reaches the top of the VZ, and moving back toward the ventricular surface during G2 to enter mitosis, or M phase (Sauer & Walker, 1959). A cardinal feature of the neuroepithelium is its upside-down orientation relative to epithelia of other tissues. This relates to the fact that the neuroepithelium forms a tube that orients the apical surface of neuroblasts in the epithelial sheet toward the lumen of the neural tube and their basal surface toward the pia.
The neurons of the peripheral nervous system (PNS) and CNS originate from the neural tube and the folds of the neural tube. The cells of the PNS are generated first, as they delaminate in a specialized region of the dorsal aspect of the neural tube called the neural crest. Neural crest neuroblasts migrate out into the developing nonneuronal tissues, where they will form the axon tracts of the periphery, as the progenitors of the CNS are beginning to establish the regions of the brain and spinal cord that will receive the sensory information from the PNS and establish brain circuitry. We will therefore discuss neurogenesis and migration in the PNS first, followed by patterns of neurogenesis and migration in the CNS.
Development of the Peripheral Nervous System
The Neural Crest is a Migratory Cell Population That Forms Multiple Derivatives
The neural crest is a transient population of cells, so named because they arise on the “crest” of the closing neural tube. This cell population is unique to vertebrates and forms most of the PNS. Neural crest cells migrate extensively along characteristic pathways and give rise to diverse and numerous derivatives. All of the dorsal root, sympathetic, parasympathetic, and enteric ganglia are derived from neural crest cells. Furthermore, most cranial sensory ganglia receive a contribution from the neural crest, with the remaining cells derived from ectodermal placodes. In addition to forming neurons and glia of the PNS, neural crest cells form melanocytes, cranial cartilage, and adrenal chromaffin cells. This wide variety of cell types arises from precursors in the neural folds and neural tube that are multipotent and have stem cell properties (Bronner-Fraser & Fraser, 1988; Stemple & Anderson, 1993). See Chapter 17 for a discussion of stem cells.
The neural crest originates at the border between the neural plate and the nonneural ectoderm by an inductive interaction between these two tissues (Selleck & Bronner-Fraser, 1995) (see Chapter 13). This induction initiates during gastrulation (Basch, Bronner-Fraser, & Garcia-Castro, 2006) even though precursors with the potential to form neural crest only become recognizable by expression of neural crest markers within the dorsal portion of the neural tube. This delay occurs as the result of epigenetic changes that allow transcription of neural crest marker genes at the proper time (Strobl-Mazzulla, Sauka-Spengler, & Bronner-Fraser, 2010). These premigratory neural crest cells subsequently emerge from the neural tube and embark upon defined pathways.
Cells in the neural tube are epithelial and look much like contiguous soda cans that have a defined top (apical) and bottom (basal) side. They are closely apposed to one another and are connected by various types of adhesive junctions. In contrast, migratory cells such as neural crest cells are mesenchymal, having a fibroblast-like morphology that facilitates their movement. Thus, precursor cells within the neural tube change from an epithelial to a mesenchymal morphology as they turn into migratory neural crest cells. Such an epithelial-to-mesenchymal transition is a common event in development during the formation of tissues and organs. The transcription factor Snail2 is expressed in premigratory and early migrating cells and represents an early neural crest marker. Snail transcription factors have been associated with epithelial–mesenchymal transitions in a number of cell types, and Snail2 function is necessary for the emigration of neural crest cells. Initiation of neural crest cell migration proceeds in a rostral-to-caudal progression along most of the neural axis, following upon the heels of the head-to-tailward closure of the neural tube. After emigration, these cells move in a highly patterned fashion through neighboring tissues and localize in diverse sites.
Initiation of Migration
As they change from epithelial to migratory mesenchymal cells, neural crest cells undergo changes in adhesive properties. While within the neuroepithelium, neural tube cells express high levels of the cell adhesion molecules N-cadherin and cadherin-6B. Migrating cells downregulate these cadherins during migration and upregulate cadherin-7. Upon coalescing into ganglia and ceasing migration, cadherin-7 is downregulated and N-cadherin are again upregulated (Nakagawa & Takeichi, 1998). This suggests that a shift in cell surface and adhesive properties may accompany the onset and cessation of migratory behavior. Neural crest cells also turn on RhoB during the initiation of migration.
After leaving the neural tube, neural crest cells encounter extracellular spaces that are rich in extracellular matrix (ECM) molecules, such as fibronectin, laminin, collagens, and proteoglycans. These may serve as a good migratory substrate, and, indeed, the neural crest cell surface has abundant integrin receptors that mediate adhesion to ECM molecules. Furthermore, administration of function-blocking antibodies to the β subunit of integrin cause severe perturbations in neural crest development in the head. They appear to prevent the migration of some neural crest cells from the cranial neural tube. In the trunk, function-blocking antibodies that interfere with the a4 subunit of integrin cause defects in neural crest cell movement but fail to alter the segmental pattern of migration through the somites. These results suggest that perturbing integrin function alters the properties of migratory cells, but not their overall metameric pattern of migration. Thus, cell–matrix interactions may play a permissive role in the migration of neural crest cells through the somites but cannot play an instructive role in directing the precise patterns or pathways for crest cell migration.
Techniques for Following Neural Crest Cells
When neural crest cells emerge from the neural tube, they first enter a cell-free space in which they are easily identifiable (Fig. 15.1). Subsequently, they invade other tissues in which they are difficult to distinguish. Therefore, it is necessary to mark neural crest cells in order to study their migratory patterns and derivatives. A variety of techniques have emerged for this purpose, ranging from transplantation of tissue containing premigratory neural crest cells to lineage tracers and molecular markers. Neural tube transplantations have provided a wealth of information about neural crest migratory pathways and, in particular, derivatives arising from this population. Although this approach was widely used in amphibians for studying numerous embryonic processes, it has been applied most successfully to the analysis of neural crest migratory pathways and derivatives in avian embryos (LeDouarin & Kalcheim, 1999). Initial experiments in birds involved transplanting a neural tube from a donor labeled with the radioactive marker [3H] thymidine into an unlabeled host (Weston, 1963). Neural crest cells generated from the labeled neural tube were also labeled and could be identified readily in the periphery. This technique yielded important information about early stages of neural crest migration, but the label became diluted with further cell division and was not useful for looking at long-term differentiation of neural crest cells into diverse derivatives.
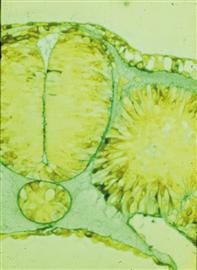
Figure 15.1 A transverse section through a chick embryo showing neural crest cells initiating migration from the dorsal neural tube and into an ECM-filled space.
Courtesy of Jan Lofberg.
To circumvent this problem, LeDouarin took advantage of the ability to perform grafts between related species of birds, such that the grafted cells were indelibly marked (Fig. 15.2). This created an interspecific chimera, from the Greek meaning “fabulous monster,” containing a donor quail portion of the neural tube and neural crest in an otherwise normal chick host embryo. Quail neural crest cells migrate away from the grafted neural tubes and can be recognized easily within the host chick embryo by staining for condensed heterochromatin, which characterizes the quail but not chick cells. This technique has been facilitated greatly by the advent of quail-specific antibodies. Recently, the availability of transgenic chickens with cell populations that are labeled with green fluorescent protein (GFP) makes it possible to perform similar grafts within the same species. Use of the these transplantation studies has made it possible to demonstrate that neural crest cell populations originating from different axial levels follow distinct migratory pathways and give rise to different progeny once they reach their destinations.
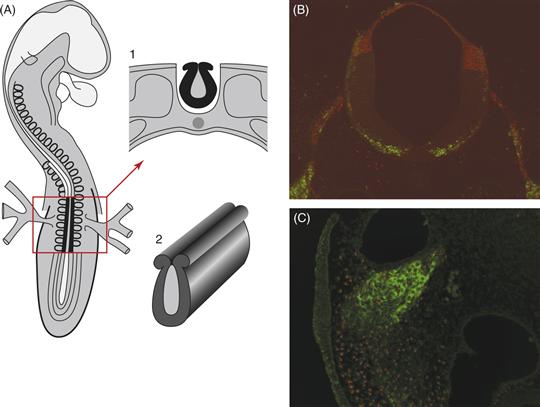
Figure 15.2 Procedures for grafting a fragment of the neural primordium from a donor quail into a host chicken embryo as used by LeDouarin and colleagues. (A) View of an avian embryo with anterior at the top. Neural folds are shown in black in the boxed region; this structure is removed and transplanted to a host embryo. (1) Cross-section through the embryo in regions shown in the box with the neural tube (2) shown in black. From LeDouarin (1982). (B) An example of a section through an embryo after grafting of a quail neural tube into a chick host. Quail cells are recognized by a quail-specific antibody (red), whereas neurons are marked in green with a neurofilament marker. (C) A higher magnification section showing quail cells (red nuclei) incorporated into a neural crest-derived ganglion, stained green with a neurofilament antibody.
Courtesy of Anne Knecht and Clare Baker.
In addition to grafting experiments, antibodies that recognize neural crest cells, such as HNK-1 and NC-1 antibodies, made it possible to identify early migrating neural crest cells without the necessity of performing microsurgery. The exclusive use of neural crest antibodies has several pitfalls because these antibodies are neither entirely specific nor stain the full complement of neural crest cells. However, they do provide important confirmatory information about the pathways followed by neural crest cells. More recently, a number of other molecular markers for early neural crest populations have become available. These include the cell adhesion molecule cadherin-6B and the transcription factor Snail2, expressed in the neural folds and neural crest cells during early stages of migration, and Sox10, expressed in premigratory, early migrating neural crest cells and later in neural crest-derived glia.
One problem with using antibodies or molecular markers to follow cell migratory patterns is that these do not represent true lineage markers. Shifts in their expression patterns could just as easily reflect up- or downregulation of these molecules as changes in cell position. Therefore, approaches for labeling small groups, as well as large populations, of neural crest cells have been employed to follow individual cell movements and interactions within the population. One successful approach has been to inject the lipophilic vital dye DiI into the neural tube or neural folds. Because the dye is hydrophobic and lipophilic, it intercalates into all cell membranes that it contacts. Injection into the neural tube marks all neural tube cells, including presumptive neural crest cells, within its dorsal aspect. Because the time and location of injection can be controlled, this provides a direct approach for following migratory pathways. When dye injections are made focally into neural folds, this technique can be used to label very small numbers of cells. It has the further advantage of being applicable to almost all vertebrates. Similarly, the availability of green fluorescent protein (GFP) and its variants has made it possible to label neural tube cells, including premigratory neural crest cells, by electroporating constructs encoding GFP into the neural tube lumen at various stages and axial levels. A disadvantage of these approaches, however, is that the dye or fluorophore becomes diluted with each cell division and, therefore, this approach is not useful for examining the long-term differentiation of neural crest derivatives.
Because most studies of cell migration in vivo look only at the beginning and end point, little is known about the dynamics of neural crest cell movement. Advances in imaging technologies have made it possible to acquire more refined images in ovo. Time-lapse movies of cranial neural crest migration can be generated by injecting DiI or electroporating GFP constructs (Yasuda et al., 2000) into the lumen of the chick neural tube, and high-resolution confocal images can be made of the labeled cells over time (Fig. 15.3). These movies make it possible to visualize the migratory behavior of neural crest cells as they emerge from the cranial neural tube and migrate toward the branchial arches. As the embryo develops, it is possible to follow individual cell movements within the branchial arches, which will give rise to the bone and cartilage of the jaw. Neural crest cells move in defined streams and appear to form distinct chains in which cells are oriented along the same trajectory and remain in contact via their processes. This suggests a large degree of intercommunication between migrating populations of cranial neural crest cells.
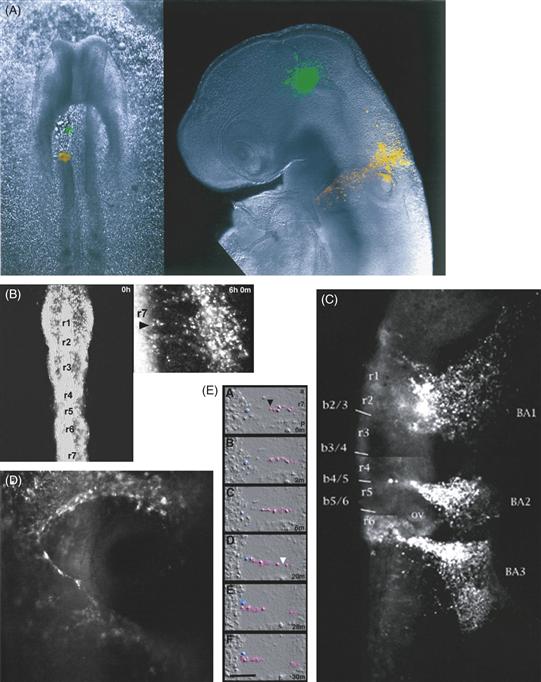
Figure 15.3 Labeling of neural crest cells with liphophilic tracer dyes. (A) On the left is a chick embryo viewed shortly after unilateral ablation of the neural folds of roughly half the neural tube at the level of the presumptive midbrain. A spot of DiO (green) was focally injected into the remaining neural tube, and DiI (red) was injected into the bordering intact neural folds. On the right, the same embryo after 48 h of further development. Cells that were originally in the neural tube had dispersed as migratory neural crest cells from both the green- and the red-labeled spots. (B–D) Different views of time-lapse movies of embryos in which the neural tube and premigratory neural crest cells were labeled with DiI. (B) Two views of the same embryo immediately after DiI labeling (left) and several hours after neural crest migration from the hindbrain. Rhombomeres (r1–r7) are indicated. (C) A similar embryo at a later time point by which time neural crest cells have migrated into the branchial arches (BA). (D) At higher magnification of neural crest streams in the branchial arches, cells seem to be in close contact as if following each other in narrow streams. (E) Following individual cells by time-lapse cinematography demonstrates close and maintained connections between individual cell pairs.
Courtesy of Paul Kulesa and Scott Fraser.
A number of different methods have proved useful for following the pathways of neural crest migration, including neural tube transplantations, vital dye labeling, GFP expression, and antibody staining. These have made it possible not only to establish the various routes of neural crest migration occurring at different axial levels but also to follow the derivatives of neural crest cells arising from distinct locations.
Regionalization of the Neural Crest along the Body Axis
Despite differences in the nature of the techniques involved, transplantation experiments, molecular markers, and direct labeling have provided similar pictures of the migratory pathways followed by neural crest cells. These migratory pathways and the derivatives formed by neural crest cells are regionalized according to their original position along the anterior/posterior axis, such that cells from a given axial level give rise to a characteristic array of progeny and follow distinct pathways from those arising at other axial levels (LeDouarin & Kalcheim, 1999; Noden, 1975). The different populations of neural crest cells arising along the neural axis have been designated as cranial, vagal, trunk, and lumbosacral (Fig. 15.4). Distinct cell types differentiate from these different populations.
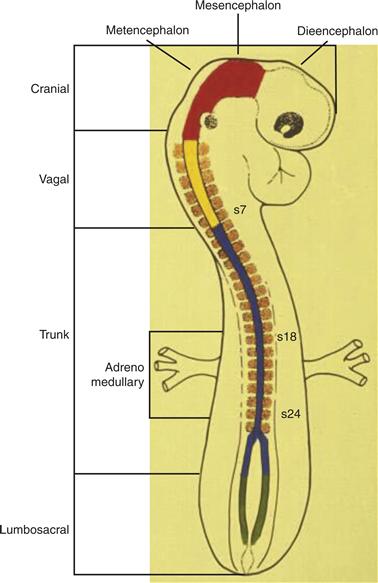
Figure 15.4 Schematic diagram showing different levels of the neural axis from which neural crest cells arise. From anterior to posterior, the neural axis can be divided into cranial, vagal, trunk, and lumbosacral levels. Each gives rise to distinct derivatives.
Drawing provided by Dr. M. Bronner.
At cranial levels, some neural crest cells contribute to the cranial sensory ganglia and the parasympathetic ciliary ganglion of the eye, whereas others migrate ventrally to form many of the cartilaginous elements of the facial skeleton. One of the interesting things about neural crest-derived bones of the head is that these are the only skeletal elements in the body that are derived from ectoderm. Precise quail/chick grafting experiments have determined the regions of neural tube from which neural crest cells arise to contribute to cartilaginous elements and cranial ganglia (Noden, 1975). Neural crest cells originating in the midbrain migrate primarily as a broad, unsegmented sheet under the ectoderm; they contribute to derivatives ranging from the skeleton around the eye, connective tissue and membranous bones of the face, to the ciliary ganglion and trigeminal ganglia (LeDouarin & Kalcheim, 1999). Neural crest cells arising in the hindbrain migrate ventrally and enter the branchial arches to form the bones of the jaw.
Vagal neural crest cells migrate long distances to form the enteric nervous system, which also receives a contribution from the lumbosacral neural crest. Within the gut, the earliest generated crest cells move as a wave from anterior to posterior to populate the bowel, which they appear to populate in sequence such that the anterior portions are populated by neural crest cells first and the cells move to progressively more posterior sites. Mice bearing a “lethal spotted” mutation lack neural crest cells in a portion of their bowel. This leads to a lack of innervation in the region called aganglionic bowel. As a consequence, food waste fails to move through this portion of the bowel, leading to a disorder called “mega-colon.” A similar defect is observed in humans leading to “Hirschsprung’s disease.” This disorder arises from a defect in molecules called endothelins and their receptors. The failure of neural crest migration in the aganglionic bowel of lethal spotted mutant mice is caused by a defect in mesenchymal components of the gut, whereas the neural crest cells themselves are normal.
Trunk neural crest cells follow two primary migratory pathways (Fig. 15.5): a dorsolateral pathway between the ectoderm and the somite and a ventral pathway through the rostral half of each sclerotome, the mesenchymal portion of the somite, which will go on to form the vertebrae. Cells following the dorsolateral stream give rise to melanocytes. Those cells following the ventral pathway give rise to the PNS of the trunk, including the chain of sympathetic ganglia and dorsal root ganglia, as well as chromaffin cells of the adrenal medulla. In addition to these neurons, these cells generate glia of the peripheral ganglia and Schwann cells that ensheathe and myelinate peripheral axons.
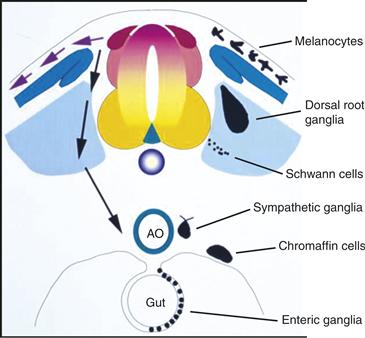
Figure 15.5 Schematic diagram of an idealized embryo in cross section showing pathways of neural crest migration in trunk and derivatives formed. Neural crest cells migrate along two primary pathways: dorsally under the skin or ventrally through the sclerotome. Dorsal migrating cells form pigment cells, whereas ventrally migrating cells give rise to dorsal root and sympathetic ganglia, Schwann cells, and cells of the adrenal medulla.
Drawn by Mark Selleck.
The neural crest can be subdivided into specific subpopulations based upon their level of origin along the neural tube. Different populations follow different migratory pathways and form characteristic types of derivatives. For example, cranial bone and cartilage only arise from the cranial neural crest.
Grafting of Neural Crest Cells to New Locations—Intrinsic Versus Extrinsic Cues?
Neural crest cells that arise and migrate at different axial levels assume different fates. Thus, it is possible that they are specified at early times to take on particular fates. For example, one possibility is that neural crest cells are preprogrammed by their axial level to populate specific derivatives; alternatively, neural crest cells might be multipotent and migrate naively into available locations, where local cues provide them with instructions about their fates. To test the role of migratory pathways in choice of fate, neural tubes from particular axial levels have been grafted to new locations. This is referred to as a “heterotopic” graft. These experiments have revealed, for example, that when vagal neural crest cells are grafted to trunk regions, they form normal trunk derivatives (dorsal root ganglia, sympathetic ganglia, etc.) and normal vagal derivatives (enteric ganglia of the gut) (Fig. 15.6). The gut is immediately ventral to the trunk region and is connected to it by a narrow piece of tissue called the dorsal mesentery. Despite their close proximity, trunk neural crest cells normally fail to invade the gut where enteric ganglia form. When vagal neural tubes are grafted in place of trunk neural tubes, however, donor vagal neural crest cells do invade the gut and form enteric ganglia. Therefore, these cells can respond to normal trunk neural crest migratory pathways, but, in addition, some cells behave in a “uniquely vagal” fashion and migrate directionally to the gut. This indicates some intrinsic differences between the two populations. Along similar lines, when cranial neural tubes are grafted in place of the trunk neural tube, donor cranial neural crest cells form some normal derivatives in the trunk-like dorsal root and sympathetic ganglia. Other cells, however, fail to migrate and instead differentiate into ectopic cartilage. In the reciprocal experiment, avian trunk neural crest cells grafted to the head region appear unable to generate cartilage at all, although they can participate in the formation of elements of the cranial ganglia (LeDouarin & Kalcheim, 1999) (Fig. 15.6).
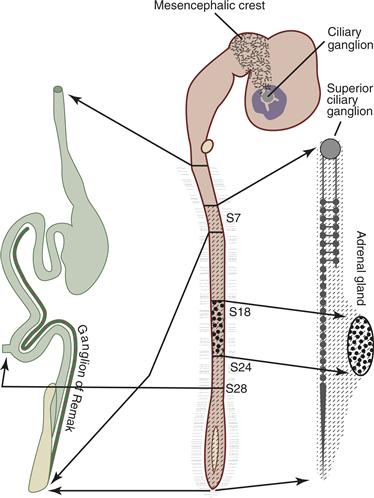
Figure 15.6 Neural crest cells at different levels along the anterior–posterior axis give rise to distinct autonomic and adrenomedullary derivatives in avian embryos. In the cephalic region (center), mesencephalic crest cells populate the ciliary ganglion. Ganglia of the sympathetic chain (right), including the superior ciliary ganglion, are formed from spinal neural crest cells originating caudal to somite 5. Cells of the adrenal medulla (right) originate exclusively from neural crest cells between somites 18 and 24. Vagal neural crest cells generated between somites 1 and 7 form enteric ganglia (left), whereas cells of the ganglion of Remak (left) are derived from the lumbosacral neural crest posterior to somite 28.
Drawing provided by Dr. M. Bronner.
These experiments suggest two things: (1) environmental factors can influence neural crest cells from different axial levels to express a broader range of fates than they would normally express when left in situ, and (2) some neural crest cells undergo their intrinsic program even when grafted to an ectopic site. Thus, some combination of intrinsic and extrinsic information is likely to govern neural crest cell fate decisions. The migratory pathways taken by neural crest cells are likely to play an important regulatory role in cell fate specification. Indeed, different migratory pathways contain different distribution patterns of important inducing factors. A number of factors, including BMPs, neuregulins, and glucocorticoids, have been demonstrated to influence the choice of neural crest cells into neural, glial, or chromaffin lineages (see Chapter 18). When added to multipotent neural crest stem cells in culture, these factors can drive these cells into neural, glial, or chromaffin lineages, respectively. Accordingly, BMP-7 is present in the dorsal aorta adjacent to the location where trunk neural crest cells differentiate into sympathetic neurons. Similarly, glucocorticoids are produced by the adrenal cortex, which surrounds the adrenal medullary cells.
In addition to specific growth factors, the timing of emigration may play an important role in eventual neural crest cell fate decisions. Neural crest cells exhibit an orderly pattern in the timing of migration, with cells initially following the ventral pathway and later contributing to progressively more dorsal derivatives. Although early and late migrating cranial neural crest cells appear to have a similar developmental potential, it is likely that the cell population does undergo a change in the range of fates that the cells can assume. For example, the last emigrating trunk neural crest cells in the bird give rise to pigment cells and appear to have a limited capacity to form sympathetic neurons. However, stem cells appear to persist in the neural tube and retain the potential to form neural crest. Well past the normal time of neural crest cell emigration, cells with the full range of neural crest potential can be isolated from the ventricular zone of the developing spinal cord (Sharma, Korade, & Frank, 1995). In the embryo, these late-emigrating cells appear to contribute to a subpopulation of neural crest derived cells in the dorsal root ganglia.
Summary
Neural crest cells are somewhat plastic with respect to their prospective fates. When put into a new environment, they sometimes behave according to their new location. However, some cells act according to their original location and thus have some “intrinsic” information. With time, the developmental potential of neural crest populations becomes restricted, although a subpopulation of “stem cells” may remain until late stages.
Segmental Migration of Neural Crest Cells
A hallmark of the developing peripheral nervous system is its inherent segmentation. After neural crest cells migrate from the neural tube and through the somites, they condense to form segmentally arranged sensory and sympathetic ganglia. For each somite, a single sensory and sympathetic ganglion forms. This exquisite and reproducible pattern suggests the presence of some inherent segmental information in the embryo that is responsible for segmental migration and gangliogenesis of neural crest cells. In longitudinal sections through early embryos, it is clear that neural crest cells migrate in a metameric pattern, moving exclusively through the rostral half of each somite while failing to enter the caudal half (Fig. 15.7).
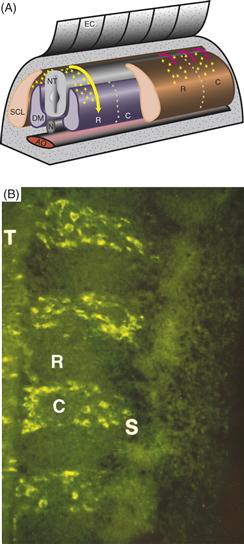
Figure 15.7 Trunk neural crest cells migrate in a segmental fashion. (A) Schematic diagram demonstrating that neural crest cells migrate through the sclerotomal portion of the somites, but only through the rostral half of the sclerotome. (B) In longitudinal section, neural crest cells (green) can be seen migrating selectively through the rostral half of each somitic sclerotome (S).
From Bronner-Fraser (1986).
Such a segmental pattern of migration could be caused by inherent cues within the neural tube that direct neural crest cells to migrate segmentally. Alternatively, tissues through which neural crest cells migrate may contain patterning information that results in segmental migration. The relationship between neural crest cells and their surrounding tissues has been explored by manipulating the neural tube and/or somites in a series of grafting experiments. Removal of the somites results in the formation of huge, unsegmented neural crest–derived ganglia, suggesting that somites are necessary for the segmental migration of neural crest cells. However, inversion of the segmental plate in the rostrocaudal dimension reverses the pattern of neural crest migration such that neural crest cells migrate through the half of the rotated sclerotome that was originally rostral but is now caudal. Motor axons, which also traverse the rostral sclerotome in normal animals, exhibit similar behavior to neural crest cells after segmental plate rotation. This suggests that the information necessary to guide neural crest cells and motor axons is intrinsic to the somites. Furthermore, these experiments show that the rostrocaudal polarity of the somites is already established at the segmental plate stage.
Other experimental manipulations demonstrate that the caudal half of each somite is inhibitory, whereas the rostral half somite is permissive for neural crest migration and motor axon guidance. If somites are constructed to contain only caudal sclerotome tissue, neural crest cells and motor axons fail to migrate altogether. Conversely, an all-rostral somite results in the absence of segmentation for both neural crest cells and motor axons. Taken together, these findings demonstrate that the segmental migration of both neural crest cells and motor axons is due to cues inherent in the somite. These cues may be caused by attractive cues in the rostral-half sclerotome, inhibitory cues in the caudal-half sclerotome, or a combination of both. The finding that somites containing all caudal-half sclerotomes cannot support neural crest migration strongly suggests that some of the guidance cues are inhibitory. This segmentally arranged stream of migrating neural crest cells in turn results in the segmental organization of the neural crest–derived ganglia of the PNS.
Although it was suggested that Eph/ephrin signaling might direct the pattern of trunk neural crest migration (Krull et al., 1997; Wang & Anderson, 1997), the Eph and ephrin mutant mice that have been examined fail to exhibit trunk neural crest migration defects (Oriolli et al., 1996; Wang, & Anderson, 1997). Another receptor expressed by neural crest cells is Neuropilin-2 (Npn2), which is expressed in premigratory and migratory neural crest in both chick and mouse. Strikingly, trunk neural crest cells no longer migrate segmentally through the somites of Npn-2 mutants and are distributed equally in rostral and caudal sclerotome. These defects are the result of Npn2/Semaphorin3F (Sema3F) signaling, as Sema3F mutants have an identical phenotype. These results demonstrate that Npn-2/Sema3F signaling is necessary to restrict neural crest migration to the rostral somite (Gammill, Gonzalez, Gu, & Bronner-Fraser, 2006). Interestingly, metameric dorsal root ganglia still form in Npn-2 mutants in the absence of segmental neural crest migration, suggesting that segmental migration may be separable from ganglion formation. However, mice lacking both Npn1 and Npn2 form abnormally fused ganglia, suggesting that Npn-1 is required for the later segregation of neural crest-derived ganglia (Fig. 15.8) (Gammill et al., 2006).
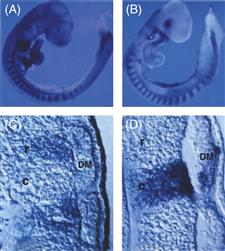
Figure 15.8 Distribution of Eph receptors (A and C) on neural crest cells in the rostral sclerotome and ephrin ligands in the caudal sclerotome (B and D) of chick embryos. Eph receptors are on neural crest cells in the rostral half of each somite, whereas inhibitory ephrin ligands are expressed in the caudal halves of each sclerotome.
From Krull et al. (1997).
As in the trunk, the migration of neural crest cells in the hindbrain is also segmented. Three broad streams of migrating cells are found adjacent to rhombomere 2 (r2), r4, and r6, whereas no neural crest cells are apparent adjacent to r3 and r5. Focal injections of DiI at the levels of r3 and r5 have demonstrated that both of these rhombomeres generate neural crest cells and that the apparent segmental pattern results from the DiI-labeled cells that originated in r3 and r5 deviating rostrally or caudally and failing to enter the adjacent preotic mesoderm or otic vesicle region.
Trunk neural crest cells move in a segmental pattern, which is controlled by inhibitory molecules present in the caudal half of each somite. Neural crest cells have Npn2 receptors on their surface. When they encounter Sema3F ligands in the caudal somite, they are diverted from this location, leading to selective migration through only the rostral-half somite.
Cessation of Neural Crest Migration
Neural crest cells exhibit an orderly pattern in their migration. Whereas the first cells to migrate tend to move most ventrally, later migratory cells contribute to progressively more dorsal derivatives. The different destinations of neural crest derivatives are therefore populated in a sequential order during development. One possibility is that the early migrating cells “fill” the more ventral sites, effectively clogging up the pathway. Alternatively, the sites themselves may change with time so that they can no longer support migration.
Surprisingly little is known about how neural crest cells know that they have reached the appropriate destination and stop their migration. This is in marked contrast to the developing CNS, where a number of mutations affect the ability of neuroblasts to cease migrating. It is clear that cell adhesion molecules such as N-cadherin and N-CAM are upregulated after cells reach their final sites and condense to form peripheral ganglia. However, there is no evidence for a causal role for cell adhesion molecules in this process.
Neurogenesis in the PNS
Some of the factors and signaling cascades that result in neurogenesis in the developing PNS are beginning to be understood (see Chapter 16). Many neural crest cells appear to be multipotent and not yet committed to a neural fate. However, particular external factors can influence their fate decisions. For example, clonal populations of neural crest cells become neural in the presence of BMPs and glial in the presence of glial growth factor. Similarly, activation of Notch signaling promotes gliogenesis at the expense of neurogenesis in neural crest cells and their derivatives. Certain transcription factors may bias cells toward certain fates; for example, while Mash-1 is essential for sympathetic neuron formation, Neurogenins are essential for sensory fates (Christiansen, Coles, & Wilkinson, 2000).
An interesting contrast between the developing PNS and the CNS is that migrating neural crest cells proliferate as they move. In fact, even after exhibiting defined neuronal characteristics, some neural crest derivatives continue to divide. For example, in the developing sympathetic ganglia, neural crest-derived cells express neurotransmitters and other proteins characteristic of sympathetic neurons but remain actively mitotic. However, other neural crest cells, such as sensory neurons, appear to withdraw from the cell cycle well before they express neuronal traits.
Summary
Neural crest cells migrate over long distances throughout the body to form diverse cell types, including neurons, glia, melanocytes, and cells of the adrenal gland. The migratory pathways of neural crest cells vary along the rostrocaudal body axis, and the local environments through which cells migrate and eventually differentiate play an important role in phenotypic specification. The migration of neural crest cells is guided by both positive (attractive or permissive) and inhibitory (repulsive) cues that are found in the environment.
Cell Migration in the CNS
The emergence of the laminar architecture of the mammalian neocortex depends on neurogenesis in germinal zones, migration of postmitotic neurons (Caviness, 1982; Del Rio, Martinez, Auladell, & Soriano, 2000; McConnell, Ghosh, & Shatz, 1994; Wood, Martin, & Price, 1992; Zecevic & Rakic, 2001), formation of transverse zones of transient neurons (the preplate, cortical plate, marginal zone, and subplate) (Caviness, 1982; Del Rio et al., 2000; Marin-Padilla, 1998), and assembly of postmigratory cortical neurons into six neuronal layers. The columnar organization of cortical regions of the CNS develops from the radial glial scaffold, which provides a substrate for directed cell migrations of the primary output neurons of the neocortex in early periods of development, and generates neurons in later stages of development. Although the anatomists Retzius, Kolliker, and Magani described a system of radial glial cells in the nineteenth century, the influence of radial glial cells on cortical histogenesis did not become apparent until the late twentieth century. The spatiotemporal pattern of neurogenesis and migration during the formation of neural layers in developing cortex emerged from the pioneering work of Richard Sidman (1970) on thymidine “birthdating.” The broad application of this approach to different brain regions and times of development demonstrated the spatiotemporal pattern of neurogenesis in the vertebrate CNS. The discovery of directed neuronal migration along glial fibers by Pasko Rakic in the 1970s (Rakic, 1972; Sidman & Rakic, 1973) set the stage for a period of intense focus on the process of neuronal migration in cortical histogenesis (Sidman & Rakic, 1973) (Fig. 15.10). Cell and molecular studies of identified neurons from the cerebellar cortex and hippocampal formation provided real-time imaging that defined the mode of movement of neurons migrating along glial fibers (Edmondson & Hatten, 1987; Gasser & Hatten, 1990a).
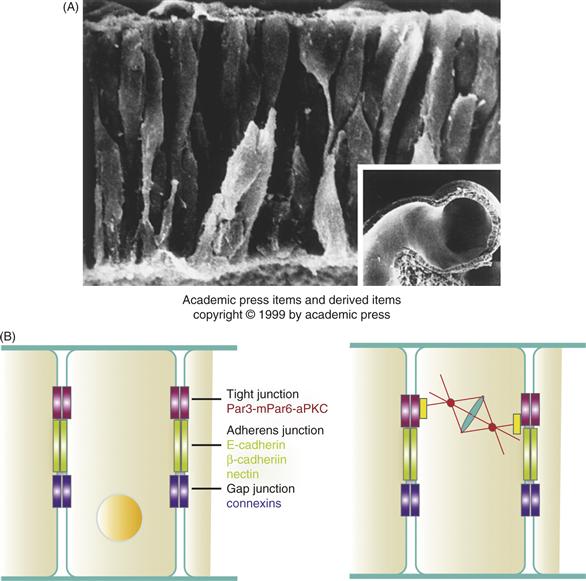
Figure 15.9 (A) The Ventricular Zone Forms a Pseudostratified Columnar Epithelium. Here neural progenitor cells have been visualized in the cerebral vesicle of a hamster embryo using scanning electron microscopy. Neuroepithelial cells are elongated bipolar cells that, at this early stage of development (E9.25), span the entire wall of the cerebrum. Some of the cells at the ventricular surface (bottom) appear spherical; these cells have retracted their cytoplasmic processes and are presumably rounding up in preparation for mitosis. Other rounded cells at the external surface (top) may be young neurons beginning to differentiate. (Inset) A low-power view of the hamster cerebral vesicle, corresponding roughly to that of a human embryo at the end of the first month of gestation. (B) Polarity Proteins and Vertebrate Epithelia. Three classes of cell-cell junctions form between cells in a vertebrate epithelium. Conserved polarity proteins function in the formation of tight junctions (red). The cadherin and nectin cell adhesion proteins are required to form adherens junctions (green) and connexins form gap junctions (purple). During cell division in the neuroepithelium, the mPar6 polarity complex positions the spindle of the dividing cell (see text for details).
From Sidman and Rakic (1973).
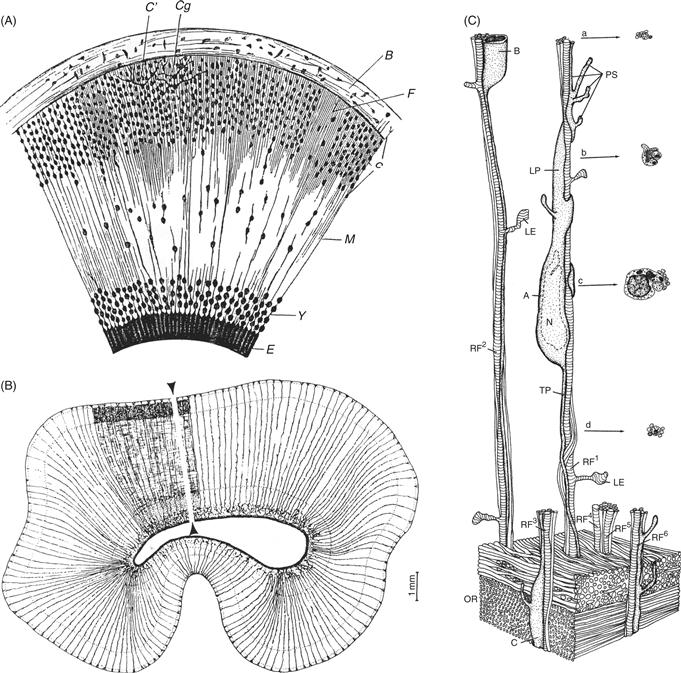
Figure 15.10 Radial Glial Cells Provide a Pathway for Neuronal Migration. (A) In the late nineteenth century, the Italian histologist G. Magini used Golgi impregnation methods to visualize a system of glial fibers that spanned the cortical wall. He proposed that the glial fibers provided a scaffold for neuronal migration. (B) In the late twentieth century, Rakic and Sidman again used the Golgi impregnation method to map radial glial cells in the developing primate neocortex. As described below, they used electron microscopy (EM) to visualize the relationship of young neurons to the glial fiber system. (C) Three-dimensional reconstruction of serial EM sections of a migrating neuron in the intermediate zone of the primate neocortex illustrate the cytology and neuron-glia relationships of migrating neurons in vivo. The cell soma of the migrating cell apposes radial glial fibers (striped vertical shafts, RF1-6), which extend short lamellate expansions (LE). Nuclei (N) of migrating neurons are elongated, and their leading processes (LP) are thicker and richer in organelles than their trailing processes (TP). The lower part of the diagram depicts the numerous parallel axons of the optic radiations (OR). These axons have been deleted from the upper portion of the figure to reveal the radial glial fibers. The leading process extends several pseudopodial endings (PS), which appear to explore the territory through which the neuron is migrating. In cross-section (a–d), a migrating neuron partially encircles the shaft of the radial glial fiber.
From Sidman and Rakic (1973).
Studies on the architectonics of human cortical malformations (Barkovich et al., 1996; Dobyns & Truwit, 1995) revealed populations of heterotopic cells and suggested that pathogenic processes disrupted normal glial-guided neuronal migration (Rakic, 1988). The hypothesis that cortical malformations resulted from migration defects became even more attractive with the analysis of spontaneously occurring neurological mutations. All of these studies underscore the critical role of neuronal migration in brain development.
Cortical Histogenesis: Formation of the Basic Embryonic Zones
During corticogenesis, early-born, polymorphic neurons settle in a narrow zone above the VZ, termed the preplate (PP) (Caviness, 1982; Del Rio et al., 2000; McConnell et al., 1994; Wood et al., 1992; Zecevic & Rakic, 2001) (Fig. 15.11). Preplate neurons include Cajal-Retzius cells, GABAergic interneurons, and future subplate projection neurons (Caviness, 1982; Del Rio et al., 2000; Marin-Padilla, 1998). During the preplate stage, preplate neurons pioneer the first axon projections of the neocortex, with some preplate neurons extending descending axons to subcortical targets and others extending corticocortical axons to targets in the ipsilateral or contralateral hemisphere via the anterior commissure. As the preplate matures, extensive cell movements occur across the dorso-ventral (DV) plane of the neuroaxis. At late preplate stages, preplate neurons undergo changes in polarity, shifting from a random orientation into a radial orientation (Schneider, Gulacsi, & Hatten, 2011). Thereafter, the preplate separates into a superficial marginal zone (MZ) of Cajal-Retzius cells and small neurons, the cortical plate (CP) (Rakic & Zecevic, 2003), and a deeper layer called the subplate (SP) (Fig. 15.11).
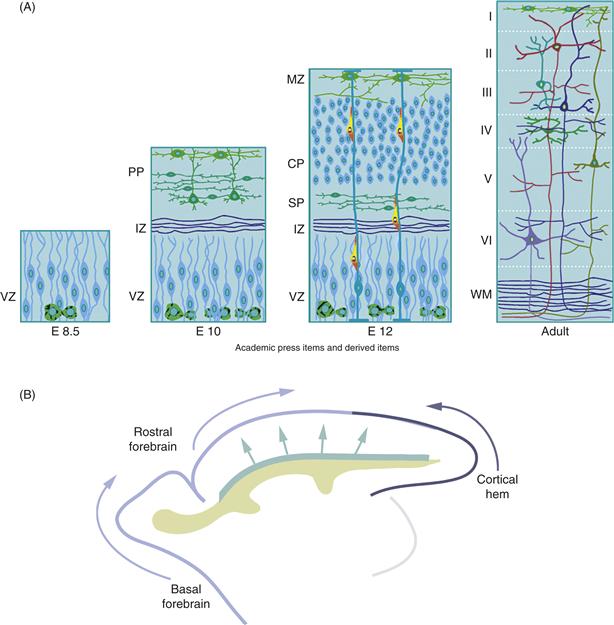
Figure 15.11 Development of the Cerebral Cortex. (A) Formation of the Fundamental Layers: The ventricular zone (VZ) contains the progenitors of neurons and glia. The first neurons to be generated establish the preplate (PP); their axons, as well as ingrowing axons from the thalamus, establish the intermediate zone (IZ). The subsequently generated neurons of cortical layers II–VI establish the cortical plate (CP), which splits the preplate into the marginal zone (MZ), or future layer I, and the subplate (SP), a transient population of neurons. After the completion of neuronal migration and differentiation, six cortical layers are visible overlying the white matter (WM) and the subplate has largely disappeared. Neural precursors in the subventricular zone (SVZ) continue to generate neurons that migrate rostrally into the olfactory bulb, even during postnatal life. (B) Emerging Complexity of the Origin of Layer 1 Neurons. Recent experiments in rodents and primates reveal multiple origins and migratory pathways of the precursor neurons in layer 1 of the developing neocortex. As discussed above, the neocortical VZ is a primary source of preplate neurons, which contains layer 1 cells. However, subsets of layer 1 neurons also arise in the rostral aspect of the neocortex, the basal forebrain, and the cortical hem. The latter progenitor cells migrate along the surface of the emerging brain from their sites of origin into the superficial layer of the neocortex. (This population of cells migrates several days before progenitors destined to become cortical interneurons). See text for details.
Studies on the genetics of cortical development underscore the importance of preplate separation to the development of cortical laminae. During corticogenesis in reeler mutant mice, the segregation of preplate neurons into two zones and formation of the cortical plate do not occur and subsequent cortical lamination fails (Caviness & Rakic, 1978; Goffinet, 1984; Tissir & Goffinet, 2003). Cell movements involved in the subdivision of the preplate into the MZ and SP and formation of the CP are also critical to the formation of cortical synaptic circuits. As first proposed by Shatz and colleagues, subplate neurons extend pioneer axons during the establishment of thalamocortical architecture and function (Dupont, Hanganu, Kilb, Hirsch, & Luhmann, 2006; Ghosh & Shatz, 1992, 1993; Ghosh, Antonini, McConnell, & Shatz, 1990; Kanold, Kara, Reid, & Shatz, 2003; Lein, Finney, McQuillen, & Shatz, 1999; Price et al., 2006). They form some of the first synapses in cortex (Blue & Parnavelas, 1983a, 1983b; Chun & Shatz, 1988; Konig & Marty, 1981; Konig, Roch, & Marty, 1975; Kostovic & Rakic, 1980; Molliver, Kostovic, & van der Loos, 1973) and provide a transient scaffold for the organization of thalamocortical projections. Thus, the neuroepithelium develops from a single layer of dividing cells to five embryonic zones, a VZ of proliferating progenitors, an intermediate zone (IZ) of immature axons, an SP containing a subset of preplate neurons, the cortical plate containing immature pyramidal cells born after embryonic day 13 (E13), and a superficial marginal zone (MZ), which contains Cajal-Retzius cells and small interneurons (Fig. 15.11).
Cortical Neurogenesis
Detailed cell cycle analyses by Takahashi and Caviness (Caviness et al., 2003) demonstrate that the murine neocortex is generated over an epoch of 11 cell cycles. Time-lapse imaging studies in cortical slices by McConnell and colleagues first suggested that progenitor cells can divide asymmetrically during the period of neurogenesis and that cleavage orientation of dividing precursors predicts the fate of the daughters (Chenn & McConnell, 1995). These studies proposed that vertically oriented divisions are symmetric, generating two identical daughters, whereas horizonal divisions are asymmetric, generating one daughter that differentiates into a neuron and another that reenters the cell cycle. The asymmetric inheritance of Notch and Numb proteins may also contribute to the control of symmetric versus asymmetric cell divisions during cortical neurogenesis. The polarity protein Numb is expressed in early phases of cortical histogenesis (Petersen, Zou, Hwang, Jan, & Zhong, 2002), and colocalizes with EGFR by a process that is actin-dependent (Sun, Goderie, & Temple, 2005). The loss of Numb and Numbl causes premature progenitor cell depletion and malformations of the neocortex (Petersen, Zou, Krauss, & Zhong, 2004), suggesting that Numb-mediated asymmetric cell divisions provide a general mechanism for cell cycle control during cell fate allocation events in the developing mammalian brain (Petersen et al., 2004).
At the cortical plate stage, two classes of neural stem and progenitor cells, the apical and basal progenitors (Farkas & Huttner, 2008; Gotz & Huttner, 2005; Noctor et al., 2002; Pinto & Gotz, 2007) are defined by their position in the cortical wall. The apical progenitors located in the VZ include the neuroepithelial cells (NECs) (Fig. 15.9) and radial glial cells (RGCs). RGCs are a specialized form of glial cell, recognized by their expression of the RC2 or BLBP antigen, that extend long radial processes toward the overlying cerebral wall (Gadisseux & Evrard, 1985; Kriegstein & Gotz, 2003; Noctor et al., 2002). As discussed below, these radial processes presage the basic columnar plan of development, providing a scaffold “in plane” with the layer of dividing cells for a subpopulation of neurons to migrate away from the primary germinal matrix. RGCs undergo symmetric divisions at early stages of cortical development, then shift to asymmetric divisions later, generating an RGC and either a neuron or a basal progenitor cell (also called an intermediate progenitor cell; IPC) that in turn divides in a more basal location, the subventricular zone (SVZ), and in most cases generates two neurons (Box 15.1, Fig. 15.14) (Farkas & Huttner, 2008; Gotz & Huttner, 2005; Kriegstein & Parnavelas, 2006; Zhong & Chia, 2008).
Box 15.1 Adult Neurogenesis
Adult neurogenesis refers to the process of generating new neurons in the adult mammalian brain (Gage, 2000). For over a century, it was believed that adult mammalian brains had no capacity to produce new neurons and that the neurons damaged in the adult brain were lost forever. However, this dogma has been challenged by the discovery of neural stem cells (NSCs) in the adult brain and by intensive research on adult neurogenesis over the past two decades. These studies of adult neurogenesis can be dated back to the 1960s, when Joseph Altman and colleagues observed [3H]-labeled neurons in brains of adult rodents injected with [3H]-thymidine, a marker for proliferative cells and their progeny (Altman & Das, 1965). A second breakthrough in the study of adult neurogenesis did not occur until the early 1990s, however, with the discovery of adult NSCs that are able to self-renew while producing daughter cells with a potential to differentiate into neurons (Reynolds & Weiss, 1992). Two discrete neurogenic niches that harbor NSCs have been consistently identified across mammalian species in vivo—the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus. NSCs in the SVZ give rise to neuronal precursors that migrate through the rostral migratory stream and differentiate into interneurons in the granular cell layer and the periglomerular layer in the olfactory bulb. In the SGZ, most of the progeny of NSCs differentiate and integrate locally as dentate granule cells. Adult neurogenesis in the hippocampus can also be observed in human brains (Eriksson et al., 1998), suggesting a therapeutic potential for utilizing adult-born neurons for treatment of neurodegenerative disease and repair of injured neural tissues in adulthood.
Adult neurogenesis starts with the proliferation of NSCs. Some progeny of NSCs remain undifferentiated as NSCs; others differentiate into neurons and glia. The newly born neurons undergo a lengthy process of morphological and physiological maturation and eventually integrate into the corresponding neural circuitry. During the differentiation and maturation processes, a significant fraction of newborn neurons are lost before their final integration. Therefore, the rate of neurogenesis is regulated at levels of NSC proliferation, neuronal fate determination, and the survival of newborn neurons. Numerous molecular factors take part in the regulation of adult neurogenesis, including growth factors, neurotrophins, morphogens, cytokines, and hormones. Moreover, distinct from embryonic neurogenesis, adult neurogenesis can also be modulated by the animals’ experiences and behaviors. For example, voluntary exercise, environmental enrichment, and hippocampus-dependent learning can enhance SGZ neurogenesis. Conversely, hippocampal neurogenesis is reduced in stressed or aged animals. Olfactory stimulus enrichment results in enhanced SVZ neurogenesis.
Because of the region-specific network integration of newborn neurons, it is postulated that SGZ neurogenesis may play a role in hippocampus-related functions, such as learning and memory and affective regulation, and SVZ neurogenesis may contribute to olfactory function. This hypothesis is supported by many studies demonstrating a positive correlation between levels of neurogenesis and performance in specific behavioral tasks. While a causal relationship between adult neurogenesis and its functions is the subject of intense investigation, current studies using a combination of molecular, cellular, genetic, system, and behavioral approaches are striving to elucidate the exact roles of newborn neurons in hippocampal and olfactory functions.
Wei Deng and Fred H. Gage
Cell Movements within the Primary Proliferative Matrix
The columnar organization of the cerebral cortex led to the hypothesis that progenitors in the neuroepithelium form a protomap for cortical organization. Two experimental approaches provided evidence for widespread tangential movements within the neuroepithelium. First, live imaging of dye-labeled cells in slices of embryonic cortex revealed extensive movements of the neuroblasts within the neuroepithelium. Labeled cells move intermittently within the plane of the epithelium at speeds between 10 and 100 µm/h (Fishell, Mason, & Hatten, 1993). Second, experiments using retroviral markers to follow the dispersion of clonally related cells in the developing brain demonstrated widespread dispersion of cells within the developing cortex (Walsh & Cepko, 1992). Thus, widespread movements occur in the neuroepthelial germinal zone of the CNS and across the plane of the outer embryonic zones of the neocortex.
Molecular Mechanisms of Cell Motility: General Features
Neuronal motility in the developing CNS, like that of all metazoan cells, occurs in response to a migration-promoting signal(s), which polarizes the cell and induces the extension of protrusions in the direction of migration (Ridley et al., 2003). These protrusions can be large, broad lamellipodia or spike-like filopodia driven by actin polymerization and microtubule dynamics, and stabilized by adhering to the extracellular matrix (ECM) in the case of migrating neural crest cells or adjacent to radial glial cells or to bundles of axons in the case of radial, glial-guided migrations and tangential migrations. Adhesions serve as traction sites during forward translocation of the cell soma (Fig. 15.13).
As key regulators of the actin and microtubule cytoskeletons, the Rho family of small GTPases plays an integral part in the morphological changes, polarization, and locomotion of migrating cells. When bound to GTP, they stimulate downstream effector molecules to regulate actin and microtubule dynamics to reorganize the cytoskeleton, as well as control cell-cycle progression, gene expression, and vesicle transport. The best-studied Rho GTPase family members are Rac, Cdc42, and RhoA. In many cell types, Rac stimulates the formation of the veil-like lamellipodial protrusions involved in membrane extension, Cdc42 promotes the formation of the thin filopodial microspikes that sense the surrounding environment, and RhoA regulates cell contractility. All three of these GTPases play a role in cell adhesion (Ridley et al., 2003). Cdc42 also has a conserved role in regulating cell polarity, along with homologs of the C. elegans polarity signaling complex Par6, and together they orient the nucleus, centrosome, and Golgi apparatus in the direction of migration (Burakov, Nadezhdina, Slepchenko, & Rodionov, 2003; Etienne-Manneville & Hall, 2001; Etienne-Manneville & Hall, 2003; Gomes, Jani, & Gundersen, 2005). The Rho GTPases orchestrate general and specific structural and functional changes required for directed motility of nonneuronal cells and neurons, as well as growth cones.
A large number of actin regulatory molecules act downstream of Rac and Cdc42 signaling to regulate the formation of lamellipodia and filopodia, respectively, during motility. Rac induces actin filament uncapping, actin polymerization, and filament branching during lamellipodial extension, and Cdc42 induces the actin polymerization of parallel bundles of actin filaments that make up filopodia. Cdc42 and Rac localize Wiskott–Aldrich syndrome protein (WASP) family members and WASP-family verprolin-homologous protein (WAVE) complexes, respectively, at the cell surface to activate the actin-related protein-2/3 (ARP2/3) complex. Arp2/3 is an actin-nucleating complex that binds to the sides or tip of a preexisting actin filament and induces the formation of a new daughter filament that branches off the mother filament (Heasman & Ridley, 2008; Ridley et al. 2003). Protrusive force by actin polymerization is generated by an “elastic Brownian ratchet” mechanism, in which thermal energy bends nascent short actin filaments, pushing the membrane forward. Thus, actin polymerization and unbending a growing actin filament against the leading edge of the cell provides a driving force for the motility of nonneuronal cells, neural crest cells and extending axons. Local activation of the Arp2/3 complex induces lamellipodial extension in a particular direction, providing the basis for directional migration.
Neuronal Migration along Radial Glial Fibers
Radial glial cells are a transient population of cells that provide a primary pathway for neuronal migrations. The histologists Albert Kölliker and Wilhelm His used silver impregnation methods to reveal the development of glial cells in the late nineteenth century. In a series of EM and Golgi studies, Rakic and Sidman provided evidence that neurons are closely apposed to radial glial fibers during the period of neuronal migration (Rakic, 1972; Sidman & Rakic, 1973) (Fig. 15.10). Rakic and Sidman postulated that the radial glia guide the migrations of young neurons from the VZ to the neural layers, in a spatiotemporal order that positioned the youngest born neurons in the most superficial layers. This “inside-out” model relied upon coordinating 3H-thymidine labeling of neuroblast proliferation in the VZ with light and EM analysis (Sidman, 1970). Cells born at successively later times after administration of labeled 3H-thymidine would be lighter than those born the day of the injection (their “birthday”), due to dilution of the label with continued proliferation.
Experiments by Nowakowski and Rakic (1979) on the developing hippocampus provided the most compelling argument for glial guidance of migration, as young neurons in the hippocampal formation followed the undulating paths of the glial fibers, rather than a straight radial line, during their migrations. Real-time imaging of the migration of identified neurons, purified from cerebellum, hippocampus, and neocortex, on glial processes provided critical support for this model (Edmondson & Hatten, 1987), and revealed the dynamics of cell movement along the glial guide described above. Cell-based assays further showed that neurons from one brain region could migrate freely on glia from other brain regions, suggesting that the glial fibers were a generic “monorail” system, rather than a track that specified the duration and direction of migration (Gasser & Hatten, 1990a, 1990b; Hatten, 1999) (Fig. 15.12).
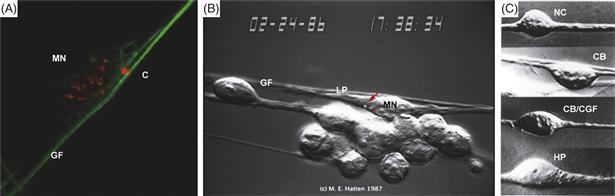
Figure 15.12 Live Imaging of Glial-Guided Neuronal Migration.
Live imaging of cells with video-enhanced differential contrast (VEC-DIC) optics. The cytology and neuron-glial relationship of a cerebellar granule neuron migrating on a glial fiber. With live imaging of neuronal migration along glial fibers, the granule neuron closely apposes the cell glial fiber (GF) along the length of the soma of the migrating neuron (MN) and extends a leading process (LP) in the direction of migration along the glial guide. The nucleus is in the posterior aspect of the cell during periods of movement, the centrosome (C) is just forward of the neuronal nucleus. (A) In migrating neurons, apparently antibodies against dynein intermediate chain (red) label the centrosome and dynein on the surface of the nucleus. Antibodies against tubulin (green) reveal a perinuclear “cage” of tubulin in migrating neurons. (B) Live imaging of a cerebellar granule neuron migrating on a glial fiber (red arrow indicates centrosome). (C) Cytology of cerebellar and hippocampal neurons migrating along homotypic and heterotypic astroglial fibers: cerebellar granule neuron migrating along a hippocampal glial fiber (CB/HP), a cerebellar granule neuron migrating on a cerebellar glial fiber (CB/CB), a hippocampal neuron migrating along a cerebellar glial fiber (HP/CB) or a hippocampal neuron migrating on a hippocampal glial fiber (HP/HP). In heterotypic co-cultures, migrating neurons have a stereotyped cytology, extending their cell soma along the glial guide and extending a leading process in the direction of migration.
Live imaging studies of neuronal migration along glial fibers revealed unique features of migrating neurons that are distinct from features of general cell and axon motility. These unique features include the extension of a highly polarized leading process in the direction of migration, the assembly of an interstitial adhesion junction beneath the cell soma (Gregory, Edmonson, Hatten, & Mason, 1988), localization of acto-myosin contractile motors in the proximal portion of the leading process (Solecki et al., 2009), and the formation of a perinuclear cage of tubulin to maintain the posterior positioning of the nucleus (Fig. 15.13). Migration involves a cycle of steps that include forward movement of the centrosome into the proximal portion of the leading process, translocation of the neuronal nucleus (Edmondson & Hatten, 1987; Solecki, Model, Gaetz, Kapoor, & Hatten, 2004), activation of acto-myosin motors in the proximal aspect of the leading process (Solecki et al., 2009), and the release of the adhesion junction. Thereafter, the neuronal cell soma glides forward along the glial fiber until a new adhesion forms.
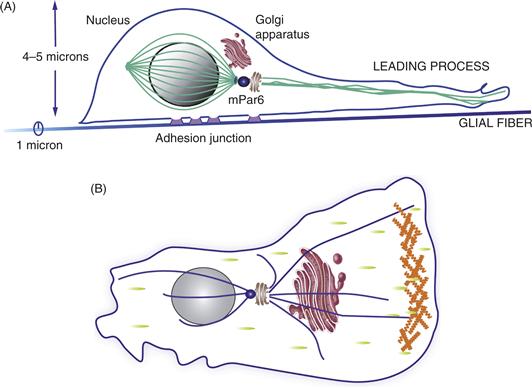
Figure 15.13 Comparison of the cellular organization of neurons migrating on glial fibers (A) and fibroblasts migrating in vivo (B). As the neuron migrates, the polarity complex mPar6, and signaling pathways, polarize the migrating cell. mPar6 localizes to the centrosome (blue) and paces the timing of the movement of the neuron along the glial fiber via Myosin IIB-mediated contraction of F-actin in the proximal aspect of the leading process. A perinuclear cage of tubulin (green) positions the nucleus in the posterior aspect of the cell. Microtubules (green) grow out of the centrosome into the leading process and support vesicle transport during movement. As the neuron moves, it forms attachments beneath the cell soma (purple), which provide traction for neuronal locomotion along the fiber. (B) The model shown represents a polarized cell that has distinct leading and trailing edges. This is a common feature of fibroblastic motility. The leading edge points in the direction of movement and is driven by actin-polymerization-mediated protrusion. Green spots represent points of interaction of the cell with the substrate. Other structures depicted include the nucleus (grey), the Golgi apparatus (brown) and the microtubule-organizing center (MTOC) (blue), from which the microtubule network (blue) radiates, as well as an actin-rich lamellipodium at the front (orange).
During glial-guided neuronal migration, Cdc42 likely polarizes migrating neurons, while Rac regulates leading process formation and protrusion. A decrease in RhoA levels and activity are required for radial migration; however, low levels likely persist to regulate actomyosin contractility to generate force during motility (Govek, Hatten, & Van Aelst, 2011). Recent studies show that two additional Rho GTPase proteins, Rnd2 and Rnd3/RhoE, inhibit RhoA signaling during radial migration to control distinct steps of the migratory process (Heng et al., 2008; Pacary et al., 2011). Thus, tight regulation of Rho GTPase levels and activity are required for proper neuronal migration, and the Rho GTPases act within multiple signaling pathways, including those associated with cortical malformations, to regulate different steps of the migration cycle.
During migration, a dynamic rearrangement of the actin cytoskeleton occurs in the proximal region of the leading process, where Myosin II motors localize. Myosin II activity, which is regulated by the conserved mPar6α polarity complex, is required for both centrosomal and somal motility (Solecki et al., 2004, 2009). The microtubule cytoskeleton is further regulated by cytoplasmic dynein and its cofactors LIS1 and Doublecortin (DCX) to couple centrosomal and nuclear movement (Coquelle et al., 2002; Faulkner et al., 2000; Mesngon et al., 2006; Smith et al., 2000; Tai, Dujardin, Faulkner, & Vallee, 2002; Tsai, Bremner, & Vallee, 2007; Tsai, Chen, Kriegstein, & Vallee, 2005; Umeshima, Hirano, & Kengaku, 2007).
Several adhesion receptor systems function in glial-guided neuronal migration, including Astrotactin (ASTN1), Neuregulin/ErbB4, and BDNF. The neuronal protein ASTN1 is a well-studied adhesion receptor for neuronal migration along glial fibers (Edmondson, Liem, Kuster, & Hatten, 1988; Fishell & Hatten, 1991) that is expressed by neurons in both the cerebellum and cerebral cortex (Zheng, Heintz, & Hatten, 1996). Mice lacking ASTN1 have slowed cerebellar granule neuron migration, a decrease in neuron-glial binding, and abnormal Purkinje cell development compared with wild-type littermates (Adams, Tomoda, Cooper, Dietz, & Hatten, 2002). Recent studies have identified a second member of the Astrotactin receptor family, ASTN2, which regulates the cell surface expression and trafficking of ASTN1 critical for granule cell migration (Wilson, Fryer, & Hatten, 2010). The importance of receptor trafficking to neuronal migration is further highlighted by the finding that inhibition of endocytosis by the small molecule dynasore reversibly arrests migration (Wilson et al., 2010; Zhou et al., 2007). Neuregulin binds ErbB4 on the glial surface and provides signals that maintain glial process formation (Anton, Marchionni, Lee, & Rakic, 1997; Rio, Rieff, Pelmin, & Corfas, 1997), and BDNF stimulates granule neuron migration (Borghesani et al., 2002). Surprisingly integrin-based adhesions, which are essential for most types of cell migration, are not required for glial-guided neuronal migration in the developing brain (Belvindrah, Hankel, Walker, Patton, & Muller, 2007; Fishell & Hatten, 1991).
As methods became available to culture acute slices of developing brain, it became feasible to acquire real-time images of neurons migrating in the complex setting of developing brain tissue. Lipophilic dyes (Rivas & Hatten, 1995), retroviral transfection, and in vivo electroporation of constructs that tag the cells with fluorescent markers such as EGFP (Bhatt, Tomoda, Fang, & Hatten, 2000) in the embryonic neocortex, followed by the generation of acute slices several days later, made it possible to follow particular cohorts of developing neurons and their fates in the emerging neocortex (Zhang, Campbell, Ting, & Tsien, 2002). Moreover, this approach facilitated real-time imaging of cells with altered levels of gene expression, achieved by the introduction of cDNAs to overexpress genes or small hairpin RNAs (shRNAs) to knockdown gene expression, in order to test the function of proteins associated with human cortical malformations (Bai et al, 2003; Tsai et al., 2005), transcription factors (Hand et al., 2005), polarity proteins (Solecki et al., 2004), cytoskeletal proteins (Tsai et al., 2005), and components of downstream signaling pathways. Methods for real-time imaging, such as spinning disc confocal imaging, and for imaging cells deep in living tissue with multiphoton systems are evolving rapidly, providing neurobiologists with the tools to study the molecular regulation of CNS migrations. These experiments have occurred in parallel with cell biological studies of the fundamental mechanisms of metazoan motility, described briefly above.
Lineage Analysis of Radial Glial Cells: Glia Generate Neurons in Late Phases of Cortical Development
During the past decade, real-time imaging of labeled radial glial cells and genetic experiments on the lineal descent of radial glia have shown that a large number of neurons are descended from glial progenitor cells. In the developing neocortex, imaging experiments (Noctor et al., 2002; Kriegstein & Noctor, 2004) revealed the surprising finding that radial glial cells can also generate neuronal precursors, which immediately, or after several rounds of cell division, migrate along the radial processes of the glial mother cells that stretch across the expanding cerebral wall (Fig. 15.14). Genetic studies by Anthony and Heintz (Anthony, Klein, Fishell, & Heintz, 2004) used Cre/loxP fate mapping and clonal analysis to demonstrate that radial glia throughout the CNS serve as neuronal progenitors and that radial glia within different regions of the CNS pass through their neurogenic stage of development at distinct time points. Thus, radial glial populations within different CNS regions are not heterogeneous with regard to their potential to generate neurons versus glia. They went on to show that the promoter region of the radial glial cell marker gene Blbp (Feng, Hatten, & Heintz, 1994) contains a binding site for the Notch effector CBF1 that is essential for Blbp transcription in radial glia (Anthony, Mason, Gridley, Fishell, & Heintz, 2005). These results identified Blbp as the first predominantly CNS-specific Notch target gene. Their results also confirmed earlier studies by Gaiano and Fishell (Gaiano & Fishell, 2002) who showed that Notch signaling regulates radial glial differentiation in the neocortex.
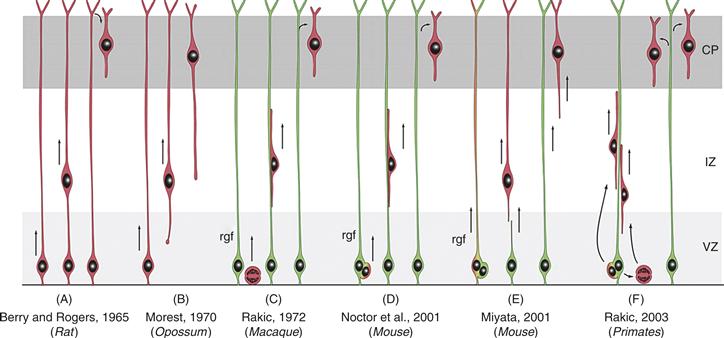
Figure 15.14 Schema of the Evolving Concepts of the Relationship between Radial Glial Cells and Migrating Neurons in the Developing Mammalian Cerebral Cortex. As discussed in the text, initial theories of neuronal migration ignored the glial cells. In the early 1970s, Sidman and Rakic proposed that neurons migrate along glial fibers. Live imaging of labeled radial glia and migrating neurons revealed that during the epoch of migration, glial cells produce neuronal progeny, which immediately or after several rounds of division use the mother glial cell as a guide for their migration. As indicated in the diagram, there are some differences in this process among vertebrate species. Neurons, red; radial glial cells, green.
Reprinted from Rakic (2003).
Tangential Migration Pathways: Cortical Interneurons, Cajal-Retzius Cells, the Rostral Migratory Stream, and the Cerebellar System
Cortical Interneurons
Although radial migration was once thought to be the primary mode of CNS migrations in cortical regions of vertebrate brain, we now realize that a small subset of the total population of neurons in the murine neocortex migrate from the neocortical VZ to form the layers of the mature cerebral cortex (Tan et al., 1995, 1998). These neurons are the large output neurons, the pyramidal cells (Parnavelas, Barfield, Franke, & Luskin, 1991), and a subpopulation of cortical interneurons. The majority of cortical interneurons originate in the basal forebrain and migrate into the neocortex (Fig. 15.15). The importance of this ventral to dorsal mode of migration is evidenced by the rather remarkable fact that the GABAergic neurons are only about 20% of the total neuronal population in the cortex of rodents (the number is far lower in humans). A detailed view of the migratory routes of cells migrating from the basal forebrain into the neocortex emerged from genetic and transplantation studies. Initial experiments with mice lacking the transcription factors Dlx1/2 suggested that cortical interneurons originate in the medial ganglionic eminence (MGE) (Anderson, Eisenstat, Shi, & Rubenstein, 1997; Tamaki, Fujimori, & Takauji, 1997). More detailed transplantation and fate mapping studies refined this original view, providing support for the general model that the majority of cortical interneurons originate in the MGE and migrate into the neocortex (Polleux, Whitford, Dijkhuizen, Vitalis, & Ghosh, 2002; Xu, Cobos, De La Cruz, & Rubenstein, & Anderson, 2004). The lateral ganglionic eminence (LGE) likely contributes a small population of interneurons to the cortical interneuron population, but current experiments show that the many LGE cells migrate into the olfactory bulb intermingling with cells that migrate from the cortical SVZ.
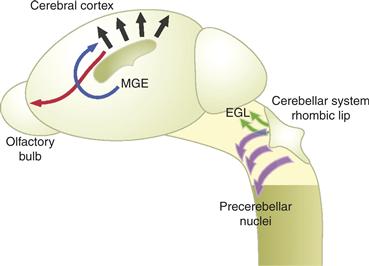
Figure 15.15 Non-Radial Pathways of Neuronal Migration. Over the past decade, a number of tangential migratory pathways have been described, in addition to radial pathways (black), including the migration of interneurons from the basal forebrain into the developing neocortex (blue), the migration of neurons from the SVZ to the olfactory bulb along the rostral migratory stream (red), and migrations of progenitors from the rhombic lip to cerebellar cortex (green) and the pre-cerebellar nuclei of the brainstem (purple). (EGL, external granule layer; MGE, medial ganglionic eminence; SVZ, subventricular zone)
Reprinted from Hatten (2002).
Another tangential migratory pathway occurs in the marginal zone of the neocortex. Although the cells of the MZ were long assumed to be the earliest generated neurons in the cortical VZ, recent evidence from genetic and molecular genetic studies supports the view that a subpopulation of MZ cells originate in the rostral forebrain (or even the basal forebrain) (Meyer, Soria, Martinez-Galan, Martin-Clemente, & Fairen, 1998), while others emerge from the cortical hem, and migrate across the surface of the developing cortex (Garcia-Moreno, Lopez-Mascaraque, & De Carlos, 2007; Meyer, 2007). Support for this view came from studies on gene expression patterns in EGFP-BAC transgenic mice from the GENSAT project (Gong et al., 2003), which showed that cells marked by the gene Pde1c migrate from the rostral forebrain back over the surface of the emerging neocortex. Similarly, studies on Wnt3a positive cells showed that cells of the cortical hem also contribute to the preplate via a superficial migratory pathway across the surface of the neocortex (Louvi, Yoshida, & Grove, 2007).
The Rostral Migratory Stream
The rostral migratory stream (RMS) is another major pathway for tangential migrations (Fig. 15.5). Between E12.5 and E14 in the mouse (E14-16 in the rat), a secondary proliferative zone is formed along the third ventricle of the forebrain (Pencea & Luskin, 2003). This progenitor zone, known as the subventricular zone (SVZ), persists in the adult, where it continues to generate neurons. The subventricular zone has been the subject of intense interest, because it continues to generate new neurons into adulthood, providing GABAergic neurons for the olfactory bulb (Luskin, 1998). As a renewable population of partially committed CNS precursors, the cells from the RMS are an ideal system for studying adult neural “stem” cells (as indicated, they are not “stem cells” in the strict definition of the term). RMS cells differentiate into interneurons when transplanted into the wide variety of target areas, including the septum, thalamus, hypothalamus, and midbrain of embryonic brain (Alvarez-Buylla, Herrera, & Wichterle, 2000). Thus, many labs are studying the molecular mechanisms that control the differentiation of these cells when placed in ectopic positions, especially in the adult brain.
RMS neurons migrate from the SVZ to the olfactory bulb by a unique mechanism. As the neurons move, they use other migrating neurons in the stream as a substrate (Lois & Alvarez-Buylla, 1994; Lois, Garcia-Verdugo, & Alvarez-Buylla, 1996). This mode of neuronal migration is termed “chain migration” because the neurons form chains within glial sheaths. Recent experiments by Muller and colleagues show that neuroblasts express integrins containing the ß1 and ß5 subunits (Belvindrah et al., 2007). Using genetic and cell biological approaches, they demonstrated that ß5 integrins are dispensable for chain migration, whereas ß1 integrins promote cell–cell interactions that link neuroblasts into chains. The ß1 integrin ligand laminin is recruited to the cell surface of migrating neuroblasts and induces chain formation in SVZ explants and the aggregation of purified neuroblasts. Thus, ß1 integrins and their laminin ligands promote the formation of cell chains in the adult RMS (Belvindrah et al., 2007).
Several secreted signaling molecules are thought to guide the migration of RMS neuroblasts to the olfactory bulb, including the diffusible chemoattractants Netrin-1 (Murase & Horwitz, 2002), the glial cell line–derived neurotrophic factor (Paratcha, Ibanez, & Ledda, 2006), and the chemorepellent Slit1/2 (Nguyen-Ba-Charvet et al., 2004; Wu et al., 1999). In addition, receptor tyrosine kinases of the Eph families and their ephrin ligands (Conover et al., 2000), and Neuregulin and Erb-B ligands (Ghashghaei et al., 2006) apparently guide neuronal migration in the RMS.
Cerebellar System
A final example of widespread tangential migrations can be found in the cerebellar system, where migrations from the rhombic lip (RL) contribute to both cerebellar histogenesis and the formation of the precerebellar nuclei of the brainstem, which project afferent connections to the cerebellar nuclei or receive efferent connections from the cerebellum (Fig. 15.5). During embryogenesis, the cerebellar territory arises from rhombomere 1, delineated by the expression of Hoxa2 (posterior) and Otx2 (anterior). In the mouse, between embryonic (E) day E9.0 and E9.5, the pontine flexure creates an area along the border of the mesencephalon and metencephalon, where the neural tube fails to close, called the RL (Harkmark, 1954). The embryonic RL is a specialized germinative epithelium that arises relatively late in development at the interface between the neural tube and the roofplate of the fourth ventricle. The rostral (cerebellar) and hindbrain domains of the RL form a continuous proliferative epithelium, which contains overlapping pools of progenitor cells that express combinatorial patterns of transcription factors. Lineage tracing experiments demonstrate that progenitor cell populations in the RL generate both cerebellar granule cells and neurons of the cerebellar nuclei (Rodriguez & Dymecki, 2000), the lateral pontine nucleus, and the cochlear nucleus (Machold & Fishell, 2005; Wang, Rose, & Zoghbi, 2005). Moreover, lineage tracing experiments define the neurons of the precerebellar nuclei that project afferent axons to cerebellar neurons (Rio et al., 1997). Thus, experiments on the allocation of cell fate among rhombic lip (RL) precursors destined for the cerebellar cortex, the cerebellar nuclei and the pre-cerebellar nuclei of the chick and mouse (Landsberg et al., 2005) illustrate the complex migrations that generate the nuclear and cortical regions of the cerebellum (Morales & Hatten, 2006). These patterns include both radial and tangential migratory pathways.
Summary
During CNS development, a remarkable range of neuronal migrations set forth the architectonics of the brain. In general, these migrations can be seen as dorso-ventral (DV) or anterior-posterior (AP) migrations, pathways thought to be prominent in lower organisms but not in vertebrates. Indeed, genes discovered in C. elegans and Drosophila provide molecular cues for guiding migrations in higher vertebrates, and conserved polarity proteins and their signaling pathways play a key role in sensing directional cues, polarizing cellular extrusions, and generating forces required to propel cells along pathways defined by specific adhesions and/or chemoattractants. The relationship of cell migrations to the establishment of the neuronal layers and brain circuitry will ultimately require novel insights into the role of the transient scaffolding systems of cortical development, the preplate, subplate, and cortical plate, which appear to pattern the connections between laminar, cortical areas of the brain, subcortical nuclei, and the formation of sensorimotor pathways.
References
1. Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972.
2. Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology. 1965;124:319–335.
3. Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: Source of neuronal precursors for brain repair. Progress in Brain Research. 2000;127:1–11.
4. Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476.
5. Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890.
6. Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes & Development. 2005;19:1028–1033.
7. Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510.
8. Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nature Neuroscience. 2003;6:1277–1283.
9. Barkovich AJ, Kuzniecky R, Dobyns WB, Jackson G, Becker LE, Evrard P. Malformations of the cortical development. Neuropediatrics. 1996;27:59–63.
10. Basch M, Bronner-Fraser M, Garcia-Castro M. Specification of neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222.
11. Belvindrah R, Hankel S, Walker J, Patton BL, Muller U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. Journal of Neuroscience. 2007;27:2704–2717.
12. Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. Journal of Anatomy. 1965;99:691–709.
13. Bhatt RS, Tomoda T, Fang Y, Hatten ME. Discoidin domain receptor 1 functions in axon extension of cerebellar granule neurons. Genes & Development. 2000;14:2216–2228.
14. Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat I Qualitative analysis. Journal of Neurocytology. 1983a;12:599–616.
15. Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat II Qualitative analysis. Journal of Neurocytology. 1983b;12:697–712.
16. Borghesani PR, Peyrin JM, Klein R, et al. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129:1435–1442.
17. Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using the monoclonal antibody HNK-1. Developmental Biology. 1986;115:44–55.
18. Bronner-Fraser M, Fraser S. Cell lineage analysis shows multipotentiality of some avian neural crest cells. Nature. 1988;335(8):161–164.
19. Burakov A, Nadezhdina E, Slepchenko B, Rodionov V. Centrosome positioning in interphase cells. Journal of Cell Biology. 2003;162:963–969.
20. Caviness Jr VS. Neocortical histogenesis in normal and reeler mice: A developmental study based upon [3H]thymidine autoradiography. Brain Research. 1982;256:293–302.
21. Caviness Jr VS, Rakic P. Mechanisms of cortical development: A view from mutations in mice. Annual Review of Neuroscience. 1978;1:297–326.
22. Caviness Jr VS, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cerebral Cortex. 2003;13:592–598.
23. Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641.
24. Christensen JH, Coles EG, Wilkinson DG. Molecular control of neural crest formation, migration and differentiation. Current Opinion in Cell Biology. 2000;12:719–724.
25. Chun JJ, Shatz CJ. Redistribution of synaptic vesicle antigens is correlated with the disappearance of a transient synaptic zone in the developing cerebral cortex. Neuron. 1988;1:297–310.
26. Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nature Neuroscience. 2000;3:1091–1097.
27. Coquelle FM, Caspi M, Cordelieres FP, et al. Lis1, CLIP-170’s key to the dynein/dynactin pathway. Molecular and Cellular Biology. 2002;22:3089–3102.
28. Del Rio JA, Martinez A, Auladell C, Soriano E. Developmental history of the subplate and developing white matter in the murine neocortex Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cerebral Cortex. 2000;10:784–801.
29. Dobyns WB, Truwit CL. Lissencephaly and other malformations of cortical development: 1995 update. Neuropediatrics. 1995;26:132–147.
30. Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83.
31. Edmondson JC, Hatten ME. Glial-guided granule neuron migration in vitro: A high-resolution time-lapse video microscopic study. Journal of Neuroscience. 1987;7:1928–1934.
32. Edmondson JC, Liem RK, Kuster JE, Hatten ME. Astrotactin: A novel neuronal surface antigen that mediates neuron-astroglial interactions in cerebellar microcultures. Journal of Cell Biology. 1988;106:505–517.
33. Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317.
34. Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498.
35. Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Current Opinion in Cell Biology. 2003;15:67–72.
36. Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Current Opinion in Cell Biology. 2008;20:707–715.
37. Faulkner NE, Dujardin DL, Tai CY, et al. A role for the lissencephaly gene L1S1 in mitosis and cytoplasmic dynein function. Nature Cell Biology. 2000;2:784–791.
38. Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908.
39. Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765.
40. Fishell G, Mason CA, Hatten ME. Dispersion of neural progenitors within the germinal zones of the forebrain. Nature. 1993;362:636–638.
41. Gadisseux JF, Evrard P. Glial-neuronal relationship in the developing central nervous system A histochemical-electron microscope study of radial glial cell particulate glycogen in normal and reeler mice and the human fetus. Developmental Neuroscience. 1985;7:12–32.
42. Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438.
43. Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annual Review of Neuroscience. 2002;25:471–490.
44. Gammill L, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires Neuropilin-2/Semaphorin3F signaling. Development. 2006;133:99–106.
45. Garcia-Moreno F, Lopez-Mascaraque L, De Carlos JA. Origins and migratory routes of murine Cajal-Retzius cells. Journal of Comparative Neurology. 2007;500:419–432.
46. Gasser U, Hatten M. Neuron-glia interactions of rat hippocampal cells in vitro: Glial-guided neuronal migration and neuronal regulation of glial differentiationsss. Journal of Neuroscience. 1990a;10:1276–1285.
47. Gasser UE, Hatten ME. Central nervous system neurons migrate on astroglial fibers from heterotypic brain regions in vitro. Proceedings of the National Academy Sciences of the United States of America. 1990b;87:4543–4547.
48. Ghashghaei HT, Weber J, Pevny L, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proceedings of the National Academy Sciences of the United States of America. 2006;103:1930–1935.
49. Ghosh A, Shatz CJ. Pathfinding and target selection by developing geniculocortical axons. Journal of Neuroscience. 1992;12:39–55.
50. Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993;117:1031–1047.
51. Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181.
52. Goffinet AM. Events governing organization of postmigratory neurons: Studies on brain development in normal and reeler mice. Brain Research. 1984;319:261–296.
53. Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463.
54. Gong S, Zheng C, Doughty ML, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925.
55. Gotz M, Huttner WB. The cell biology of neurogenesis. Nature Reviews Molecular Cell Biology. 2005;6:777–788.
56. Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Developmental Neurobiology. 2011;71(6):528–553.
57. Gregory WA, Edmonson JC, Hatten ME, Mason CA. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. Journal of Neuroscience. 1988;8:1728–1738.
58. Hand R, Bortone D, Mattar P, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62.
59. Harkmark W. Cell migrations from the rhombic lip to the inferior olive the nucleus raphe and the pons A morphological and experimental investigation on chick embryos. Journal of Comparative Neurology. 1954;100:115–209.
60. Hatten ME. Central nervous system neuronal migration. Annual Review of Neuroscience. 1999;22:511–539.
61. Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–1663.
62. Heasman SJ, Ridley AJ. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nature Reviews Molecular Cell Biology. 2008;9:690–701.
63. Heng JI, Nguyen L, Castro DS, et al. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455:114–118.
64. Hinds JW, Ruffett TL. Cell proliferation in the neural tube: An electron microscopic and Golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat. 1971;115:226–264.
65. His, W. (1886). Zur Geschichte des menschlichen Ruckenmarks und der Nervenwurzeln Ges Wissensch. BD 13 S, 477.
66. Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525.
67. Konig N, Marty R. Early neurogenesis and synaptogenesis in cerebral cortex. Bibliotheca Anatomica. 1981;19:152–160.
68. Konig N, Roch G, Marty R. The onset of synaptogenesis in rat temporal cortex. Anatomy and Embryology. 1975;148:73–87.
69. Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. Journal of Neurocytology. 1980;9:219–242.
70. Kriegstein AR, Gotz M. Radial glia diversity: A matter of cell fate. Glia. 2003;43:37–43.
71. Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends in Neurosciences. 2004;27:392–399.
72. Kriegstein AR, Parnavelas JG. Progress in corticogenesis. Cerebral Cortex. 2006;16:i1–i2.
73. Krull CE, Lansford R, Gale NW, et al. Interactions between Eph-related receptors and ligands confer rostrocaudal polarity to trunk neural crest migration. Current Biology. 1997;7:571–580.
74. Landsberg RL, Awatramani RB, Hunter NL, Farago AF, DiPetrantioio HJ, Dymecki SM. Hindbrain rhombic lip is comprised of disrete progenitor cell populations allocated by Pax6. Neuron. 2005;48:933–947.
75. LeDouarin NM. The neural crest New York, NY: Cambridge University Press; 1982.
76. LeDouarin NM, Kalcheim C. The neural crest New York, NY: Cambridge University Press; 1999.
77. Lein ES, Finney EM, McQuillen PS, Shatz CJ. Subplate neuron ablation alters neurotrophin expression and ocular dominance column formation. Proceedings of the National Academy Sciences of the United States of America. 1999;96:13491–13495.
78. Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148.
79. Lois C, Garcia-Verdugo J-M, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981.
80. Louvi A, Yoshida M, Grove EA. The derivatives of the Wnt3a lineage in the central nervous system. The Journal of Comparative Neurology. 2007;504:550–569.
81. Luskin MB. Neuroblasts of the postnatal mammalian forebrain: their phenotype and fate. Journal of Neurobiology. 1998;36:221–233.
82. Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24.
83. Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends in Neurosciences. 1998;21:64–71.
84. McConnell SK, Ghosh A, Shatz CJ. Subplate pioneers and the formation of descending connections from cerebral cortex. Journal of Neuroscience. 1994;14:1892–1907.
85. Mesngon MT, Tarricone C, Hebbar S, et al. Regulation of cytoplasmic dynein ATPase by LIS1. Journal of Neuroscience. 2006;26:2132–2139.
86. Meyer G. Genetic control of neuronal migrations in human cortical development. Advances in Anatomy, Embryology, and Cell Biology. 2007;189:1–111 1 p preceding 1.
87. Meyer G, Soria JM, Martinez-Galan JR, Martin-Clemente B, Fairen A. Different origins and developmental histories of transient neurons in the marginal zone of the fetal and neonatal rat cortex. Journal of Comparative Neurology. 1998;397:493–518.
88. Molliver ME, Kostovic I, van der Loos H. The development of synapses in cerebral cortex of the human fetus. Brain Research. 1973;50:403–407.
89. Morales D, Hatten ME. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. Journal of Neuroscience. 2006;26:12226–12236.
90. Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. Journal of Neuroscience. 2002;22:3568–3579.
91. Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971.
92. Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. Journal of Neuroscience. 2004;24:1497–1506.
93. Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. Journal of Neuroscience. 2002;22:3161–3173.
94. Noden DM. An analysis of the migratory behavior of avian cephalic neural crest cells. Developmental Biology. 1975;42:106–130.
95. Nowakowski RS, Rakic P. The mode of migration of neurons to the hippocampus: A Golgi and electron microscopic analysis in foetal rhesus monkey. Journal of Neurocytology. 1979;8:697–718.
96. Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. The EMBO Journal. 1996;15:6035–6049.
97. Pacary E, Heng J, Azzarelli R, et al. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibion of RhoAsignaling. Neuron. 2011;69:1069–1084.
98. Paratcha G, Ibanez CF, Ledda F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Molecular and Cellular Neuroscience. 2006;31:505–514.
99. Parnavelas JG, Barfield JA, Franke E, Luskin MB. Separate progenitor cells give rise to pyramidal and nonpyramidal neurons in the rat telencephalon. Cerebral Cortex. 1991;1:463–468.
100. Pencea V, Luskin MB. Prenatal development of the rodent rostral migratory stream. Journal of Comparative Neurology. 2003;463:402–418.
101. Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nature Neuroscience. 2004;7:803–811.
102. Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934.
103. Pinto L, Gotz M. Radial glial cell heterogeneity – the source of diverse progeny in the CNS. Progress in Neurobiology. 2007;83:2–23.
104. Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160.
105. Price DJ, Kennedy H, Dehay C, et al. The development of cortical connections. The European Journal of Neuroscience. 2006;23:910–920.
106. Rakic P. Mode of cell migration to the superficial layers of fetal monkey cortex. Journal of Comparative Neurology. 1972;145:61–84.
107. Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Progress in Brain Research. 1988;73:15–37.
108. Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia. 2003;43:19–32.
109. Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cerebral Cortex. 2003;13:1072–1083.
110. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710.
111. Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709.
112. Rio C, Rieff H, Pelmin Q, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50.
113. Rivas R, Hatten M. Motility and cytoskeletal organization of migrating cerebellar granule neurons. Journal of Neuroscience. 1995;15:981–989.
114. Rodriguez CI, Dymecki SJ. Origin of the precerebellar system. Neuron. 2000;27:475–486.
115. Sauer ME, Chittenden AC. Deoxyribonucleic acid content of cell nuclei in the neural tube of the chick embryo: evidence for intermitotic migration of nuclei. Experimental Cell Research. 1959;16:1–6.
116. Sauer ME, Walker BE. Radioautographic study of interkinetic nuclear migration in the neural tube. Proceeding of the Society for Experimental Biology and Medicine. 1959;101:557–560.
117. Schneider D, Gulacsi A, Hatten ME. Lrp12/Mig13a reveals changing patterns of preplate neuronal polarity during corticogenesis that are absent in reeler mutant mice. Cerebral Cortex. 2011;21:134–144.
118. Selleck MAJ, Bronner-Fraser M. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development. 1995;121:526–538.
119. Sharma K, Korade Z, Frank E. Late-migrating neuroepithelial cells from the spinal cord differentiate into sensory ganglion cells. Neuron. 1995;14:143–152.
120. Sidman RL. Autoradiographic methods and principles for study of the nervous system with thymidine-H3. In: Nauta WJ, Ebbesson SOE, eds. Contemporary research techniques of Neuroanatomy. New York, NY: Springer-Verlag; 1970;252–274.
121. Sidman RL, Rakic P. Neuronal migration with special reference to developing human brain: A review. Brain Research. 1973;62:1–35.
122. Smith DS, Niethammer M, Ayala R, et al. Regulation of cytoplasmic dynein behavior and mictotubule organization by mammalian Lis1. Nature Cell Biology. 2000;2:767–775.
123. Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nature Neuroscience. 2004;7:1195–1203.
124. Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80.
125. Stemple DL, Anderson DJ. Lineage diversification of the neural crest: In vitro investigations. Developmental Biology. 1993;159:12–23.
126. Strobl-Mazzulla PH, Sauka-Spengler T, Bronner-Fraser M. Histone demethylase JmjD2A regulates neural crest specification. Developmental Cell. 2010;19:460–468.
127. Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886.
128. Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in LIS 1 kinetochore function. Journal of Cell Biology. 2002;156:959–968.
129. Tamaki K, Fujimori E, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. Journal of Neuroscience. 1997;17:8313–8323.
130. Tan S-S, Faulkner-Jones B, Breen SJ, Walsh M, Bertram JF, Reese BE. Cell dispersion patterns in different cortical regions studied with an X-inactivated transgenic marker. Development. 1995;121:1029–1039.
131. Tan SS, Kalloniatis M, Sturm K, Tam P, Reese B, Faulkner-Jones B. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304.
132. Tissir F, Goffinet AM. Reelin and brain development. Nature Reviews Neuroscience. 2003;4:496–505.
133. Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nature Neuroscience. 2007;10:970–979.
134. Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. Journal of Cell Biology. 2005;170:935–945.
135. Umeshima H, Hirano T, Kengaku M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proceedings of the National Academy Sciences of the United States of America. 2007;104:16182–16187.
136. Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. Journal of Cell Science. 2005;118:4917–4919.
137. Walsh C, Cepko CL. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex [see comments]. Science. 1992;255:434–440.
138. Wang HU, Anderson DJ. Roles of Eph family transmembrane ligands in repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–396.
139. Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43.
140. Weston JA. A radiographic analysis of the migration and localization of trunk neural crest cells in the chick. Developmental Biology. 1963;6:279–310.
141. Wilson P, Fryer RH, Hatten ME. Astn2, A Novel member of the Astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. Journal of Neuroscience. 2010;30:8529–8540.
142. Wood J, Martin S, Price DJ. Evidence that the earliest generated cells of the murine cerebral cortex form a transient population in the subplate and marginal zone. Developmental Brain Research. 1992;66:137–140.
143. Wu W, Wong K, Chen J, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336.
144. Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. Journal of Neuroscience. 2004;24:2612–2622.
145. Yasuda K, Momose T, Takahashi Y. Applications of microelectroporation for studies of chick embryogenesis. Development, Growth and Differentiation. 2000;42(3):203–206.
146. Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. Journal of Neuroscience. 2001;21:5607–5619.
147. Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nature Reviews Molecular Cell Biology. 2002;3:906–918.
148. Zheng C, Heintz N, Hatten ME. CNS gene encoding Astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–419.
149. Zhong W, Chia W. Neurogenesis and asymmetric cell division. Current Opinion in Neurobiology. 2008;18:4–11.
150. Zhou P, Porcionatto M, Pilapil M, et al. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68.
Suggested Readings
1. Bielas S, Higginbotham H, Koizumi H, Tanaka T, Gleeson JG. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annual Review of Cell and Developmental Biology. 2004;20:593–618.
2. Gao L, Macara IG. Isoforms of the polarity protein par6 have distinct functions. Journal of Biological Chemistry. 2004;279:41557–41562.
3. Gierdalski M, Sardi SP, Corfas G, Juliano SL. Endogenous neuregulin restores radial glia in a (ferret) model of cortical dysplasia. Journal of Neuroscience. 2005;25:8498–8504.
4. Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638.
5. Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568.