Balint’s syndrome and feature binding Balint’s syndrome is a rare disorder that can occur with lesions to both parietal lobes of the human brain (specifically inferior parietal and dorsal occipital cortex) and characterizes the most severe spatial deficit observed in neuropsychology (Balint 1909/1995). It results in what Balint termed functional blindness, meaning that even when primary visual areas (*V1) are functionally intact patients behave as if they are blind in their daily life. The spatial information of the external world is all but lost, while perception of personal body space remains intact (e.g. determining right limb from left). However, the relationship between the patient’s body and objects and/or features in the external world are absent (Robertson et al. 1997). Clinically, one of the syndrome’s defining features is the inability to perceive more than one object at any given time, accompanied by an inability to reach in the correct location (optic ataxia) and a fixated gaze (optic apraxia). The perception of only one object is more than simply an inability to apprehend the whole from a set of parts (both are often called simultanagnosia, but this refers to very different phenomena). Rather, the patient may see a pencil in an examiner’s hand, but will not perceive the hand that holds it. Note that the parts of the pencil itself appear to be integrated (which remains a puzzle). However, its location is unknown. While the perceived item may be large or small, complex or simple, or appear within foveal or peripheral vision, it cannot be localized accurately above chance levels (either by naming, pointing, reaching, or gesturing). As a result, nearly constant care is required to accomplish basic everyday tasks. For example, without seeing both a plate and a fork on the dinner table, or knowing where the fork is relative to the body, it is nearly impossible for these individuals to feed themselves. Fortunately, some of the most severe aspects of Balint’s syndrome tend to improve slowly over time, although the symptoms reappear under time limited conditions even after years after the insult (Robertson et al. 1997).
1. Property binding and parietal damage
2. Property binding and consciousness
3. Summary
For the purposes of this entry, we will focus on another prominent perceptual dysfunction in individuals with Balint’s syndrome: namely, a deficit in *binding basic features (e.g. size, colour, motion, shape) together in perception. The features that produce binding errors have been associated with specialized neural populations of the primate brain. This form of binding has been termed property binding by Treisman (1996), and can be distinguished from other types of binding (e.g. binding parts to form a shape or binding properties to location). Although the parts of a single object may be bound together and identified accurately (i.e. the four lines and corners of a square), without an accurate spatial map of the external world, basic features that are properties of an object such as size and colour are not bound accurately to the perceived shape (Freidman-Hill et al. 1995, Humphreys et al. 2000). In fact, features from other items in a display can be incorrectly attached to the shape that one sees (e.g. a display containing a blue T and green X may be seen as a green T or blue X). This type of error is known as an illusory conjunction, and in Balint’s patients can occur even under free viewing conditions in which no time limitations are imposed. It is also consistent with reports by Balint’s patients themselves about their perceptual experiences in everyday life (e.g. a house by a busy street appearing to move even when the patient denies seeing any vehicles or even a street).
Despite the loss of spatial awareness in Balint’s patients, there is now substantial evidence that they can detect basic feature properties with relative ease. For example, a patient with a classic case of Balint’s syndrome due to bilateral parietal/occipital lesions (R. M.) was able to detect a unique feature target among related distractors in a visual search display (e.g. a red X among a number of green O and green X distractors). Furthermore, his ability to detect a feature was independent of the number of distractors in the display, a well-known perceptual phenomenon called pop-out in which a unique feature is detected without the necessity for a serial attentional search through the items in the display. However, when a target contained features shared by the distractors in the search display (e.g. a red X among red O and green X distractors; conjunction search display), R. M. was unable to detect the target any better than chance. This problem in property binding occurred in displays with as few as one, three, or five distractors (Robertson et al. 1997), and again was observed under free viewing conditions. Thus, unique features (at least those associated with specialized neural populations) capture attention even when the external representation of space is lost, but the ability to voluntarily search for a conjunction of two features (which requires serial search for the properly bound item) is severely impaired. In either case, Balint’s patients are unable to spatially locate the item they report seeing. Spatial deficits can be so severe that even reporting whether a target is located to the left or right of central fixation on a computer monitor can be at chance levels (Friedman-Hill et al. 1995), Robertson et al. 1997).
These deficits are consistent with attentional theories proposing that feature and conjunction processing are qualitatively different. While feature detection does not require spatial attention and is often considered preattentive (Treisman and Gelade 1980), detection of conjunctions requires accurate binding of features in multi-item arrays through spatial attention. Balint’s syndrome extends this conclusion to consciousness by showing that properties of objects of which the patient is unaware can migrate to an object of which the patient is aware, as shown in the case of illusory conjunctions.
The perceptual deficits evident in Balint’s syndrome support the hypothesis that dorsal stream spatial processes are critically involved in binding properties such as colour and shape that are encoded in the ventral stream of the human brain (Treisman 1996, Robertson 2003). They further show that this binding process (the binding of properties to the shapes of the objects themselves) appears to rely critically upon perceptual awareness of space itself.
One of the most challenging questions of consciousness is how and why it came to be. But another issue is whether consciousness has a role to play in cognition, or is simply the end product of a massive amount of preprocessing that precedes it. Mechanistic views posit (whether explicitly or implicitly) that consciousness is an *epiphenomenon that reflects the underlying biological and/or cognitive processes.
While not denying underlying biological processing, the case of Balint’s syndrome suggests that accurate binding (at least of features on dimensions such as colour and shape) requires conscious awareness of the space in which features coexist. Thus, consciousness seems to play a role in properly binding biologically distributed properties in a way that reflects the spatially segregated structure of the external world. Balint’s syndrome demonstrates that without awareness of the dimension of space, binding between non-spatial properties is altered. In this way Balint’s syndrome suggests that one job of consciousness is to individuate objects (in this case in space) in order for the features in a scene to be properly bound together. Objects that disappear from conscious awareness when dorsal spatial maps are damaged nevertheless continue to supply feature information that drives neural encoding of their properties. However, the brain has no place to put them without a spatial map, resulting in the random selection of features for perception. The implications for normal consciousness are that it has a crucial role in cognition (or at least in perception) in establishing and maintaining segregation of features and their proper location.
In sum, the evidence from patients with Balint’s syndrome is consistent with a central role for spatial awareness in accurately binding features that are properties of objects. When this breaks down, features from objects that are outside awareness can intrude on objects of awareness. These features have been associated with ventral coding, suggesting a network for binding that includes interactions between ventral and dorsal posterior areas of the cortex. Spatial maps that guide controlled spatial attention appear necessary to accurately bind at least some types of features together in conscious perceptual awareness.
LYNN C. ROBERTSON AND THOMAS VAN VLEET
Balint, R. (1909/1995). ‘Seelenlahmung des ‘Schauens’, optische Ataxie, raumliche Storung der Aufmerksamkeit’. Monatshrift fur Psychiatrie und Neurologie, 25; transl. Cognitive Neuropsychology, 12.
Friedman-Hill, S., Robertson, L. C., and Treisman, A. (1995). ‘Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions’. Science, 269.
Humphreys, G. W., Cinel, C., Wolfe, J., Olson, A., and Klempen, N. (2000). ‘Fractionating the binding process: neuropsychological evidence distinguishing binding of form from binding of surface features’. Vision Research, 40.
Robertson, L. C. (2003). ‘Binding, spatial attention and perceptual awareness’. Nature Reviews Neuroscience, 4.
——, Treisman, A., Friedman-Hill, S., and Grabowecky, M. (1997). ‘The interaction of spatial and object pathways: evidence from Balint’s syndrome’. Journal of Cognitive Neuroscience, 9.
Treisman, A. M. (1996). ‘The binding problem’. Current Opinion in Neurobiology, 6.
—— and Gelade, G. (1980). ‘A feature-integration theory of attention’. Cognitive Psychology, 12.
Bereitschaftspotential See READINESS POTENTIALS AND HUMAN VOLITION
bicameral mind The term ‘bicameral mind’ was coined by psychologist and archaeologist Julian Jaynes (1920–1997) in a controversial book (Jaynes 1976). His hypothesis was that early humans, and even the ancient Greeks, were not conscious in the way we are now; they had a two-part or ‘bicameral’ (literally meaning ‘two-chambered’) mind, and consciousness arose only in the last few thousand years when this two-part structure broke down, long after the evolution of language and with the development of modern language and thought. This means that Jaynes’s theory puts the origin of consciousness as far more recent than almost all other theories.
Jaynes argued that the earliest text from which we can deduce the nature of mind was the Iliad, an epic story of revenge, blood and tears, written about 900 or 850 BC. He explores the question ‘What is mind in the Iliad?’ and answers that ‘There is in general no consciousness in the Iliad’ (Jaynes (1976:69)). He bases this conclusion on the fact that he can find no words in the Iliad that can be translated directly as ‘consciousness’, and no descriptions of mental acts either; words such as psyche or thumos, which later came to mean ‘mind’ or ‘soul’, usually referred to concrete things like blood or breath. There is also no word for ‘will’, and no concept of free will. Most curiously, when the heroes of the Iliad carry out their great acts of revenge, abduction, deception, or generosity they are not described as having their own plans and intentions, or even reasons and motives, but as hearing the gods telling them what to do.
Although this sounds very strange to the modern mind it is, claims Jaynes, just what we should expect from a bicameral mind. One chamber deals with action (including volition, planning, and initiative), while the other deals with perception. These are not integrated into one whole and so when the action system decides what to do the perception system hears this in the form of voices. We modern humans would call these *hallucinations, but for the early Greeks they were the voices of the gods. The voices were obeyed, and had to be obeyed, because bicameral man could not work out what to do by himself.
To try to imagine what this is like, Jaynes points out that we can easily drive a car without awareness, doing all sorts of complex actions in response to events in the outside world, while our conscious self is busy with something else. Bicameral man was even more split than that and without a conscious self; unconsciously hearing voices and unconsciously obeying them.
According to Jaynes, the bicameral mind began to break down when language led to the use of analogies and metaphors. Modern consciousness, he claims, operates by way of constructing an analogue space with an analogue ‘I’ that can observe that space and move metaphorically within it. The whole thing is an invented world with an invented self, built on analogies with both the outside world and observed behaviours. We may feel as though we are a continuous self having conscious experiences but according to Jaynes ‘The seeming continuity of consciousness is really an illusion’ (1976:24). This is a modern illusion. Bicameral man had ‘no awareness of his awareness of the world, no internal mind-space to introspect upon’, no self, no free will, and no subjectivity.
Evidence to support the theory includes the importance of hallucinations, oracles, and divination in contemporary bicameral societies, the role of idols and burial practices found in archaeology, the changing use of words in literature and linguistics, and the neuropsychology of hallucinations and religious behaviour.
The theory has been described as preposterous (indeed Jaynes himself uses this word) and has been controversial since its first publication (Cavanna et al. 2007). Nevertheless it continues to be widely read and cited. A second edition was published in 1990, the book has been reprinted many times, and there is even a Julian Jaynes Society.
SUSAN BLACKMORE
Cavanna, A. E., Trimble, M., Federico, C., and Monaco, F. (2007). ‘The ‘bicameral mind’ 30 years on: a critical reappraisal of Julian Jaynes’ hypothesis’. Functional Neurology, 22.
Jaynes, J. (1976). The Origin of Consciousness in the Breakdown of the Bicameral Mind.
binding problem Binding, in the most general sense of the word, refers to a process or an underlying mechanism of integration that results in the overall unity of an entity, or to the emergence of its holistic features.
1. Binding and consciousness
2. Binding in neuroscience
3. Binding in cognitive science
4. Local and global unity of consciousness
5. Different approaches to the phenomenal binding problem
6. Cognitive and neural theories of binding mechanisms
7. Conclusion
The contents of conscious experience are unified. An object visually perceived, such as a red ball rolling towards you, is experienced as a unified package of visual features, where motion, colour, and form are coherently bound together. Binding is thus required for the *unity of consciousness. Some believe that a solution to the binding problem is the key to solving the entire problem of consciousness, because consciousness is taken to be fundamentally unified. Others argue that binding and consciousness are two different and dissociable problems and therefore a solution to one does not necessarily shed any light to the other.
There are many varieties of binding and therefore also many different binding problems. When it comes to consciousness, one variety of binding is the process or mechanism that brings about the unity of *phenomenal consciousness or the holistic features of subjective phenomenal experience (phenomenal unity, phenomenal binding). Thus, the phenomenal binding problem is the problem of explaining how exactly the unity of consciousness is brought about in the brain or the mind. The problem is considered difficult and persistent because it is not at all obvious how it could be solved, or whether it will be solved at all. The phenomenal binding problem deals purely with the unity of subjective experience and is therefore in principle independent of external physical stimuli or brain responses to them. Thus, the phenomenal binding problem applies equally well to externally evoked percepts as to internally generated images such as *dreams (Revonsuo and Tarkko 2002).
In neuroscience and cognitive science, the binding problem has been formulated in ways that do not refer to the phenomenal unity of consciousness. In neuroscience, binding is the neural process or mechanism that integrates the activities of single neurons to functional groups and neural assemblies. The problem here stems from the fact that any stimulus object appearing in the visual field will activate a huge number of neurons across a wide range of spatially separated cortical areas. Although the response properties of *single neurons in the visual cortex are relatively well known, it remains unclear how thousands or even millions of spatially separate neurons in the cortex interact to form a functionally unified group when they all simultaneously respond to different features of the same object.
This neural binding problem as such thus deals with purely neurophysiological unity: the mechanisms of the holistic features of neural activity that represent an external stimulus as a coherent object. Whether or not such activity is correlated with conscious experience is largely irrelevant. In fact many experiments exploring the mechanisms of neural binding have been conducted by using anaesthetized, unconscious animals as subjects. It is possible to present visual stimuli for an unconscious animal and to detect neural responses in the animal’s visual cortex. Although the animal is unconscious, coherent large-scale neural activity elicited by the stimulus in the animal’s visual cortex may still reflect the holistic properties of the stimulus and covary with the appearance of such properties in the visual field. This kind of coherent neural activities may be the solution to the purely neural binding problem, but they do not address the phenomenal binding problem directly, as no phenomenal experiences, unified or non-unified, exist for the anaesthetized animal during the experiment.
In cognitive science, the binding problem has been formulated in terms of the integration of representation or information processing. As cognitive systems, humans have several modular input systems that process sensory information independently of each other, in an isolated manner. Thus, within the input systems, sensory information originating in a single stimulus object is mostly represented in a non-unified manner. The different features of the object, such as its shape, colour, motion, location, and sound, are handled by separate processing modules, in parallel, at least in the early stages of input processing. By contrast, in the more central systems dealing with selective attention, decision-making, declarative memory, and the control of our interactions with the environment, object representations are complex and holistic, unifying information within and across the modular input systems. At those stages of processing, complex and integrated representations of the world must be formed, because they are required to control and guide coherent behaviour. The cognitive binding problem thus is to explain how the widely distributed, isolated, independently processed streams of modular input information become bound together to integrated representations of the world. The cognitive binding problem deals with purely cognitive unity. The question whether the representations or streams of information being bound together are at some point accompanied by subjective experience or consciousness is largely irrelevant. Thus, the cognitive binding problem is considered to be no different in non-conscious information-processing systems such as robots or machine vision systems from what it is in conscious humans beings.
Although consciousness is largely irrelevant to the neural and the cognitive binding problems, cognitive and neural mechanisms are highly relevant when it comes to the phenomenal binding problem. Thus, it may be that some specific types of cognitive and neural binding in the conscious human brain might in fact underlie phenomenal unity and therefore solve the phenomenal binding problem. But in fact phenomenal unity itself is rather complex and comes in different forms (Revonsuo 1999, Bayne and Chalmers 2003). Before we explore the underlying mechanisms of phenomenal binding in more detail, we need to take a closer look at phenomenal unity itself.
Roughly, the phenomenal unity of consciousness may be divided to two different varieties: local and global. First, consider the local unity of particular contents of consciousness. A paradigmatic example of this is a unified visual percept where a number of different parts and visual features such as colour, shape, and motion are integrated to form a single well-defined phenomenal object. A concrete example: when tracking a flying bird or a colourful butterfly with your gaze, the visual experience caused by the stimulus constitutes a locally integrated percept where all the separate visual parts and features are bound coherently together in a single well-defined region in the subjective visual field.
Second, there is the global unity of consciousness. This refers to the entire phenomenal field where all simultaneous experiences, such as the phenomenal body image, phenomenal visual field, phenomenal auditory space, and the rest of our external sense experiences and internal images and thoughts, form a single experiential space. All simultaneously present phenomenal contents are thus fundamentally interrelated with each other within a unified spatiotemporal context. A concrete example of this is the intuitive feeling of being one single person within one single world, as the philosopher Thomas Metzinger (2003) has put it. The global unity of consciousness forms the constant phenomenal background unity that pervades all subjective experience.
In consciousness research the binding problem is the problem of explaining how the phenomenal unity of consciousness comes about. What kind of underlying process or mechanism could bring about locally unified phenomenal experiences, where a number of different phenomenal parts or features of an experience hang coherently together, or the globally unified phenomenal field that underlies our all-embracing experience of being constantly present within a unified world? The difficulty with this problem lies in the fact that the processes and mechanisms that could, even in principle, account for phenomenal unity remain unknown.
Three different directions in which a solution to the phenomenal binding problem has been sought can be identified.
Elimination of phenomenal unity. According to this line of thought, the phenomenal unity of consciousness is an illusion. The most radical version argues that there is no definable time and space in the brain (or anywhere else) where phenomenal events happen or phenomenal features come together in a ‘Cartesian theatre’. Phenomenal consciousness does not exist, therefore phenomenal unity is an illusion and phenomenal binding is unnecessary, because there is nothing there to be bound together for a unified phenomenal presentation to a subject, and moreover there are no known mechanisms in the brain that could account for such binding. This line of thought can be found e.g. in Daniel Dennett’s work. Typically, empirical data from *change blindness and *inattentional blindness experiments are invoked to support the idea that we are simply mistaken about the unity of consciousness. A somewhat less radical version argues that phenomenal consciousness does exist, but in a non-unified manner. Semir Zeki has defended this line of thought under the label of *microconsciousness theory (Zeki 2003). According to this, the different features of phenomenal experience come about in an isolated manner in different parts of the cortex: each functionally specialized module produces its own microconsciousness (e.g. phenomenal colour or motion), but whether and to what extent these isolated phenomenal features ever become integrated anywhere in the brain remains doubtful.
Purely phenomenological description of phenomenal unity. According to this line of thought, to investigate the experiential unity of consciousness, we need not assume that anything external to the experiences themselves exist. Thus, data from neuroscience, brain anatomy, or experimental psychology is irrelevant to the task. This approach has been advocated by Barry Dainton (2000), who argues that the unity of consciousness can be described and explained by an experiential relation of synchronic co-consciousness. Experience is self-unifying, in that to understand the unity we find within experience, we need not go beyond experience itself.
Mechanistic explanation of phenomenal unity. According to this line of thought, phenomenal unity must be taken seriously as a real feature of experience. In order to explain the holistic features of phenomenal consciousness, the underlying cognitive and neural mechanisms of binding must be exposed and described (Revonsuo 2006). The difficulty of solving the binding problem in this manner arises from the disunity of the input mechanisms and cortical sensory representations of stimulus information. The different features of a stimulus object are separately represented in a number of specialized cortical areas and maps, but there seems to be no brain area or mechanism that would put all the information back together again, resembling the manner in which the information is experienced as phenomenally unified. There are, however, a number of theoretical ideas about the potential cognitive and neural mechanisms suggested to account for phenomenal unity. Most of them refer to a large-scale integration of information that could take place within the densely interconnected thalamocortical system in the brain (e.g. Llinás 2001).
The feature integration theory (FIT; Treisman 1996) proposes the following cognitive mechanism to account for the binding of different perceptual features together. In addition to the input modules that process information representing the separate features of the stimulus, there is a master map of locations, representing the entire perceptual space, and a window or spotlight of attention, scanning the location map. The stimulus features that fall within the spotlight of attention and are connected to the same location become bound together. They form a single unified package of information called an object file. Outside the window of attention bindings may also happen, but only randomly and temporarily. Empirical evidence that can be interpreted to support FIT (but is consistent with other binding theories as well) comes from *Balint’s syndrome (Robertson 2003): patients with this neuropsychological syndrome have bilateral damage in the posterior parietal cortex and therefore the master map of locations is deficient. Consequently, patients with Balint’s syndrome report seeing random and rapidly changing illusory bindings of features, not knowing which feature in reality belongs together with which others. Coherent, stable visual objects cannot be held together in the absence of the required cognitive mechanisms.
According to Treisman (2003:103), ‘attention provides a window for consciousness through which we become aware of a small subset of real bindings among a throng of illusory phantom objects’. Thus, the cognitive binding mechanism proposed in FIT may account for the local unity of visual consciousness, as the mechanism is supposed to produce coherent object representation that emerge into consciousness.
The neural mechanism that typically has been proposed to underlie perceptual binding is temporal coding or the synchronicity of neural activity. The basic idea is that spatially separated neurons that all respond to the same stimulus object will start to fire in temporal synchrony so that the same rhythm of activity is shared by all the neurons representing that object, and the rhythm is also unique to the neurons representing that object. Thus, the spatially distributed neural assembly becomes a higher-level coherent functional entity defined by its unique synchronous activity pattern. There is a growing body of empirical evidence from animal and human experiments that neural synchronicity correlates with the perception of coherent visual objects and with the *gestalt principles of perceptual grouping (Singer and Gray 1995). Human electroencephalography (EEG) studies have shown that there is a transient increase in high-frequency or *gamma-band power around 300 ms after stimulus onset, and that this response is larger for coherent visual objects, even when the coherence is merely illusory, not present in the physical stimulus (Tallon-Baudry 2003).
In a now classic paper, Crick and Koch (1990) first put forward the idea that there may be a connection between neural binding through synchronization and the phenomenal unity of objects in visual consciousness. According to their hypothesis there is an attentional mechanism that temporarily binds the relevant neurons together by synchronizing their spikes in 40 Hz oscillations, and this results in a coherent object representation in consciousness. Engel and Singer (2001) developed the synchronicity hypothesis further. They propose that synchronization may be the neural mechanism of several different aspects of consciousness, such as arousal, perceptual organization, the short-term stability of the contents of focal attention and working memory, and even the global unity of the self and the world (Engel and Singer 2001).
Whereas the above neural synchronicity theories mostly deal with potential cortical mechanisms of neural synchrony, Rodolfo Llinás (2001) suggests that the key mechanisms of binding consist of the bidirectional loops of thalamocortical connectivity. His theory describes two different types of thalamocortical loops: the specific loop is assumed to be responsible for the binding of distributed sensory fragments into single coherent objects, and the non-specific loop is assumed to provide the overall context or the conscious state where the individual objects are related to each other. The interaction between the two loops through synchronous oscillatory activity around 40 Hz is proposed to bind all the simultaneous contents into one coherent experience. Thus, this theory attempts to give an account of both local and global phenomenal binding. It furthermore develops into a more general neural theory of consciousness. Llinás (2001:126) suggests that our subjectivity is generated by the dialogue between the thalamus and the cortex, a temporally coherent sphere of activity: ‘It binds, therefore I am’.
Overall, the problems related to binding and the unity of consciousness have not yet been solved. During the brief history of modern consciousness research, at least some progress has been made in understanding what the problem is all about. We now see that the term ‘binding’ refers to several different processes at different levels of description (phenomenal, cognitive, neural). Therefore, the binding problem is a whole group of related problems. We also understand that the unity of consciousness is an enormously complex achievement, and that there are many different kinds of unity—as well as many different kinds of disunity—of consciousness. The theories proposed to solve the binding problem are still quite speculative, but at least empirically testable. To some extent the currently available data support the idea that high-frequency neural synchronization is correlated with some aspects of phenomenal unity. More detailed theories as well as new empirical data directly testing such theories are required before we can expect to understand how the phenomenal unity of consciousness is brought about by neurocognitive mechanisms in the brain.
ANTTI REVONSUO
Bayne, T. and Chalmers, D. J. (2003). ‘What is the unity of consciousness?’ In Cleeremans, A. (ed.) The Unity of Consciousness.
Crick, F. and Koch, C. (1990). ‘Towards a neurobiological theory of consciousness’. Seminars in the Neurosciences, 2.
Dainton, B. (2000). Stream of Consciousness.
Engel, A. K. and Singer, W. (2001). Temporal binding and the neural correlates of sensory awareness. Trends in Cognitive Sciences, 5, 16–25.
Llinás, R. (2001). I of the Vortex.
Metzinger, T. (2003). Being No One.
Revonsuo, A. (1999). ‘Binding and the phenomenal unity of consciousness’. Consciousness and Cognition, 8.
—— (2006). Inner Presence.
—— and Tarkko, K. (2002). ‘Binding in dreams’. Journal of Consciousness Studies, 9.
Robertson, L. C. (2003). ‘Binding, spatial attention and perceptual awareness’. Nature Reviews Neuroscience, 4.
Singer, W. and Gray, C. M. (1995). ‘Visual feature integration and the temporal correlation hypothesis’. Annual Review of Neuroscience, 18.
Tallon-Baudry, C. (2003). ‘Oscillatory synchrony as a signature for the unity of visual experience in humans’. In Cleeremans, A. (ed.) The Unity of Consciousness.
Treisman, A. (1996). ‘The binding problem’. Current Opinion in Neurobiology, 6.
—— (2003). ‘Consciousness and perceptual binding’. In Cleeremans, A. (ed.) The Unity of Consciousness.
Zeki, S. (2003). ‘The disunity of consciousness’. Trends in Cognitive Sciences, 7.
binocular rivalry Binocular rivalry refers to a perceptual phenomenon that occurs when very different visual patterns are presented to each eye simultaneously. In normal vision, the two eyes receive corresponding views of the world from slightly different perspectives, yet the visual system successfully interprets and synthesizes them into a coherent, stable perceptual experience. However, under certain (often artificial) circumstances, retinal projection patterns can be beyond the integrative capacity of the brain. This can be demonstrated by showing differently oriented or coloured patterns, directions of motion, or even different photographs to each eye in isolation. In these cases the brain proves incapable of arriving at a stable and satisfying interpretation of the retinal input. Perhaps surprisingly, the result is not a transparent superposition of the dissimilar patterns, but rather an unstable and wavering perception, with one eye’s view dominating for a few seconds before being replaced by its rival from the other eye. Accordingly, an observer will typically experience a sequence of stochastic alternations that proceeds as long as the sensory conflict is present.
Binocular rivalry has fascinated humans throughout the centuries. The first known written account of the phenomenon was from Neapolitan polymath Giambattista della Porta, who in 1593 bemoaned the fact that he was unable to read more than one book at a time by using each eye independently. Subsequent centuries witnessed further anecdotal references to the rivalry phenomenon. When Charles Wheatstone introduced the mirror stereoscope in the 1830s, the systematic testing of binocular rivalry became possible, and from this point on binocular rivalry became the subject of intense scientific research. Up to this day, several dozens of studies on binocular rivalry are published each year, either exploring the phenomenon itself or using it as a research tool to study conscious perception.
While the experience during binocular rivalry is often characterized as a simple switching between the right eye’s and left eye’s views, which might produce a percept similar to opening and closing each eye in succession, this is a considerable oversimplification of what subjects actually observe. Particularly with larger and more complex visual stimuli, rivalry perception can proceed as a fluid sequence of ever-changing mosaics (so-called piecemeal percepts), consisting of an interleaved patchwork of portions of the two eyes’ views. Several studies have revealed that the structure of this patchwork at each point in time is determined by a combination of local inter-ocular competition, *gestalt grouping principles, and waves of ocular dominance that are likely to reflect coherent spatiotemporal activity patterns in the visual cortex. Also, when competing rivalry patterns are both very low in their contrast, perception can be marked by a superposition of the two eyes’ views, something that is never observed when one or both of the patterns are of higher contrast. Likewise, very short presentations of conflicting stimuli are frequently perceived as being fused.
When rivalry suppression does occur, the suppressed area is rendered completely invisible. At the same time, suppression is surprisingly superficial when measured psychophysically, since the threshold for detecting test probes presented to the non-dominant eye is only minimally elevated by suppression. Moreover, the temporal dynamics of rivalry alternations are determined by the characteristics of the suppressed, rather than dominant, stimulus. At each point in time the expected dominance duration is predicted by the strength (i.e. contrast, speed, brightness) of the suppressed pattern. These findings suggest that unperceived stimuli in the suppressed eye are still processed to a large extent by the visual system and not simply blocked at an early processing stage. It is for this reason that binocular rivalry became a primary research tool for investigators interested in the differential effects of consciously perceived and suppressed visual stimuli.
Initial studies on the brain mechanisms underlying binocular rivalry were focused on the activity of *single neurons in monkey visual cortex. It was found that isolated neurons throughout visual cortex change their firing rate whenever the percept changes during rivalry. It turned out the proportion of neurons that do so differs drastically between visual cortical areas. While only few cells modulate their activity with perceptual alternations in primary visual cortex (*V1), there is an increasing frequency of neurons with percept-related activity as one goes up the classical visual hierarchy. Recordings in both monkeys and humans showed that at the latest stages of visual processing, the majority of neurons alter their activity during perceptual switches. This activation pattern is not explainable by a fixed network of neurons with a closer link to perception than other cells, since different stimuli elicit perceptual modulation among different neuronal populations.
Human *functional brain imaging (fMRI) studies agree that there are widespread activity changes throughout the brain at the moment of a spontaneous perceptual reversal, particularly in the *frontal and parietal lobes. Activity in higher cortical areas (such as that responsible for face processing) is modulated so consistently during rivalry that it can be used to read out the actual perception of an observer. It has also been shown that the unperceived, perceptually suppressed stimuli are not entirely erased form processing at these stages since their impact is still measurable. Disagreement exists, however, between imaging and single-unit data on the participation of the earliest stages of cortical processing in the formation and maintenance of a perceptual state during rivalry. While single neurons in monkeys show minimal correspondence with the perceptual state, human neuroimaging studies demonstrated that the fMRI signal in primary visual cortex is closely coupled with the visibility of a pattern during rivalry. Recent findings hint at a resolution of this conflict by suggesting that the fMRI signal is more related to the synaptic membrane currents than local spiking activity, and perceptual suppression has a profound impact on the first but not the latter. How much primary visual cortex contributes to binocular rivalry remains an important question to explore.
Putative evidence for the involvement of other cortical areas in binocular rivalry is its close relationship to the bistable perception induced by other ambiguous geometrical patterns that give rise to more than one valid perceptual solution. The temporal dynamics of rivalry alternations are nearly identical with that of the famous Necker cube, whose structure similarly appears to change spontaneously every few seconds (see MULTISTABLE PERCEPTION). Most attempts to simulate binocular rivalry in neuronal networks can be generalized to other bistable stimuli. These models generally assume mutual inhibition between neuronal populations as the ultimate cause of perceptual instability (further assuming that a population being more active is somehow causing one of the alternative percepts). This simple ‘flip-flop’ circuitry is the easiest way to generate oscillations in a model. The randomness of the perceptual state changes then are explained by ‘noise’ that is added to the system. Other models assume more complex mechanisms related to dynamical systems theory such as local minima in an abstract energy space or chaotic fluctuations in a self-organized system.
Another indication for a common mechanism of perceptual alternation is that the temporal dynamics of binocular rivalry and other bistable visual phenomena are all connected to several cognitive variables. In particular, the switching frequency can vary by an order of magnitude between observers but is consistent within an observer over multiple testing sessions, declining slowly with age. A large number of studies have attempted to link IQ and personality type to alternation rate with bistable figures, with limited success. While voluntary control over alternation is limited, observers can improve their ability to control reversal with practice, but never learn to inhibit it altogether. Neurostimulants, mood disorders, *meditative states, and *brain damage, particularly to the right frontal cortex, can all impact the rate of reversal. While the connection between these factors and rivalry is by no means clear, these findings support the assumption that perceptual switching is induced or at least influenced by modulating factors outside the sensory domain.
Binocular rivalry also appears to be closely related to spontaneous fluctuations observable in some dim visual patterns, where two components of the pattern can alternate in their visibility—a phenomenon misleadingly termed monocular rivalry. Despite these similarities, binocular rivalry appears to be unique in its generality among different stimuli, as well as in the completeness of its perceptual suppression. It is possible that these unique qualities are related to its relevance in natural vision. Binocular vision in a cluttered three-dimensional environment involves zones of inter-ocular discrepancy, including a vast zone outside of Panum’s fusional area where there is no interocular correspondence at all. Under these conditions, the brain is often forced to choose one eye’s view while completely suppressing the other’s. This commonplace suppression may be directly related to that observed in binocular rivalry.
Binocular rivalry has always attracted students of diverse disciplines. It has been used as a tool to study the human unconscious, to assess cognitive abnormalities, and to learn more about binocular vision and perception in general. Thus, it has served as an unlikely common ground for philosophers, biologists, psychologists, and physicists, who all seem captivated by the implications for subjective experience. While a great deal is known about rivalry, it is, perhaps surprisingly, the big picture questions that are the still the most contentious. With technical advances in neuroscience, passive-correlational approaches are slowly being complemented by causal manipulations. Such interventions may ultimately provide a clearer picture of the neuronal mechanisms underlying perceptual alternation and visual suppression during rivalry. Understanding the neuronal mechanisms causing binocular rivalry may have direct implications for our understanding of how a percept gets established and supported in the brain. It thus has been and continues to be a vital and fascinating paradigm for the scientific study of visual awareness.
ALEXANDER MAIER AND DAVID A. LEOPOLD
Blake, R. and Logothetis, N. K. (2002). ‘Visual competition’. Nature Reviews Neuroscience, 3.
Crick, F. and Koch, C. (1998). ‘Consciousness and neuroscience’. Cerebral Cortex, 8.
Leopold, D. A. and Logothetis, N. K. (1996). ‘Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry’. Nature, 379.
Logothetis, N. K. and Schall, J. D. (1989). ‘Neuronal correlates of subjective visual perception’. Science, 245.
Lumer, E. D., Friston, K., and Rees, G. (1998). ‘Neural correlates of perceptual rivalry in the human brain’. Science, 280.
Sheinberg, D. L. and Logothetis, N. K. (1997). ‘The role of temporal cortical areas in perceptual organization’. Proceedings of the National Academy of Sciences of the USA, 94.
Tong, F., Nakayama, K., Vaughan, J. T., and Kanwisher, N. (1998). ‘Binocular rivalry and visual awareness in human extrastriate cortex’. Neuron, 21.
Wilson, H. R., Blake, R., and Lee, S.-H. (2001). ‘Dynamics of travelling waves in visual perception’. Nature, 412.
biological naturalism Biological naturalism is an account of the relation of consciousness, as well as other mental phenomena, to the brain and the rest of the body. It rejects both *dualism and materialism (see PHYSICALISM), while attempting to preserve what is true in each. The theory begins with a common-sense definition of ‘consciousness’, one that is intended to identify the target rather than try to provide a scientific or philosophical analysis of the sort that can only come at the end of the investigation. ‘Consciousness’ is defined as those qualitative states of sentience or feeling or awareness that typically begin in the morning when we wake from a dreamless *sleep, and continue throughout the day until we fall asleep again or otherwise become unconscious. *Dreams, on this definition, are a form of consciousness. Consciousness, so defined, does not necessarily imply a higher order level of *self-consciousness. Humans and many species of animals are conscious, but we do not yet know how far on the phylogenetic scale consciousness goes.
The first feature to notice about consciousness is that there is a qualitative feel or character to every conscious state, a ‘what it feels like’, or ‘*what it is like’ aspect of consciousness. You can see this by reflecting on the difference between, for example, drinking wine and listening to a string quartet. These may occur simultaneously, but they have a different qualitative character. A second feature of consciousness is that it is subjective in the ontological sense that it can only exist when experienced by a human or animal subject. Its subjective, first-person ontology makes it differ from other features of the world such as mountains, molecules, and tectonic plates, that have an objective, or third-person, ontology. Third, any conscious state, such as feeling of pain or thinking about one’s income tax, can only exist as part of a unified, conscious field. You do not just think about your income tax, feel a pain, and taste the wine you are drinking, but you have all of these experiences as parts of one large experience that constitutes your entire conscious field at this very moment. These three features of consciousness—qualitativeness, *subjectivity, and *unity—appear to be distinct from each other, but if we reflect on them we can see that in fact each implies the next. There is no way that a state could be qualitative in the sense described without it being subjective, and there is no way that it could be subjective without occurring as part of a unified conscious field.
About consciousness so defined and so characterized, biological naturalism makes the following four claims:
1. Consciousness is real. It is a real part of the biological world and cannot be reduced to something else, nor eliminated by any kind of reduction. It cannot be reduced to any third-person phenomena because it has a first-person or subjective ontology, and this ontological feature would be lost if we reduced consciousness to neuron firings or any other such third-person phenomena.
2. Consciousness is entirely caused by neuronal processes. We do not know the exact mechanisms that cause consciousness, but we are sure, beyond a reasonable doubt, that these are neurobiological mechanisms in the brain, and perhaps parts of the rest of the central nervous system. Consciousness so defined is thus causally reducible but not ontologically reducible to brain processes. It is causally reducible because there is no feature of consciousness which is not causally explained by neuronal behaviour, but it is not ontologically reducible because the first-person ontology of consciousness prevents it from being reduced to a third-person ontology of the rest of neurobiology.
3. Consciousness is entirely realized in the brain as a higher-level or system feature. Though a state of consciousness is caused by the behaviour of neurons, individual neurons and synapses by themselves are not conscious. Just as individual H2O molecules are not liquid or solid, and yet the behaviour of the molecules accounts for liquidity and solidity as features of systems of molecules, so individual neurons are not conscious, yet the behaviour of the neurons accounts for the existence of consciousness as a feature of a system of neurons. You cannot point to a single neuron and say: ‘This one is conscious’, any more than you can point to a single water molecule and say: ‘This one is wet’.
4. Consciousness functions causally in producing behaviour. You consciously decide to raise your arm, for example, and then your intention-in-action causes your arm to go up. Consciousness, like other real features of the real world, stands in cause and effect relationships to other phenomena.
Biological naturalism can best be understood by comparing it with dualism and materialism, the two traditionally most widely accepted theories of mind–body relationships. According to the biological naturalist it is possible to preserve what is true in both of these theories, while discarding with what is false.
Materialists say truly that the universe consists entirely of physical particles (or whatever entities the ultimately true physics comes up with) in fields of force, and these are typically organized into systems, and on our Earth some carbon-based systems have evolved into the present human animal and plant species. But, according to the biological naturalist, materialists claim falsely that there are no irreducible mental phenomena in the world. Consciousness really does exist, so it cannot be eliminated, but it has a first-person ontology and therefore cannot be reduced to something that has a third-person ontology.
Dualists say truly that consciousness really does exist as part of the universe and cannot be eliminated or reduced to anything else. Dualists say falsely that recognition of the irreducible character of consciousness commits us to the existence of two distinct realms of being, the realm of the physical and the realm of the mental. According to the biological naturalist, once we discard the Cartesian heritage of talking about the mental and the physical as distinct metaphysical realms, we can see that the so-called mental is really just a higher level of biological, and therefore physical, feature of neurobiological systems. But in order to say that, we have to abandon the traditional usage of the Cartesian heritage according to which anything irreducibly mental must be in a different metaphysical realm from anything physical. According to the biological naturalist, if we just state the facts as they are stated in propositions 1–4, we can see how it is possible to perceive the true claim of dualism with the true claim of materialism, while abandoning the false claims in each.
In light of this comparison with the traditional views, let us now consider the four defining propositions of biological naturalism:
1. The reality and irreducibility of consciousness. In our philosophical tradition there is supposed to be a distinction between *eliminative reductions that show that the reduced phenomena never existed, at all, and noneliminative reductions that show that the phenomenon does exist, but it is nothing but something else, the reducing phenomenon. An example of an eliminative reduction is the reduction of rainbows which shows that they do not really exist as arches in the sky. A non-eliminative reduction is water to H2O molecules which shows that water consists of H2O molecules, but does not show that it does not exist. The problem with attempting to do a non-eliminative reduction of consciousness is that it turns out to be eliminative because any attempt to reduce something that has an essential first-person ontology to something with a third-person ontology will eliminate the essential trait. So to grant the existence of consciousness is already to grant that it cannot be reduced or eliminated by the standard reductive methods.
2. The neurobiological causation of consciousness. Many writers in the dualist tradition think it is impossible that we should ever be able to explain how the brain causes consciousness, or, at least, that we cannot possibly explain it with our present conceptual apparatus. To the biological naturalist this is unwarranted pessimism. We now know, for a fact, that brain processes do cause consciousness, and though we are still struggling to figure out exactly how they do it, there has been a remarkable amount of progress in neurobiology in the past several decades and it is not at all unlikely that in this century we will have an explanation of how brain processes cause consciousness. But whether we get a scientific explanation or not is in a sense beside the point. We know that in fact it happens. There is no doubt that all of our conscious states are entirely caused by neuronal processes, and in that sense consciousness is causally reducible to brain processes.
3. Realization in the brain of consciousness. We know that consciousness exists, we know that it is a real phenomenon. Where is it? Where is it in real space-time? All of our conscious states exist between our ears. To understand this point we have to see that consciousness does not exist on the level of neurons and synapses, much less at the level of atoms and electrons, but at a much higher systemic level. There are lots of features of nature that are like that. Consider liquidity and solidity, for example. Individual water molecules are neither liquid nor solid, it is only the system of which the individual molecule is a component that can be liquid or solid.
4. The causal functioning of consciousness. A normal person intends to raise her arm and then she does so intentionally. Her conscious intention-in-action causes the arm to raise. Indeed, in that particular context, we can suppose that the intention-in-action was causally sufficient for the arm to go up. But we know independently that in order for her arm to go up, there has to be a series of purely physical causal factors such as the secretion of acetylcholine at the axon end-plates of her motor neurons. It is sometimes presented as an objection to biological naturalism that it appears to be causal overdetermination. On the one hand, the arm going up is caused by her intention; on the other, it is caused by neuronal electrochemical processes. The objection is that this leaves us with too many causes. This would be a case of causal overdetermination. To the biological naturalist, however, this seems to strengthen his position, because it shows us, indeed offers us implicitly a proof that the conscious intention-in-action is part of a complex neuronal event that also has chemical properties. In fact, we can prove this point by simple deduction: (1) The conscious intention-in-action causes the arm to go up. (2) Anything that caused the arm to go up must have the necessary chemical and electrical properties (enough to contract the arm muscles). (3) Therefore the conscious intention-in-action must have these properties.
The existence of a complex event that can be described causally at higher and lower levels is not at all unusual in the real world. Consider, for example, the operation of a car engine. We can describe the operation of the car engine either at the level of the passage of electrons between the electrodes of the spark plug, or we can describe the same operation in terms of the explosion of the fuel mixture in the cylinder; one event, different levels of description. In the same way, we can describe the one event of my intentionally raising my arm in terms of the conscious intention-in-action or in terms of the lower-level biochemical features.
Biological naturalism is a form of naturalism because it insists on the fact that consciousness and other mental properties are parts of the natural world. It is biological in the sense that it insists that the peculiarity of consciousness is that it is caused by certain sorts of biological systems, and it exists in those biological systems. It is a natural property of humans and certain animal species, and thus is a natural, biological property.
JOHN R. SEARLE
blindness, recovery from In 1688 William Molyneax sent a letter to John Locke asking whether a man, born blind, who had learnt to distinguish a globe and cube by touch, would be able to distinguish them by vision alone if sight were ever restored. This question, known as Molyneax’s problem, played an important role in the seventeenth and eighteenth century development of the empiricist argument that perceptual concepts are not present from birth but require the presence of sensory experience. Empiricists such as Locke were well aware that our sensations have a complex and occasionally indirect relationship to the physical world. However, Locke believed that the sensations of shape and distance involved in seeing a cube were primary sensations that originated more directly from the object than secondary sensations such as those of colour. Locke therefore believed that a sight-recovery patient would have the same visual sensations as a person with normal visual experience (Locke 1690), but would not know that these sensations were of the same ‘cube’ objects for which he had previous tactile experience.
Fifty years later, Cheselden’s (1728) publication of a sight-recovery case study revealed that his patient had acute difficulty interpreting his two-dimensional retinal image as a three-dimensional world. Condillac (1746) and Diderot (1749) questioned whether the blind man would even have the same visual sensations as someone with a normal visual history. Both philosophers emphasized the role of active experience in making sense of vision. For example, Diderot, in his Letter on the Blind, suggested that some visual sensations (such as those of shape) might need to be disambiguated by tactile experience to be understandable. Later, Reid (1764) made the distinction between sensations (e.g. ‘feeling a smooth surface’) which belong to a particular sense, and perceptions, an awareness of external objects that could be mediated by more than one sense: one can ‘perceive a cube’ through either touch or vision. According to Reid, perceptions were innate ideas about the external world that were awakened by sensations. This is of course very similar to the nativist tradition originally rejected by Locke. Thus, as far as the epistemological issue of how our sensations, perceptions, and cognitive constructs might be related to the outside world, within a hundred years Molyneux’s question had led philosophers full circle.
However, the study of Molyneux’s question did lead philosophers to make increasingly fine distinctions between different types of internal psychological events, such as sensations, perceptions, and cognitive constructs. These distinctions were often based on prescient behavioural observations and played a significant role in guiding later experimental research. Condillac, in discussing the development of understanding in a statue progressively provided with smell, hearing, touch, and sight, recognized that intentional action is crucial in relating sensations to external objects. Diderot, considering how a three-dimensional world is inferred from the two-dimensional retinal image, suggested that the development of certain sensations, such as those of shape and size, may be acquired in a developmental process that depends on tactile experience. Both of these observations have since been supported by infant development studies suggesting that the understanding of three-dimensional shape develops in parallel with grasping behaviour between four and six months of age (Piaget 1952). For a comprehensive description of the philosophical issues raised by Molyneux’s question, see Morgan (1977).
This philosophical debate has not been seriously troubled by an overabundance of empirical data. Although the first report of recovery from blindness was in AD 1020 and the first clinical study of sight recovery after long-term blindness was carried out in 1728 (Cheselden 1728), only sporadic cases have been studied over the last three centuries: e.g. S. B. (Gregory and Wallace 1963), H. S. (Valvo 1971), H. B. (Ackroyd et al. 1974), Virgil (Sacks 1995), and M. M. (Fine et al. 2003). None of these cases should be considered as examples of ‘pure’ sight recovery as defined by no light perception from birth to adulthood, and in many cases the patients were only studied some months after sight recovery had occurred. Nonetheless, some consensus has gradually emerged about the restored visual abilities of those who have lost their sight early in childhood.
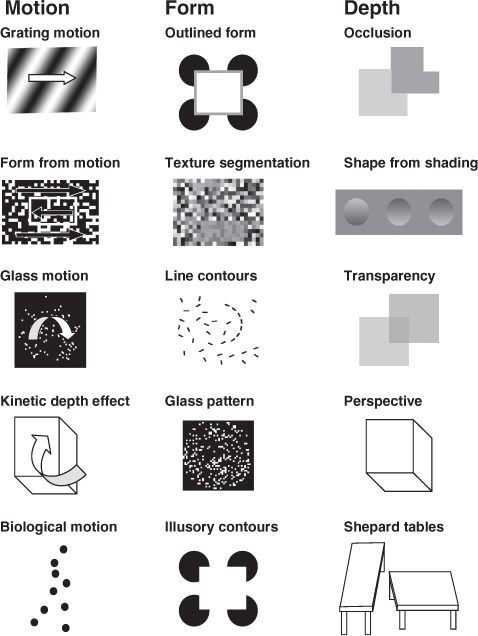
Most sight-recovery patients can name colours easily once they have learned the correct colour names, and can distinguish fine differences in hue (Ackroyd et al.’s patient H. B. had difficulty with colour naming, but her English, though fluent, was a second language). Colour processing for M. M. was essentially normal. Similarly, motion processing also appears to be relatively spared. It was said of S. B. ‘His only signs of appreciation were to moving objects, particularly the pigeons in Trafalgar Square.’ Similarly, for H. B., ‘She could see the pigeons as they alighted in Trafalgar Square but she said that they appeared to vanish as they came to rest.’ M. M. had no difficulty on motion tasks that included identifying the direction of motion of simple and complex plaids (see Fig. B1), the barber’s pole *illusion, segregating textured fields based on motion, and using motion cues to compute the three-dimensional shape of a rotating cube. M. M. was also sensitive to biological motion, recognizing a point-light Johansson figure (a dynamic representation of a walking person represented by dots of light at the walker’s joints, see Fig. B1), and was even able to make sense of the fine motion cues that differentiate male and female gaits.
Sight-recovery patients also have few difficulties with recognizing two-dimensional shapes. M. M. could segment texture patterns based on luminance contrast, could identify whether a field of line contours contained a sequence of nearly collinear line segments, and could discriminate Glass patterns from random noise (see Fig. B1). The only two-dimensional task M. M. had difficulty with might be considered a three-dimensional one: though he recognized outlined two-dimensional shapes, he could not identify the same shapes in Kanisza illusory contours.
In contrast, sight-recovery patients seem to have little understanding of three-dimensional shape. It was reported of Sacks’ patient Virgil, ‘Sometimes surfaces of objects would seem to loom … when they were still quite a distance away; sometimes he would get confused by his own shadow … [Steps] posed a particular hazard. All he could see was a confusion, a flat surface of parallel and crisscrossing lines’. H. S. described his initial experiences after sight recovery: ‘I had no appreciation of depth or distance; street lights were luminous stains stuck to window panes, and the corridors of the hospital were black holes.’ (H. S. was not deprived of sight until the age of 15, and recovered his ability to interpret the world in three dimensions over a matter of months postoperatively.) M. M. could exploit occlusion cues but not shading, transparency, or perspective. He could not identify wire drawings of stationary Necker cubes or pyramids, describing the cube as ‘a square with lines’. M. M. was also immune to illusions based on perspective cues such as the Shepard tables.
Presumably as a consequence of these difficulties in constructing a three-dimensional percept, sight-recovery patients have trouble recognizing even ‘familiar’ objects and faces. Cheselden (1728) describes this confusion in his patient: ‘Having often forgot which was the cat and which the dog, he was ashamed to ask, but catching the cat which he knew from feeling, he was observed to look at her steadfastly and then … have said, So puss, I shall know you another time.’
Why is it that colour, two-dimensional shape, and motion perception are relatively unaffected by deprivation, while three-dimensional processing is dramatically impaired? One hypothesis is that some abilities might be more innate, as evidenced by developing early in infancy, and might therefore be more robust to deprivation (Lewis and Maurer 2005). However, two-dimensional tasks such as contour integration develop relatively late in infancy, yet remain relatively undisturbed by deprivation. Another suggestion is that non-spatial temporal information (flicker), which has been preoperatively available to all the sight-recovery patients studied to date, results in a sparing of motion pathways. But chromatic and two-dimensional form processing also seem to be spared from the effects of visual deprivation. Spared chromatic and two-dimensional form processing is also left unexplained by the hypothesis that ventral ‘what/recognition’ pathways are more affected by deprivation than dorsal ‘where/action’ pathways, since chromatic and two-dimensional information is thought to be predominantly processed within ‘what’ pathways (see VISUAL STREAMS: WHAT VS HOW). It has been suggested that sight-recovery patients might only have the ability to understand those sensations for which there is a tactile equivalent: yet they have no difficulty discriminating colours. One intriguing possibility is that almost the opposite is true. As suggested by Diderot, and confirmed by later infant development studies, the development of some visual perceptions such as those of shape may depend on tactile experience. Perhaps sight-recovery patients have no difficulties with purely visual sensations (colour, two-dimensional shape, and motion) but, without the developmental experience of disambiguating visual experience with the help of touch, find it impossible to construct a three-dimensional world from a two-dimensional retinal image.
IONE FINE, CORDELIA FINE, AND KIT FINE
Ackroyd, C., Humphrey, N. K., and Warrington, E. K. (1974). ‘Lasting effects of early blindness. A case study’. Quarterly Journal of Experimental Psychology, 26.
Cheselden, W. (1728). ‘An account of some observations made by a young gentleman, who was born blind, or who lost his sight so early, that he had no remembrance of ever having seen, and was couch’d between 13 and 14 years of age’. Philosophical Transactions of the Royal Society of London, 35.
de Condillac, E. B. (1746). Essai sur l’origine des connaissances humaines.
Diderot, D. (1749). Lettre sur les aveugles.
Fine, I., Wade, A., Boynton, G. M. B., Brewer, A., May, M., Wandell, B., and MacLeod, D. I. A. (2003). ‘The neural and functional effects of long-term visual deprivation on human cortex’. Nature Neuroscience, 6.
Gregory, R. L. and Wallace, J. G. (1963). Recovery from Early Blindness: a Case Study. Experimental Psychology Society Monograph No. 2
Lewis, T. L. and Maurer, D. (2005). ‘Multiple sensitive periods in human visual development: evidence from visually deprived children.’ Developmental Psychobiology, 46.
Locke, J. (1690). An Essay Concerning Human Understanding.
Morgan, M. J. (1977). Molyneux’s Question: Vision, Touch and the Philosophy of Perception.
Piaget, J. (1952). The Origins of Intelligence in Children.
Reid, T. (1764). An Inquiry into the Human Mind on the Principles of Common Sense.
Sacks, O. (1995). ‘To see and not to see’. In An Anthropologist on Mars.
Valvo, A. (1971). Sight Restoration After Long-Term Blindness: the Problems and Behavior Patterns of Visual Rehabilitation.
blindsight Blindsight is an oxymoron—surely one cannot be blind and sighted at the same time? But if not all sight is conscious, and ‘blind’ refers to a lack of conscious sight, visual functions would be possible although one would not experience oneself as seeing. This occurs in patients with fields of cortical blindness caused by lesions of the primary visual cortex (*V1, striate cortex). Their ability to detect, localize, and discriminate between visual stimuli they avow not to see demonstrates that sight can indeed be blind. The term ‘blindsight’ captures this dissociation (Weiskrantz et al. 1974) which ‘knocked the stuffing out of the “obvious” assumption that awareness of a signal is necessary for an intentional response to that signal’ (Churchland 1984:45–46). The phenomenon has intrigued neuroscientists, psychologists, and philosophers who try to understand its implications for conscious and unconscious vision, their function(s) and neuronal bases.
Lesions that destroy or denervate the primary visual cortex cause cortical blindness in the region of the visual field that was represented in that area. Complete cortical blindness results from bihemispheric destruction of V1 and is fortunately rare; most patients suffer unilateral damage that affects the same region of the contralateral visual hemifield in both eyes. V1 lesions induce widespread degeneration in the neuronal structures that lose their connections to the damaged region. Nevertheless, visual input from the blind field still reaches the retinorecipient nuclei, and these transmit it, directly or via other nuclei, to visual cortical areas beyond V1 (Fig. B2). Physiological recordings in monkeys whose V1 was ablated or cooled revealed that neurons in the occipitoparietal (dorsal) visual processing stream respond more vigorously to stimuli presented to the affected visual field (see Bullier et al. 1994 for review) than those in the occipitotemporal (ventral) stream (see VISUAL STREAMS: WHAT VS HOW). The lesion’s anatomical and functional repercussions depend on the age at lesion; earlier lesions cause more degeneration, but at the same time induce more plastic alterations which are expressed in unusual anatomical pathways and close-to-normal neuronal responses in extrastriate visual cortical areas. The information on the neuroanatomical and neurophysiological consequences of V1 lesions in animals has been confirmed and extended by pathological and, more recently, structural and *functional neuroimaging studies of patients.
Fig. B2. A simplified schema of the visual system shows that only the extra-geniculostriate projections, from and to parts of the thalamus, escape the effects of the V1 lesion indicated by vertical grey bars. Both the affected hemiretina and its projection to the dorsal lateral geniculate nucleus also undergo partial degeneration (n. sc., suprachiasmatic nucleus; PGN, pregeniculate nucleus; pulv., pulvinar nuclei; sup. coll., superior colliculus; PT, pretectal nuclei; AOS, accessory optic system).
The neuronal pathways that survive destruction of V1 support a variety of visually guided behaviours. This was first clearly shown by Heinrich Klüver’s studies of monkeys with bilateral removal of occipital cortex; investigators who include the Pasiks, Weiskrantz, Humphrey (see HELEN, ‘A BLIND MONKEY WHO SAW EVERYTHING’), Cowey, Keating, Mohler, and Wurtz have followed in his footsteps. The visual functions they have demonstrated include detection, manual localization, and saccadic localization as well as visually guided navigation, but also discrimination of total contour, shapes, patterns, and chromatic differences (for reviews, see Pasik and Pasik 1982, Stoerig and Cowey 1997).
The monkeys’ visual capacities have always and necessarily been tested with non-verbal behavioural methods, and were interpreted in terms of residual conscious sight because the majority of human patients with striate cortical damage consistently declared that they did not see anything in their blind field. In humans, but not monkeys, conscious sight thus depended on the integrity of V1. The apparent contrast between sight in monkeys and blindness in humans was regarded as evidence for stronger corticalization: only in humans, it was posited, is cortex required for conscious vision. This hypothesis ruled as long as the patients were asked only whether they were aware of stimuli presented within their blind field. But it was undermined when behavioural methods previously only used on monkeys ‘forced’ the patients to respond to targets presented within their blind field. Although the patients claimed to be ‘just guessing’, they performed much better than expected by chance (Pöppel et al. 1973, Weiskrantz et al. 1974). The reports on unconscious vision in humans met with surprise bordering on disbelief. They also sparked methodological critiques attributing human ‘blindsight’ to eye movements, stray light falling on to the normal parts of the retina, and near-threshold vision (Campion et al. 1983). However, despite careful experimental control measurements that excluded artefacts, e.g. by showing that the same stimuli failed to elicit better-than-chance performance when they were presented so as to fall on to the receptor-free optic disc (the natural blind spot), visual functions continued to be demonstrated in the cortically blind field. In addition to saccadic and manual localization of blind-field targets, they include detection and discrimination of stimuli differing in flux, contour, orientation, motion, spatial frequency, shape, and wavelength (for review see Weiskrantz 1996, Stoerig and Cowey 1997). Humans and monkeys thus show largely similar visual functions in their cortically blind fields.
Together with the similar functional neuronanatomy of the human and simian visual systems, this raised the question of whether monkeys, like the human patients, have blindsight rather than the residual conscious sight originally attributed to them. To tackle this question, three monkeys with complete unilateral V1 ablation and a control monkey were first tested in a target localization task, where they manually localized small visual stimuli that could appear briefly in any one of the four corners of a touch-sensitive monitor at better than 90% correct in both hemifields. Then the paradigm was changed so as to allow the possibility of signalling ‘no target’. This was done by introducing blank (no light) trials which were presented unpredictably among target trials. Whenever a blank trial occurred, the correct response was to touch a constantly outlined ‘no target’ area on the screen. Having this option did not affect responses to good-field targets which remained close to perfect. It did, however, radically alter the responses to the targets presented in the hemianopic field: despite their excellent localization performance, the monkeys now treated these stimuli as ‘no target’ (Cowey and Stoerig 1995). The results thus suggested that the monkeys had blindsight rather than (possibly poor) conscious sight, a hypothesis strengthened when the same combination of forced-choice localization and signal detection tasks subsequently differentiated between blind and poor sight in human patients. In line with their reporting awareness of a percentage of targets presented to the relatively blind region, the ‘poor-sight’ patients not only performed well in the localization but in the detection task as well (see Fig. B3). Conversely, patients tested in regions of absolute blindness performed well in the localization task, but indicated ‘no target’ whenever a blind-field target was presented in the signal detection test (Stoerig et al. 2002). Like the monkeys, the patients with blindsight thus showed a behavioural difference in their responses to blind-field targets that depended on whether or not the response options included a ‘notarget’ one. Offering this option thus appeared to capture the dissociation between localization performance and stimulus unawareness.
Fig. B3. Percentage correct localization (black), percentage aware responses (striped), and percentage correct detection of targets presented within the affected hemifield (grey). H. K. was tested in his absolutely blind hemifield, and like monkey Dracula performed well in the localization, but indicated ‘no target’ on almost all trials in the detection task. In contrast, monkey Rosie more resembled G. Y. who was tested in his relatively blind field, and eventually detected a sizeable proportion of the blind-field targets she localized so accurately.
Interestingly, Rosie, a hemianopic monkey who also participated in the latter experiment, correctly localized 6% of the blind-field targets even when she had the option to signal ‘no target’. Her behaviour thus somewhat resembled that of the human patients who were tested in regions of relative blindness; more strikingly, with continued testing she eventually raised the proportion of blind-field trials to which she responded by signalling a target instead of a blank stimulus to about 50%. Does this indicate that she learned to discriminate unseen stimulus and blank trials in her blind field? Or that she recovered some conscious sight in her hemianopic field?
Both options are possible. That blindsight requires and improves with practice has been documented in simian (Humphrey 1974) and human subjects (e.g. Stoerig 2006), and is important in several ways. First, it shows that the prevalence of blindsight, higher in monkeys and varying widely in humans, depends not only on factors including extent and type of lesion, age at lesion, and the precise task to be performed, but also on whether subjects have had sufficient practice; monkeys, by virtue of the training required to inform them what to do, generally have much more practice. Second, by furthering the capacities of the blind field, training of blindsight may improve subjects’ functional outcome, opening a route to rehabilitation. Third, the evidence for learning through practice shows that blindsight is not simply the sum of functions that remain when conscious vision is lost, but develops slowly and for a long time as long as it continues to be challenged (Humphrey 1974). The alternative option, recovery of conscious sight, was already suggested when repeated visual field perimetry in monkeys who participated in studies of blindsight revealed a shrinkage or filling-in of the blind field (Cowey 1967). More recently, Moore et al. (1996) interpreted the excellent localization performance of their infant-lesion hemianopic monkeys as indicative of conscious sight, because, unlike that of their adultlesion brethren, it persisted even when no central and visible cue announced the presentation of a target. That the younger system’s greater plasticity enables better recovery from occipital lesions was also found in a longitudinal study of veterans with fields of cortical blindness. Those who had suffered their lesions in their teens were much more likely to recover sight spontaneously than those injured later in life. Nevertheless, spontaneous recovery from cortical blindness can also occur in subjects who suffered their lesions later in life. As this opportunity is restricted to a period of up to six months after injury, during which recovery is attributed to the particular lesion’s at least partially transient effects, it cannot account for the changes in the size and/or density of the field defect that occur long after this period.
Nevertheless, such changes continue to be documented in cases where the blind field’s capacities are persistently challenged. Practice thus not only improves blindsight performance, it can also provoke pronounced reductions in the size and/or the density of the blind field of monkeys (Cowey 1967) and patients (Zihl and von Cramon 1985, Sahraie et al. 2006, Stoerig 2006). Although an early age at lesion is likely to promote them, such changes have also been found in older lesion subjects. They may thus have contributed to the marked reduction in ‘no target’ responses made by our monkey Rosie, who was 4 years at lesion, to stimuli presented to the hemianopic field; they may also have contributed to the improved performance a different adult lesion monkey eventually showed in the uncued localization task of Moore and colleagues (1996). They make it easy to confound poor sight and blindsight, a task not made simpler by the improvements in performance that can occur with and without any stimulus awareness.
Blindsight recruits the parts of the visual system that escape the effects of the V1 lesion. These may include V1 neurons that survive next to or even within the damaged region, but blindsight does not depend on these. Histology carried out on monkeys has only occasionally shown that lesions were less complete than intended; more often they were total or extended beyond V1. As far as can be judged from functional neuroimaging studies that repeatedly failed to reveal any activation within the lesion, the same is true for human patients. Instead of demonstrating a necessity for V1 neurons surviving in the damaged region, such studies have consistently revealed that visual information from the blind field can activate intact extrastriate visual cortical areas, implying that blindsight is not blind by virtue of relying exclusively on subcortical processes. Moreover, by also revealing that areas in the occipitotemporal processing stream may respond to colours and objects presented to the blind field, they have shown that in practised patients blindsight invokes occipitoparietal areas which are known to be important for visuomotor behaviour. It is noteworthy that stimulation of fields of recovered, albeit sometimes poor, sight yielded similar activation patterns regardless of whether the recovery occurred spontaneously or in response to practice. If a failure to reveal evidence for activation with functional imaging indicates an absence of possibly weak responses from within the lesion, extrastriate cortical activation is sufficient to mediate not only blindsight but also the conscious sight that can remain or recover in fields of cortical blindness (Kleiser et al. 2001, Schoenfeld et al. 2002).
What are the implications of blindsight research? Regarding the quest for the neuronal correlates of conscious sight, it seems that destruction of the primary visual cortex disables the mysterious transition from neurochemical and electrical events to visual *qualia not because the computations V1 normally performs are indispensable, but because this area gates the vast majority of retinal input to the higher visual cortical areas. In this scenario, blindsight is blind because the retinal input from the blind field that informs visually guided responses fails to activate these areas sufficiently effectively to enable even poor conscious sight. If blindsight training induces changes that increasingly engage these cortical areas, training-induced recovery of varying degrees of conscious sight would result once the extrastriate cortical activation reached a critical level and/or pattern. The pronounced extrastriate cortical activation observed in patients who recovered sight spontaneously (Schoenfeld et al. 2002) or, more painstakingly, in the course of extended practice (Kleiser et al. 2001) support this conjecture. Support of a different kind comes from the visual *hallucinations that patients with fields of absolute cortical blindness can experience in their blind fields. They may appear as vague moving shapes, geometric patterns, or scenes, and are attributed to strong endogenous activation of higher visual cortices. In addition to showing that blind but veridical and conscious but non-veridical sight are also dissociable, they indicate that extrastriate areas can retain the capacity to generate non-veridical conscious vision in the absence of V1. Recovery of veridical conscious sight in fields of cortical blindness should result when sufficient retinal input informs higher visual cortical areas.
Does blindsight imply that conscious vision is *epiphenomenal, because monkeys and patients with destruction of V1 can learn to initiate non-reflexive visually guided actions in the absence of a phenomenal representation (Churchland 1984)? Careful observation of blindsight-guided behaviour shows it to be distinct from that displayed by sighted individuals. It resembles the fast motor responses we know from sports, where balls are hit precisely although they move too fast to allow conscious perception, and often deteriorates when responses are pondered. While ‘unseen’ information is sufficient to initiate a variety of responses (manual, saccadic, verbal, navigational), it is insufficient for recognition and for considered, representational responses. Normal-sighted monkeys are clearly capable of such responses, but like human patients lose this capacity through destruction of primary visual cortex. In addition, animals and humans show closely similar visual functions in the affected fields. Both may respond effectively to stimuli they indicate non-verbally are invisible when given this option; both improve with practice; and both may eventually stop signalling ‘no target’ on every blind-field trial. Although only the patients can verbally report concomitant stimulus awareness or unawareness, the overwhelming similarities between human and simian blindsight and the way it differs from conscious sight at least to us appear very difficult to reconcile with views that deny conscious sight to normal monkeys.
See also HELEN, ‘A BLIND MONKEY WHO SAW EVERYTHING’
PETRA STOERIG AND ALAN COWEY
Bullier, J., Girard, P., and Salin, P.-A. (1994). ‘The role of area 1 in the transfer of information to extrastriate visual cortex’. In Peters, A. and Rockland, K. S. (eds) Cerebral Cortex, Vol. 10.
Campion, J., Latto, R., and Smith, Y. M. (1983). ‘Is blindsight an effect of scattered light, spared cortex, and near-threshold vision?’ Behavioural and Brain Sciences, 6.
Churchland, P. M. (1984). Matter and Consciousness.
Cowey, A. (1967). ‘Perimetric study of field defects in monkeys after cortical and retinal ablations’. Quarterly Journal of Experimental Psychology, 19.
—— and Stoerig, P. (1995). ‘Blindsight in monkeys’. Nature, 373.
Humphrey, N. K. (1974). ‘Vision in a monkey without striate cortex: a case study’. Perception, 3.
Kleiser, R., Wittsack, J., Niedeggen, M., Goebel, R., and Stoerig, P. (2001). ‘Is V1 necessary for conscious vision in areas of relative cortical blindness?’ Neuroimage, 13.
Moore, T., Rodman, H. R., Repp, A. B., Gross, C. G., and Mezrich, R. S. (1996). ‘Greater residual vision in monkeys after striate cortex damage in infancy’. Journal of Neurohysiology, 76.
Pasik, P. and Pasik, T. (1982). ‘Visual function in monkeys after total removal of visual cerebral cortex’. Contributions to Sensory Physiology, 7.
Pöppel, E., Held, R., and Frost, D. (1973). ‘Residual visual function after brain wounds involving the central visual pathways in man’. Nature, 243.
Sahraie, A., Trevethan, C. T., MacLeod, M. J., Murray, A. D., Olson, J. A., and Weiskrantz, L. (2006). ‘Increased sensitivity after repeated stimulation of residual spatial channels in blindsight’. Proceedings of the National Academy of Sciences of the USA, 103.
Schoenfeld, M. A., Noesselt, T., Poggel, D. et al. (2002). ‘Analysis of pathways mediating preserved vision after striate cortex lesions’. Annals of Neurology, 52.
Stoerig, P. (2006). ‘Blindsight, conscious vision, and the role of primary visual cortex’. Progress in Brain Research, 155.
—— and Cowey, A. (1997). ‘Blindsight in man and monkey’. Brain, 120.
——, Zontanou, A., and Cowey, A. (2002). ‘Aware or unaware? Assessment of cortical blindness in four men and a monkey’. Cerebral Cortex, 12.
Weiskrantz, L. (1996). Blindsight: a Case Study and its Implications, 2nd edn
——, Warrington, E. K., Sanders, M. D., and Marshall, J. (1974). ‘Visual capacity in the hemianopic field following a restricted occipital ablation’. Brain, 97.
Zihl, J. and von Cramon, D. (1985). ‘Visual field recovery from scotoma in patients with postgeniculate damage. A review of 55 cases’. Brain, 108.
blind spot See FILLING-IN
blinking Humans blink spontaneously about 15 times per minute. Voluntary, spontaneous, and reflex blinks all have similar stereotyped kinematics, though voluntary blinks tend to be slightly greater in amplitude than spontaneous or reflex blinks. Blink duration ranges from 200 to 400 ms. The down-phase of a blink, when the eyelids close, typically lasts half as long as the up-phase, when the eyelids reopen, and the maximum velocity of the down-phase is twice as fast as that of the up-phase. During blinks the pupil is fully occluded by the eyelid for 100–140 ms.
Although blinks occur frequently in normal vision, they are rarely noticed, even though external darkening of the visual scene of similar durations is readily apparent. Continuity of conscious vision during blinks does not depend on continuous activity in early visual areas, as *single neurons in monkey early visual cortex show rapid and significant decreases in firing during a blink (Gawne and Martin 2002). These reductions in firing may even exceed the reduction caused by an equivalent external darkening. Interestingly, a significant minority of early visual cortex neurons show a strong transient response to the shutting off of visual input, whatever its cause. Some of these neurons respond differently depending on what caused the visual stimulus to disappear. Continuity of conscious vision during blinking may therefore rely on the suppression of these neuronal transients, which otherwise signal that the visual stimulus has disappeared.
Responses of the human visual system during blinks have been investigated using an ingenious device that bypasses the physical consequences of eyelid closure, by stimulating the retina via a fibre-optic cable placed in the mouth (Volkmann et al. 1980; see Fig. B4). This illuminates the retina via the palatine bone and allows retinal stimulation to be maintained during a blink, permitting measurement of visual sensitivity unconfounded by the purely mechanical effects of eyelid closure. In such circumstances, visual sensitivity is nevertheless reduced about tenfold during a blink; and this reduction commences just before the descent of the eyelid (Volkmann 1986). This indicates an active suppression of visual sensitivity associated with the motor commands underpinning eyelid closure. The loss of visual sensitivity mainly affects the magnocellular visual pathway that carries information about changes in luminance and responds to low spatial frequency, high temporal frequency, low contrast, and achromatic stimuli (Ridder and Tomlinson 1993). This suggests that visual suppression that occurs during blinks has evolved to minimize our ability to detect the eyelid descending across the pupil, a low spatial frequency stimulus, and the reduction in visual input, a change of luminance, that occur during blinks. This loss of visual sensitivity is thought to be caused by a neural signal from the motor regions controlling blinking (an efferent copy or corollary discharge) produced in parallel with the motor commands that cause the blink, and sent to the visual system causing the reduction in sensitivity and allowing the blink to go unnoticed.

Fig. B4. Technique devised by Volkmann et al. (1980). Light is passed into the mouth via a fibre-optic cable and transilluminates the retina via the palatine bone (see Colour Plate 4).
Consistent with these psychophysical studies, noninvasive *functional brain imaging using a very similar experimental apparatus to stimulate the retina shows that the response to visual stimulation in early visual cortex is reduced during blinking (Bristow et al. 2005a, 2005b). This reduction of activity is especially apparent in retinotopic area V3, which has a very strong magno-cellular input. In contrast, enhanced signal can be measured in visual cortex when blinking occurs in darkness. This pattern of effects of blinking on activity in visual cortex is very similar to that seen during saccadic eye movements, with suppression of visually evoked activity and an enhanced signal in darkness (Sylvester et al. 2005). The activation in darkness may reflect a motor signal associated with blinking (and saccades), perhaps a form of ‘corollary discharge’ that accompanies the motor command.
In addition to finding effects of blinking on early visual cortex, areas of prefrontal and parietal cortex also show significant reductions in activity during blinking when visual stimulation was held constant. These changes in activity in higher cortical areas may simply reflect the reduced output of earlier visual areas feeding forward. However, activation of similar *frontal and parietal regions is also reliably associated with changes in the *contents of consciousness (see FUNCTIONAL BRAIN IMAGING). Thus, reduction in the activity of such areas during blinking may indicate suppression of structures required to consciously register changes in the content of consciousness, preventing awareness of loss of visual continuity during a blink.
DAVINA BRISTOW AND GERAINT REES
Bristow, D., Frith, C., and Rees, G. (2005a). ‘Two distinct neural effects of blinking on human visual processing’. Neuro-image, 27.
Bristow, D., Haynes, J. D., Sylvester, R., Frith, C. D., and Rees, G. (2005b). ‘Blinking suppresses the neural response to unchanging retinal stimulation’. Current Biology, 15.
Gawne, T. J. and Martin, J. M. (2002). ‘Responses of primate visual cortical neurons to stimuli presented by flash, saccade, blink, and external darkening’. Journal of Neurophysiology, 88.
Ridder, W. H. and Tomlinson, A. (1993). ‘Suppression of contrast sensitivity during eyelid blinks’. Vision Research, 33.
Sylvester, R., Haynes, J. D., and Rees, G. (2005). ‘Saccades differentially modulate human LGN and VI responses in the presence and absence of visual stimulation’. Current Biology, 15.
Volkmann, F. C. (1986). ‘Human visual suppression’. Vision Research, 26.
——, Riggs, L. A., and Moore, R. K. (1980). ‘Eyeblinks and visual suppression’. Science, 207.
body image and body schema The terms body image and body schema are used in a variety of disciplines, including psychology, neurology and medicine, philosophy, and psychoanalysis, to explain how the body maintains spatial orientation, controls movement, and organizes somatosensory information and body awareness. The origins of these concepts can be traced to 19th-century neurology. Various neurologists postulated the existence of ‘images’ or ‘schemas’ stored in the sensorimotor cortex for the spatial awareness of the body and the control of movement. Henry Head (1920) proposed the concept of a body schema as a postural model that actively organizes sensory impressions to dynamically represent current body position with reference to previous position. These postural schemas are not conscious images but non-conscious functions, generated and controlled by cortical representations that register postural changes, in the service of motor control. The same neural activations, however, may generate a conscious sense of bodily position and movement.
Terminological and conceptual confusions soon developed around these concepts. Paul Schilder (1935), for example, claims to be in agreement with Head, yet equates body schema with the conscious sensation of position and uses the terms ‘body image’ and ‘body schema’ interchangeably. Confused usage of these terms continues, despite some justified criticisms and attempts at conceptual reform (e.g. Poeck and Orgass 1971). The fact that these terms continue to be used in various literatures, despite the confusion, suggests that they can be useful tools for understanding the dynamics of bodily movement and experience (De Preester and Knockaert 2005, Gallagher 2005; see Tiemersma 1989 for a good review of the literature).
From both behavioural and neurological perspectives, body image and body schema can be understood, respectively, as two different, albeit closely related, interactive and coordinated systems. As such, the body image is characterized as a system of inconstant perceptions, feelings, and beliefs where the object of such intentional states is one’s own body. The body schema, in contrast, consists of a system of sensorimotor capacities designed for motor and postural control without awareness or the necessity of perceptual monitoring. Just as having a perception of one’s body is different from having a capacity to move one’s body, so a body image is different from a body schema. Most of the time the body schema functions when the intentional object of perception is something other than one’s own body. For example, when I reach to open the door, I may consciously perceive the doorknob, or I may be attending to my reason for opening the door, or to the person I expect to see on the other side, but I am not attending to, and in most cases, I am not aware of my hand, the specific details of its grasp or my reach, or the posture that I take (Campbell 1995, O’Shaughnessy 1995).
Although perceptual consciousness of one’s own movement (or someone else’s movement) can be interrelated with one’s actions, so that processes connected with body image may be interrelated with body-schematic processes, there is good empirical support for the conceptual distinction. In some cases of unilateral neglect, for example, body-schematic processes for the neglected side of the body may remain intact despite the disruption of the body image for the neglected side. In contrast, in cases of deafferentation, subjects who have lost tactile and proprioceptive input from the neck down are able to control their limb movements only by visual guidance (see PROPRIOCEPTION). They use their body image in a unique way to compensate for the loss of body-schematic function (Cole and Paillard 1995). Such dissociations provide empirical reasons for thinking that the conceptual distinction between body image and body schema points to a real difference.
The conceptual distinction is also useful for understanding recent findings in neuroscience, which themselves help to explain how body image and body schema interact on the behavioural level. Areas of the brain responsible for motor control are activated not only when we engage in intentional action (serving body-schematic functions) but also when we observe others act, or when we imagine ourselves acting (which involves the body image). On the behavioural level we may exploit our body image to learn new movements, or to correct movements when things go wrong, in which case the body image clearly informs or modifies the body schema.
There is a substantial amount of literature on pathologies that involve the body image. There is general agreement, for example, that anorexia nervosa involves distortions of the body image, although there continues to be some debate about whether the distortions are affective or perceptual in nature, or to what extent such distortions are connected with cultural and socially determined ideals of acceptable body shape. Body dysmorphic disorder (BDD) is defined as a disturbance of body image that involves an extreme dissatisfaction with the appearance of one’s body. BDD can manifest in compulsive mirror checking, social withdrawal, excessive plastic surgeries, or even voluntary amputation.
Disorders that involve degrees of disembodied experience manifest themselves as an ambiguous presence of the body as object. The body may appear as something alien or as something to consciously control, although these feelings of alienation from the body do not advance to the point where the subject fails to acknowledge it as his or her own body. Cotard’s syndrome involves a delusional belief about various states of one’s body, e.g. that it is dead, rotting, or missing certain internal organs. Other disorders involve disruptions of the body image in which there is no sense of presence or ownership, as in the case of unilateral neglect mentioned above. In some neglect subjects there are complications from paralysis and they may also misidentify their arm or leg. Such patients famously complain that there is a strange leg in their bed, or that they cannot understand whose hand it is that is lying next to them (this is not to be confused with *anarchic hand syndrome; see below). In most cases of neglect, however, subjects pay no attention to the affected side of their body, and it seems not to belong to their embodied self-image. *Anosognosia often accompanies neglect and involves a lack of awareness of any problem with the body. The anosognosic patient will claim that he is indeed using the limb when in fact it is paralysed and he cannot move it (Berti et al. 2005). Autotopagnosia (somatotopagnosia) is another form of body-image related agnosia that involves the inability to name or point to various parts of one’s body on command.
Body-schematic functions in motor control normally tend to be non-conscious or tacit, and the body-in-action tends to efface itself phenomenologically. In *schizophrenia, however, it is sometimes the case that the normally tacit aspects of automatic body-schematic processes become explicit (what Sass 1998 calls a ‘hyperreflexive’ awareness of one’s body). In some cases, a disruption in processes of action preparation (corresponding to neurological problems in the generation of motor commands and efference copy) may disrupt the normal sense of agency for such action and motivate delusions of control, involving misattributions of agency to some other person. Anarchic hand syndrome, due to lesions in the supplementary motor area (SMA), involves a similar loss of the sense of agency, although the subject does not misattribute agency to someone else (Della Sala et al. 1994). Body-schematic motor control of the hand is neurologically disconnected from intentional control mechanisms, yet the hand makes complex purposive movements that are not intended by the subject. Environmental affordances likely elicit the hand’s behaviour and the subject is unable to inhibit it.
SHAUN GALLAGHER
Berti, A., Bottini, G., Gandola, M. et al. (2005). ‘Shared cortical anatomy for motor awareness and motor control’. Science, 309.
Campbell, J. (1995). ‘The body image and self-consciousness’. In Bermúdez, J. L., Marcel, A., and Eilan, N. (eds) The Body and the Self.
Cole, J. and Paillard, J. (1995). ‘Living without touch and peripheral information about body position and movement: studies with deafferented subjects’. In Bermúdez, J. L. et al. (eds) The Body and the Self.
Della Sala, S., Marchetti, C., and Spinnler, H. (1994). ‘The anarchic hand: a fronto-mesial sign’. In Boller, F. and Grafman, J. (eds) Handbook of Neuropsychology, Vol. 9.
De Preester, H. and Knockaert, V. (eds) (2005). Body Image and Body Schema: Interdisciplinary Perspectives on the Body.
Gallagher, S. (2005). How the Body Shapes the Mind.
Head, H. (1920). Studies in Neurology, Vol. 2.
O’Shaughnessy, B. (1995). ‘Proprioception and the body image’. In Bermúdez, J. L. et al. (eds) The Body and the Self.
Poeck, K. and Orgass, B. (1971). ‘The concept of the body schema: a critical review and some experimental results’. Cortex, 7.
Sass, L. (1998). ‘Schizophrenia, self-consciousness and the modern mind’. Journal of Consciousness Studies, 5.
Schilder, P. (1935). The Image and Appearance of the Human Body.
Tiemersma, D. (1989). Body Schema and Body Image: an Interdisciplinary and Philosophical Study.
brain Although several scientists and philosophers used to think otherwise a long time ago, consciousness is in the brain. The possibility to acquire information about the world from the senses, the capability to experience feelings, the complexities of language and motor output as well as many other facets of conscious experience can and should be attributed to activity in the brain (Crick 1994, Koch 2005). Therefore, an important focus of the scientific research efforts to elucidate a mechanistic explanation for consciousness is the search for the neural *correlates of consciousness (NCC). The simple observation that consciousness needs to be accounted for in terms of brain processes embodies the difficulties involved in explaining consciousness and at the same time points to the road towards an eventual possible solution. The mystery arises because the brain is a physical system; a complex physical system indeed, but a physical system nonetheless. Therefore, phenomena related to consciousness need to be ultimately linked to a material substrate to provide a scientific explanation. The laws of physics, chemistry, and biology, through molecules and neurons, need to produce *qualia and the mind.
In order to explain consciousness, scientists need to decipher the inner workings of the brain. In spite of major progress in neuroscience in recent years, the brain remains one of the most challenging and fascinating objects of scientific study. The human brain, for example, contains about 1011 neurons (Kandel et al. 2000). Brains of other species such as non-human primates, mice, and dogs show an approximately similar complexity. There are organisms with a much smaller nervous system that play a pivotal role in the development of neuroscience; one such example is the C. elegans worm where all neurons can be counted and identified. Unfortunately, the usefulness of such species for the study of consciousness remains highly unclear (see EVOLUTION OF CONSCIOUSNESS). Most neurons communicate with other neurons through connections called synapses. There are thousands of synapses per neuron. In contrast to modern computers where the circuit diagram is clearly known (by design), the sheer number of synapses in the primate brain makes the task of mapping the connectivity pattern a daunting one.
There are many different types of neurons in the brain (Koch 1999, Kandel et al. 2000). A basic distinction can be made between so-called pyramidal neurons and interneurons; these two classes of neurons can be distinguished based on their morphology, the type of neurotransmitters they release, their connectivity properties, and their firing patterns. The codes that neurons and networks of neurons use to represent information are still not fully understood (Kreiman 2004). Both the number of spikes and the pattern of spikes fired by each neuron seem to matter for conveying information. There are also network properties including synchronization and *gamma-band oscillations that can play important roles in information transmission across and within brain areas (Engel and Singer 2001, Bichot et al. 2005). Deciphering the signatures or codes that neuronal networks use to represent information in general may provide important insights into the study of consciousness. It is possible that specific firing patterns or network properties are particularly relevant for representing the contents of consciousness (Koch 2005).
The brain has two hemispheres and four main lobes: the occipital lobe, the parietal lobe, the *frontal lobe, and the temporal lobe. The two hemispheres communicate with each other at several points, the most prominent of which is the corpus callosum. In some patients with severe *epilepsy, neurosurgeons may need to conduct a resection of one or more of the connections across hemispheres. This procedure may lead to a failure of information transmission between the two hemispheres. These *commissurotomy (split-brain) patients can show remarkable properties and, under appropriate circumstances, they can be shown to function as if they had two independent brains (Sperry 1982).
Over the years, it has become clear that functions are specialized within different regions of the brain. Scientists have mapped the approximate locations for many different functions. For example, parts of the cerebral cortex may be specialized for processing auditory information; other parts are specialized in processing olfactory information. Yet other areas, such as the hippocampus, are necessary to transfer short-term memories into long-term *memories (Zola-Morgan and Squire 1993). Within the visual system, investigators have discovered that there are about twenty or more distinct areas that process different aspects of visual information and are segregated into two main *visual streams (Felleman and Van Essen 1991). Therefore, in the same way that distinct brain regions may be specialized to process information from different sensory modalities, different behaviours, or different cognitive processes, it is conceivable that different parts of the brain may play very distinct roles with respect to the representation of conscious information (Koch 2005).
Several different tools are used to study the brain (Kreiman 2004). These different tools span wide ranges of spatial and temporal resolutions. At the smaller spatial scales, scientist study, for example, the structure and function of specific ion channels at the angstrom resolution (10–10 of a metre). At the larger spatial scales, several studies make inferences about the function of the brain as a whole or use tools with very coarse special resolution such as the scalp *electroencephalogram (EEG) or *magnetic encephalography (MEG). At the faster temporal scales, electrophysiological measurements including EEG, MEG, and also neuronal spike recordings can analyse activity at the sub-millisecond level. At the other extreme other tools such as *functional magnetic resonance imaging (fMRI) provide information with a resolution of seconds. In other cases, scientists may be interested in the effects of learning or ageing across months or years.
What the appropriate spatial and temporal scale to study consciousness should be is not necessarily trivial. In terms of temporal scales, the subjective impression is that conscious percepts are continuous in time. Even though this notion has been challenged (Dennett 1991, Chalmers 1996, Koch 2005), it still seems that high temporal resolution will be important to study the neuronal dynamics that give rise to consciousness. A scale of seconds or minutes may be too slow for us to be able to comprehend how consciousness arises from the activity of networks of neurons. In terms of spatial scales, the more detailed mechanistic and quantitative description of brain phenomena are typically rooted at the level of single neurons or small networks of neurons. Coarser techniques that average the activity of multiple neuronal types and across large areas may fail to unveil some key aspects of how conscious percepts arise. The devil is in the detail. The difficulty arises from two methodological aspects: the technological challenge of studying large ensembles of neurons at the single-neuron level and the invasive nature of the single-neuron studies. The invasive nature of single-neuron electrophysiology generally makes it necessary to study animal models such as the *single-cell studies in monkeys. Under special circumstances, including the study of patients with intractable epilepsy and patients with Parkinson’s disease, it has been possible to study *single-neuron activity in the human brain.
In addition to the many tools used to measure brain activity, another important piece of evidence relating to the function of a given brain area has been the study of lesions. In animal models, scientists can induce rather specific brain lesions in order to understand the function of specific circuits. In humans, these studies are limited to naturally occurring lesions (see BRAIN DAMAGE). The study of lesions has provided the first demonstrations of functional specialization in the brain, going back to the initial studies in Broca’s language area (Broca 1861, Finger 2000). Other important insights into the nature of consciousness that came through the studies of lesions or other brain abnormalities include the research into *blindsight, neglect (see ANOSOGNOSIA), *schizophrenia, and epilepsy. In the future, it is likely that tools that rely on molecular biology may be used to induce reversible and high-resolution microlesions in specific circuits in animal models including primates. These high-resolution and reversible microlesion studies may eventually help provide a link between the correlative physiological measurements and the neuronal causes of consciousness.
Lesion studies provide support to assess whether specific brain areas may be necessary for consciousness. Questions about sufficiency are extremely hard to ask in the context of consciousness. For example, is the activation of neurons in inferior temporal cortex sufficient for the perception of complex visual objects? The closest one can get to answers to such questions comes from studies that use brain stimulation tools including *transcranial stimulation (TMS) and electrical neural stimulation. Several studies in animal models have shown that electrical microstimulation of relatively small clusters of neurons can bias or even induce specific percepts (Salzman et al. 1990, Romo et al. 1998, Brecht et al. 2004). Electrical stimulation in human epileptic patients also suggests that specific and transient sensory experiences can be elicited by direct stimulation of the brain (Penfield, 1937, Ojemann and Mateer 1979).
The brain is perhaps one of the body parts that have been subject to very strong evolutionary selective pressure. It is therefore not surprising to find important changes across species in brain function and structure. Still, there are also strong similarities between the brains of non-human primates and humans. For example, many visually selective brain areas in the macaque monkey brain have a corresponding homologue region in the human brain. A detailed study of the way in which the brain evolved over long periods of time and the important differences that distinguish humans from other species may yield important insights into the evolution of consciousness.
At any given time, many brain areas show intense activity, even in the absence of concomitant sensory input. Most neurons in the brain have a sustained level of spontaneous activity which can be modulated dependent on the time of day and other environmental circumstances. What this spontaneous activity represents (if anything at all) is still strongly debated. What is clear is that even under conditions with no sensory input, many brain areas can be strongly activated. Examples of such situations include *sleep and *anaesthesia. It can be argued that brain activity during non-dream sleep and anaesthesia is not correlated with conscious percepts. Thus, the study of brain activation during these states can provide interesting insights into the neuronal correlates of consciousness. In general, these studies (and many other such studies) show that humans are not aware of a large fraction of the activity in their brains.
Given that many brain processes may be uncorrelated with conscious perception, an important focus of research in the search for the neuronal correlates of consciousness has been the attempt to elucidate which brain areas correlate with subjective perception. The use of situations where perception is dissociated from sensory input allows us to distinguish between inputrelated activation and subjective activation. One important experimental paradigm that follows this idea is the study of *binocular rivalry where two different images are shown to the two eyes. Under these circumstances, perception alternates in a seemingly random fashion between the two images. At the level of the retina, activity should be essentially constant given that the sensory input is constant where the competition between the two images for conscious perception takes place in higher visual areas.
GABRIEL KREIMAN
Bichot, N. P., Rossi, A. F., and Desimone, R. (2005). ‘Parallel and serial neural mechanisms for visual search in macaque area V4’. Science, 308.
Brecht, M., Schneider, M., Sakmann, B., and Margrie, T. (2004). ‘Whisker movements evokes by stimulation of single pyramidal cells in rat motor cortex’. Nature, 427.
Broca, M. (1861). ‘Remarques sur le siège de la faculté du langage articule, suivies d’une observation d’aphémie (perte de la parole)’. Bulletin de la Société Anatomique, 6.
Chalmers, D. (1996). The Conscious Mind: in Search of a Fundamental Theory.
Crick, F. (1994). The Astonishing Hypothesis.
Dennett, D. (1991). Consciousness Explained.
Engel, A. and Singer, W. (2001). ‘Temporal binding and the neural correlates of sensory awareness’. Trends in Cognitive Sciences, 5.
Felleman, D. J. and Van Essen, D. C. (1991). ‘Distributed hierarchical processing in the primate cerebral cortex’. Cerebral Cortex, 1.
Finger, S. (2000). Minds Behind the Brain. A History of the Pioneers and Their Discoveries.
Kandel, E., Schwartz, J., and Jessell, T. (2000). Principles of Neural Science, 4th edn.
Koch, C. (1999). Biophysics of Computation.
—— (2005). The Quest for Consciousness.
Kreiman, G. (2004). ‘Neural coding: computational and biophysical perspectives’. Physics of Life Reviews, 1.
Ojemann, G. and Mateer, C. (1979). ‘Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems’. Science, 205.
Penfield, W. (1937). ‘The cerebral cortex in man. I. The cerebral cortex and consciousness’. Archives of Neurology and Psychiatry, 40.
Romo, R., Hernandez, A., Zainos, A., and Salinas, E. (1998). ‘Somatosensory discrimination based on cortical microstimulation’. Nature, 392.
Salzman, C., Britten, K., and Newsome, W. (1990). ‘Cortical microstimulation influences perceptual judgements of motion direction’. Nature, 346.
Sperry, R. (1982). ‘Some effects of disconnecting the cerebral hemispheres’. Science, 217.
Zola-Morgan, S. and Squire, L. R. (1993). ‘Neuroanatomy of memory’. Annual Review of Neuroscience, 16.
brain damage Progress in intensive care efforts has increased the number of patients who survive severe acute brain damage. The most frequent causes of coma are traumatic or ischaemic brain damage. Although most of these patients recover from coma within the first days after the insult, some permanently lose all brain function (brain death), while others evolve to a state of ‘wakeful unawareness’ (vegetative state, VS). Those who recover, typically progress through different stages before fully or partially recovering consciousness (minimally conscious state, MCS; see Fig. B5). Bedside evaluation of residual brain function in severely brain-damaged patients is difficult because motor responses may be very limited or inconsistent. In addition, consciousness is not an all-or-none phenomenon and its clinical assessment relies on inferences made from observed responses to external stimuli at the time of the examination. We here review the major clinical entities of altered states of consciousness following severe acute brain damage.
Fig. B5. Flowchart of the different conditions that follow a cerebral insult. Classically vegetative state follows a coma; after 1 month the term persistent vegetative state is used; after 3 months (non-traumatic insult) or 1 year (traumatic insult) some authors use the term permanent vegetative state which implies no chance of recovery.
1. Brain death
2. Coma
3. Vegetative state
4. Minimally conscious state
5. Locked-in syndrome
The concept of brain death as defining the death of the individual is largely accepted. Most countries have published recommendations for the diagnosis of brain death, but the diagnostic criteria differ from country to country. Some rely on the death of the brainstem only, others require death of the whole brain including the brainstem. However, the clinical assessments for brain death are very uniform and based on the irreversible loss of all brainstem reflexes and the demonstration of continuing apnoea in a persistently comatose patient. Since the first definition of the neurological criteria of death in the mid 1960s, no patient in apnoeic coma properly declared brain (or brainstem) death has ever regained consciousness.
*Functional imaging using cerebral perfusion tracers and single-photon emission computed tomography (SPECT) or cerebral metabolism tracers and positron emission tomography (PET) typically show a ‘hollow skull phenomenon’ in brain death patients, confirming the absence of neuronal function in the whole brain.
Some authors have proposed that death be defined by the permanent cessation of the higher functions of the nervous system that demarcate humans from the lower primates. This neocortical or higher brain death definition has been mainly developed by philosophers and its conceptual basis rests on the premise that consciousness, cognition, and social interaction, not the bodily physiological integrity, are the essential characteristics of human life. On the basis of this definition, vegetative patients following an acute injury or chronic degenerative disease and anencephalic infants are considered dead. This neocortical definition of death has never convinced either medical associations or courts (for a recent review see Laureys 2005).
Coma is characterized by the absence of arousal and thus also of consciousness. It is a state of unarousable unresponsiveness in which the patient lies with the eyes closed and has no awareness of self and surroundings. The patient lacks the spontaneous periods of wakefulness and eye-opening induced by stimulation that can be observed in the VS. Coma can result from diffuse bihemispheric cortical (e.g. after cardiac arrest) or white matter damage secondary to diffuse neuronal or axonal injury (e.g. after deceleration traffic accidents), or from focal brainstem lesions that affect the pontomesencephalic tegmentum and/or paramedian thalami bilaterally (e.g. after stroke or haemorrhage). To be clearly distinguished from syncope, concussion, or other states of transient unconsciousness, coma must persist for at least one hour. In general, comatose patients who survive begin to awaken and recover gradually within 2–4 weeks. This recovery may go no further than VS or MCS, or these may be stages (brief or prolonged) on the way to more complete recovery of consciousness.
The prognosis of coma survivors following brain anoxia is worse than following trauma. Clinically, absent or stereotyped motor responses and absent pupillary reflexes often indicate bad outcome. Paraclinically, isoelectrical (‘flat’) or ‘burst suppression’ *electroencephalogram (EEG) and the bilateral absence of somatosensory evoked potentials (SEPs) in primary cortex (called N20 potential) are strong indicators of death or irreversible VS. In contrast, auditory oddball evoked potentials showing an intact mismatch negativity (MMN) effect predicts an outcome better than death or VS.
In patients with coma of traumatic or hypoxic origin, PET studies show that, on average, grey matter metabolism is 50–70% of normal values. Cerebral metabolism has been shown to correlate poorly with the level of consciousness, as measured by the Glasgow Coma Scale, in mild to severely brain-damaged patients. A global depression of cerebral metabolism is not unique to coma. When different *anaesthetics are titrated to the point of unresponsiveness, the resulting reduction in brain metabolism is similar to that observed in comatose patients. Another example of transient metabolic depression can be observed during deep *sleep (stages III and IV). In this daily physiological condition, cortical cerebral metabolism can drop to nearly 40% of normal values (Fig. B6; Laureys et al. 2004).
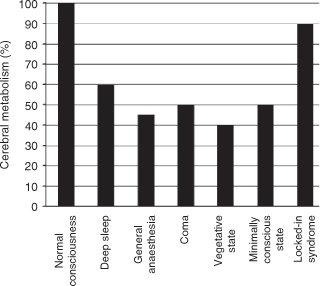
Fig. B6. Overall cortical metabolism in coma, VS, MCS, and LIS as compared to slow-wave sleep and anaesthesia.
Patients in a VS are awake but are unaware of self or of the environment. Bryan Jennett and Fred Plum cited the Oxford English Dictionary to clarify their choice of the term ‘vegetative’: to vegetate is to ‘live merely a physical life devoid of intellectual activity or social intercourse’ and vegetative describes ‘an organic body capable of growth and development but devoid of sensation and thought’. Persistent VS has been arbitrarily defined as a vegetative state still present one month after acute traumatic or non-traumatic brain damage, but does not imply irreversibility. Permanent VS denotes irreversibility. Current UK medical guidelines consider that 6 months following a non-traumatic brain damage and 12 months after traumatic injury, the condition of VS patients may be regarded as ‘permanent’ (Jennett 2005).
The terms apallic syndrome and neocortical death have previously been used to describe patients in a VS. However, as discussed below, functional imaging studies have shown that vegetative patients are not apallic— that is, they still may show preserved activation in islands of functional ‘pallium’ or cortex.
It has been shown that more than one out of three patients initially diagnosed as being in a VS in fact shows some signs of awareness when carefully examined. Although these studies on the misdiagnosis of the VS are distressing, they also show that more careful examinations by skilled clinicians can identify very subtle signs of awareness in severely brain-damaged patients who remain unable to communicate verbally or non-verbally. To reliably make the diagnosis of the vegetative or minimally conscious state, especially adapted standardized consciousness scales should be employed (reviewed in Majerus et al. 2006). The chances of recovery from VS are better in children than in adults; better after traumatic than non-traumatic brain injury; and worse as the time spent in VS passes. Neither clinical nor complementary tests such as EEG, event-related potentials (ERP), structural or functional imaging alone can reliably predict the individual patient’s potential for recovery. At present, no treatment has been shown to increase the chances of recovery from VS (or MCS).
In the VS the brainstem is relatively spared, whereas the grey and/or white matter of both cerebral hemispheres are widely and severely damaged. Overall cortical metabolism of vegetative patients is 40–50% of normal values. Characteristic of VS patients is a relative sparing of metabolism in the brainstem (encompassing the pedunculopontine reticular formation, the hypothalamus, and the basal forebrain). The functional preservation of these structures allows for the preserved arousal and autonomic functions in these patients. The other hallmark of the vegetative state is a systematic impairment of metabolism in the polymodal associative cortices (bilateral prefrontal regions, Broca’s area, parietotemporal and posterior parietal areas and precuneus; see also AROUSAL VS AWARENESS). These regions are known to be important in various functions that are closely related to consciousness, such as attention, memory, and language. It has long been controversial whether the observed metabolic impairment in this large frontoparietal cortical network reflects an irreversible structural neuronal loss, or functional and potentially reversible damage. However, in the rare cases where VS patients recover awareness of self and environment, PET shows a functional recovery of metabolism in these same cortical regions. Moreover, the resumption of long-range functional connectivity between these associative cortices and between some of these and the intralaminar thalamic nuclei parallels the restoration of their functional integrity. The cellular mechanisms which underlie this functional normalization remain putative: axonal sprouting, neurite outgrowth, and cell division (known to occur predominantly in associative cortices in normal primates) have been proposed as candidate processes. The challenge is now to identify the conditions in which, and the mechanisms by which, some vegetative patients may recover consciousness.
In addition to measuring resting brain function and connectivity, neuroimaging studies have identified which brain areas still ‘activate’ during external stimulation in vegetative patients. In cohort studies of patients unequivocally meeting the clinical diagnosis of VS, noxious somatosensory and auditory stimuli have shown robust activation of primary sensory cortices and lack of activation in higher-order associative cortices from which they were functionally disconnected. For example, high-intensity noxious electrical stimulation activated midbrain, contralateral thalamus, and primary somatosensory cortex in each and every one of the 15 VS patients studied, even in the absence of detectable cortical evoked potentials (Laureys et al. 2002). However, the rest of the ‘pain matrix’ (encompassing secondary somatosensory, insular, posterior parietal, and anterior cingulate cortices) failed to show activation. Moreover, the activated primary somatosensory cortex was shown to exist as an island, functionally disconnected from the higher-order associative cortices in VS. Similarly, auditory stimuli activated bilateral primary auditory cortices in VS patients, but hierarchically higher-order multimodal association cortices were not activated. Moreover, a cascade of functional disconnections was observed along the auditory cortical pathways, from primary auditory areas to multimodal and limbic areas (Laureys et al. 2000). These studies suggest that the observed residual cortical processing in the VS does not lead to integrative processes which are thought to be necessary for awareness.
The clinical criteria for MCS, formally proposed only in 2002, subcategorize patients above VS but unable to communicate consistently. To be considered as minimally conscious, patients have to show limited but clearly discernible evidence of consciousness of self or environment, on a reproducible or sustained basis, by at least one of the following behaviours: (1) following simple commands; (2) gestural or verbal yes/no response (regardless of accuracy); (3) intelligible verbalization; and (4) purposeful behaviour (including movements or affective behaviour that occur in contingent relation to relevant environment stimuli and are not due to reflexive activity). The emergence of MCS is defined by the ability to use functional interactive communication or functional use of objects (Giacino et al. 2002). Further improvement is more likely than in VS patients. However, some patients remain permanently in MCS.
Akinetic mutism is an outdated term that is better avoided and is now considered to be a subcategory of MCS. The term was first introduced in 1941 to describe a condition characterized by severe poverty of movement, speech, and thought without associated arousal disorder or descending motor tract impairment. Typical for akinetic mutism is the complete or near-complete loss of spontaneity and initiation so that action, ideation, speech, and emotion are uniformly reduced. The absence of internally guided behaviour allows attention to be passively drawn to any environmental stimulus that the patient is exposed to. The preservation of spontaneous visual tracking and occasional, albeit infrequent, speech and movement to command, help differentiate akinetic mutism from VS.
Because criteria for the MCS have only recently been introduced, there are still few functional imaging studies of patients in this condition. Overall cerebral metabolism is decreased to values slightly higher but comparable to those observed in the VS. Metabolic activity in the medial parietal cortex (precuneus) and adjacent posterior cingulate cortex seems to best differentiate minimally conscious from vegetative patients. Interestingly, these areas are among the most active brain regions in conscious waking and are among the least active regions in *altered states of consciousness such as general *anaesthesia, *sleep, *hypnotic state, *dementia, and Wernicke–Korsakoff’s or post-anoxic *amnesia. It has been suggested that this richly connected multimodal posteromedial associative area is part of the neural network subserving human awareness (Fig. B7).
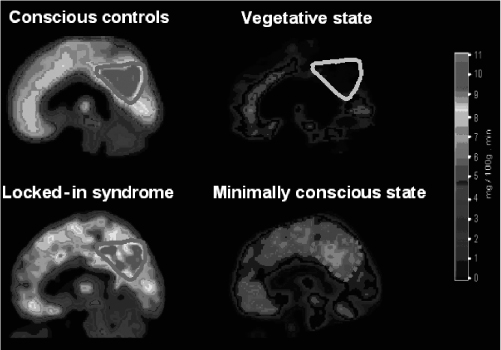
Fig. B7. In normal conscious waking, the medial posterior cortex (encompassing the precuneus and adjacent posterior cingulate cortex, delineated by a red line) is the metabolically most active region of the brain; in waking vegetative patients, this same area (delineated by a blue line) is the metabolically least active region. In the locked-in syndrome, no supratentorial brain region shows significant decreases in metabolism. In the minimally conscious state, the precuneus and posterior cingulate cortex shows an intermediate metabolism, higher than in vegetative patients, but lower than in conscious controls. We hypothesize that this region represents part of the neural network subserving (human) consciousness (see Colour Plate 5). Reproduced from Laureys et al. (2004).
Simple auditory stimulation has been shown to induce a more widespread activation in minimally conscious than in vegetative patients. In the former, activation encompassed not only primary but also higher-order associative areas, suggesting a more elaborate level of processing. Moreover, cortico-cortical functional connectivity is more efficient in the MCS, compared to the VS, between auditory cortex and the frontoparietal ‘*global neuronal workspace’considered critical in awareness. Such findings encourage ongoing developments of neuromodulatory and cognitive revalidation therapeutic strategies in MCS patients.
In response to natural language stimuli (e.g. meaningful sentences), fMRI activation patterns of MCS patients exhibiting command-following were examined by Schiff et al. (2005) during presentation of forward and backward narratives read in a familiar voice and containing personally meaningful content. Components of the cortical language networks showed selective activation compared to baseline conditions. Presentation of the narratives time-reversed (played backward), which shared most of the physical properties of the sounds, activated the same networks as forward narratives in the normal control subject, but failed to activate the networks in the MCS patients. These findings correlate with low resting metabolic activity and suggest that a residual capacity to activate large integrative networks may remain in some MCS patients. Preservation of large-scale networks in MCS patients may underlie rare instances of late recoveries of verbal fluency in such patients.
The term locked-in syndrome (LIS) was introduced by Fred Plum and Jerome Posner in 1966 to reflect the quadriplegia and anarthria brought about by the disruption of corticospinal and corticobulbar pathways respectively. It is defined by (1) the presence of sustained eye opening (bilateral ptosis should be ruled out as a complicating factor); (2) preserved awareness of the environment; (3) aphonia or hypophonia; (4) quadriplegia or quadriparesis; and (5) a primary mode of communication that uses vertical or lateral eye movement or blinking of the upper eyelid to signal yes/no responses (Plum and Posner 1983).
Classically, structural brain imaging (MRI) may show isolated lesions (bilateral infarction, haemorrhage, or tumour) of the ventral portion of the basis pontis or midbrain. According to some authors, electroencephalography (EEG) and evoked potentials do not reliably distinguish the LIS from the VS. PET scanning has shown significantly higher metabolic levels in the brains of patients in a LIS compared to patients in the VS. Voxel-based statistical analyses show that no supratentorial cortical areas show a significantly lower metabolism in LIS patients when compared to healthy controls. These findings emphasize the need for speed both in making the diagnosis and in recognizing the terrifying situation of patients with intact awareness of self and environment in acutely locked-in immobile bodies. Health-care workers should adapt their bedside behaviour and consider pharmacological anxiolytic therapy, taking into account the intense emotional state acute LIS patients go through. With appropriate medical care, life expectancy may be several decades and even if the chances of motor recovery are very limited, computer-based communication methods have drastically improved the quality of life of chronic LIS patients (Laureys et al. 2005).
STEVEN LAUREYS AND MÉLANIE BOLY
Giacino, J. T., Ashwal, S., Childs, N. et al. (2002). ‘The minimally conscious state: definition and diagnostic criteria’. Neurology, 58.
Jennett, B. (2005). ‘30 years of the vegetative state: clinical, ethical and legal problems’. In Laureys, S. (ed.) The Boundaries of Consciousness: Neurobiology and Neuropathology.
Laureys, S. (2005). ‘Science and society: death, unconsciousness and the brain’. Nature Reviews Neuroscience, 6.
Laureys, S. and Boly, M. (2008). ‘The changing spectrum of coma’. Nature Clinical Practice Neurology, 4.
——, Degueldre, C. et al. (2000). ‘Auditory processing in the vegetative state’. Brain, 123.
——, ——, Peigneux, P. et al. (2002). ‘Cortical processing of noxious somatosensory stimuli in the persistent vegetative state’. Neuroimage, 17.
——, Owen, A. M., and Schiff, N. D. (2004). ‘Brain function in coma, vegetative state, and related disorders’. Lancet Neurology, 3
——, Pellas, F., Van Eeckhout, E. et al. (2005). ‘The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless?’ Progress in Brain Research, 150.
Majerus, S., Gill-Thwaites, H., Andrews, K., and Laureys, S. (2006). ‘Behavioral evaluation of consciousness in severe brain damage’. In Laureys, S. (ed.) The Boundaries of Consciousness: Neurobiology and Neuropathology.
Owen, A. M., Coleman, M. R., Boly, M., Davis, M. H., Laureys, S., and Pickard, J. D. (2006). ‘Detecting awareness in the vegetative state’. Science, 313.
Plum, F. and Posner, J. B. (1983). The Diagnosis of Stupor and Coma, 3rd edn.
Schiff, N. D., Rodriguez-Moreno, D., Kamal, A. et al. (2005). ‘fMRI reveals large-scale network activation in minimally conscious patients’. Neurology, 64.
brain death See BRAIN DAMAGE