This chapter explains the physical properties of electromagnetic radiation, the different types that exist, and the units of measurement—such as watts and volts—that are used throughout the remainder of this book. If you would rather skip this chapter, doing so will not impair your ability to read and appreciate any of the book’s remaining content.
Back in 1998, four men were just finishing up the 16th hole at a golf course in Colorado, when, without warning, an electrical storm erupted. Seemingly out of nowhere, lightning struck a tree under which the men were standing. One of the golfers sustained serious burns. Two were knocked unconscious. The fourth suffered no external burns nor showed any evidence of having been hit by lightning. Yet, puzzling his doctors, he suffered cardiac arrest and died about three weeks later.
How could something like this occur? How could a man be affected by lightning when the lightning did not actually make contact with his body? As researchers concluded in the June 13, 1998, issue of the Lancet, the answer is EMF, or electromagnetic fields.
Electromagnetic fields are invisible forces that surround us— increasingly so in our modern, electrically powered world. The underlying science of electromagnetic radiation can be complex. In this chapter, I’ll break down the most important concepts in EMF to enhance your understanding of the issues discussed in this book. Electromagnetic fields (as the name implies) emerge from a combination of two commonly experienced and well-understood forces in nature: electricity and magnetism.
ELECTRICITY
All matter is composed of atoms (such as carbon and iron), and all atoms are made up of the same fundamental particles that are negative (electrons), positive (protons), or uncharged (neutrons). The particles in an atom are in the form of a solar system with a nucleus containing the much heavier protons and neutrons in the center, and the much lighter electrons orbiting around the nucleus. A model of an atom of lithium is shown below with three electrons orbiting around the nucleus containing three protons. Different substances are characterized by the number of protons in the nucleus (e.g., carbon has 6 and iron has 26). Since atoms have the same number of electrons as protons, they are uncharged. However, electrons are relatively light and atoms can easily gain or lose electrons to become charged.

Electricity is a phenomenon that occurs because of these charges. Electric currents occur due to the flow of electrons or of atoms that assume positive or negative charges due to gaining or losing electrons. These charged particles are called ions. A lightning storm demonstrates that electricity occurs naturally all around us, but we also have learned how to generate, harness, and transport electricity for our benefit.
In grade school we learned how Benjamin Franklin, in 1752, used a kite to demonstrate that lightning is a form of electricity. Based on this knowledge, Franklin designed the metallic lightning rod to protect wooden structures from lightning-sparked fires by rapidly conducting the electricity to ground.

MAGNETS
The “magnetic” part of electromagnetic radiation refers to the same type of magnetic fields that emanate from those little pieces of metal that you have sticking to your refrigerator door. Certain materials exhibit magnetic properties (stemming from a particular ordering of the atoms that make up the magnet), enabling them to attract or repel other magnetic objects, or to be attracted to or repelled by other magnets. The needle in a compass is a magnet that points north because it interacts with the magnet that is part of our planet. The space in which the attractive and repulsive forces exert their influence is called the magnetic field. And as anyone who has played with two magnets knows, the strength of magnetic fields decreases with distance from the magnet. Because magnetic fields exert influence without making physical contact, physicists refer to magnetism as “action at a distance.”
As mentioned above, the earth itself is one giant magnet, with magnetic poles on the north and south ends of the planet. This is why compasses work and why certain species of birds are able to fly such great distances with such accuracy. Human beings also generate magnetic fields (such as those that can be seen in electrocardiograms due to electrical currents in the heart). We measure the strength of magnetic fields in units of gauss (G) or tesla (T). For some perspective, a typical refrigerator magnet has a magnetic field of 50 G (or 5 millitesla or 5 mT), while your brain emits a magnetic field of approximately .0000001 G.
EMF
Electricity flowing in a current generates magnetic fields. An electrical current (moving charges) in a wire is always accompanied by a magnetic field around the wire. The magnetic fields that result from charge flows are known as electromagnetic fields (EMFs) or electromagnetic radiation (EMR).
In practical applications involving the effects of emissions from cell phone towers or from cell phone antennas, the strength of EMFs is measured in units of power density, which tells us how much power is hitting a particular area. Power density can be measured in watts per square meter (W/m 2) or microwatts per square centimeter (μW/cm2), a unit that is 100 times smaller. It is therefore very important to keep track of the units.
While power density measurements inform us how strong a field is, power density does not tell us how much of that power is absorbed by what it comes in contact with, such as a human being. The measure of how much EMF is absorbed by a given area in the field is the specific absorption rate (SAR), measured in watts per kilogram (W/kg). Because SAR represents the measurement of radiation absorption at a specific point, it is usually averaged over greater areas, such as the head or body. This is how radiation from cell phones is commonly—though not comprehensively—measured. This approach assumes that radiation is uniformly absorbed throughout body tissue, which is very unlikely.
FREQUENCY
Although the many different types of electromagnetic radiation can be shown as waves, they differ from each other in that they have different frequencies, or wavelengths. Frequency is measured in units called hertz (Hz). Named after 19th-century German physicist Heinrich Hertz (the first to conclusively prove the existence of electromagnetic waves), Hz quantifies the cycles per second of electricity. It’s a measurement that we are all familiar with because it’s used to identify the frequencies used by stations in radio broadcasting.
AM radio bands start at around 520 and go up to around 1,610. These are frequencies of EMF (specifically, radio frequencies, or RF), where 520 on the AM dial is a signal of EMF radiation vibrating at 520 kilohertz (kHz) and 1,610 is a signal broadcast at a frequency of 1,610 kHz, or 1.61 megahertz (MHz). On the FM dial, you’ll find a similar spectrum of stations, which start on your radio around 87.5 MHz and top out near 108 MHz.

A radio dial showing FM (upper scale in MHz) and AM (lower scale in kHz) frequencies used in radio broadcasting. The dials tune into specific frequencies of electromagnetic radiation that are used to transmit audio signals.
Similarly, the rainbow of colors that make up the range of visible light are defined by individual frequencies. Visible light is a type of EMF—the earliest type of EMF recognized by people. Each color is different because the light vibrates with different frequencies. Red has the lowest frequency of visible light (in the range of 400–484 terahertz, or THz), orange a bit higher (484–508 THz), yellow a bit higher still (508–526 THz), all the way up to violet, which has the highest frequency of any color in the visible light spectrum (668–789 THz). When we say violet has a higher frequency than red, this means that the electromagnetic waves that generate violet vibrate at a faster rate than those waves that make the color red.
We have described waves in terms of their frequency, but one could just as easily describe them in terms of wavelength, as is often done. The product of frequency and wavelength of any wave is equal to the speed at which it advances, and for electromagnetic waves the product is equal to the speed of light, a fundamental constant of nature.
frequency (F) x wavelength (L) = speed of light (C)
This equation also tells us that because the speed of light is a constant, when the frequency increases, the wavelength decreases (and vice versa).
THE EM SPECTRUM
Radio frequency (RF) and visible light are just two ranges of EMF on a broad spectrum of electromagnetic energy. The electromagnetic spectrum (also known as the EM spectrum) contains all known frequencies of electromagnetic radiation, from radio waves toward the lower frequency end, through the visible light spectrum, all the way up to gamma rays.
RF is pretty low on the spectrum. Below it, we find extremely low frequency (ELF) radiation, such as what is emitted by the power lines and electrical circuits we use to supply electricity to our homes. Above RF, we find microwave (MW) radiation, which is what your microwave oven uses. Above MW is infrared (IR), such as that emitted by motion sensors or remote controls. And, on the other side of visible light, above violet (the highest frequency of color in the visible spectrum), we find ultraviolet radiation, X-ray radiation, and gamma radiation. The EM spectrum is important because different frequencies of EMF radiation are used in many different practical applications.
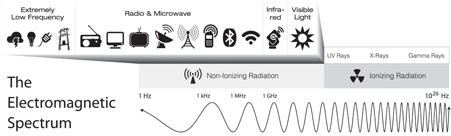
The electromagnetic (EM) spectrum extends from extremely low frequency radiation (pictured here on the far left), up through very high-energy gamma rays (on the far right). Different technologies use EMF radiation from different sections of the EM spectrum.
MAN-MADE AND NATURAL EMF
EMF comes from both natural and man-made sources. Visible light is one type of natural EMF that is generated by the sun. Modern devices like cell phones and WiFi networks generate man-made EMF, as do less novel devices like hair dryers and lightbulbs. As described above, human-made sources of EMF generate different frequencies of EM radiation across the spectrum, generally in the non-ionizing range. Everyday appliances, such as your desk lamp or hair dryer (as well as the power lines that provide electricity to these appliances), generate low-level frequencies of EM radiation in the ELF range. Radio broadcasts are in the RF range. Televisions, cell phones, and their towers emit a higher frequency of EMR called microwave radiation. Humans have been exposed to increasing levels of man-made EMF since the days when electricity was first harnessed. Anything that runs on electricity generates electromagnetic radiation, and our daily lives depend more and more on such products.
Natural EM radiation can hurt you, as anyone who has suffered a nasty sunburn (which is caused by ultraviolet EM radiation) can attest. And some modern conveniences can exacerbate natural EM radiation. For example, when you fly in a plane at an altitude of 20,000 feet, you are exposed to far more cosmic EM radiation (the type of radiation that hits earth from extraplanetary sources and which our atmosphere helps reflect) than your body has evolved to handle. This may help explain why flight crews have a higher risk of developing cancer. One of many such studies, for example, indicates that women who have been on flight crews for more than five years have double the normal occurrence of breast cancer.1
POWER AND ENERGY
Two terms frequently used in discussing electricity and EMF are energy and power. In common use, these terms are often interchangeable. In physics, however, these are distinct concepts, and for the purposes of EMF science and safety standards, it is important to understand the difference.
Energy reflects the ability to perform work. The higher the frequency of EMF, the more energy it has (and the more work it can do). So visible light has more energy than RF, which has more energy than ELF (and so on, across the EM spectrum). Power (measured in W) reflects the rate at which work can be performed. The higher the power of a given energy, the more work the electricity can perform. So an EMF signal of 300 Hz could be generated with 5 W or 50,000 W, in which case the same signal is being radiated with very different levels of power (the 300 Hz signal generated with 50,000 W of power could travel a much greater distance).
It is a long-held belief that the less energetic EM radiation in the lower end of the EM spectrum is less damaging to the body than higher energy frequencies. ELF is less damaging than RF or MW, and X-rays are more damaging than ELF, RF, and MW. This is why criteria for safety standards are different in each range of the EM spectrum. However, we know that even low-energy EMF can cause bodily damage. A high-powered ELF field can deliver enough current to kill a person (such as a lightning strike or the electric chair), while a person does not even feel a low-powered radio signal (such as the transmission from a nearby baby monitor) that is composed of waves that can be a million times more energetic.
The fact that significant biological responses to EMF can occur across the EM spectrum, shows that the focus on energy levels in the discussion of EMF public health and safety is largely irrelevant. Nevertheless, it has been used to justify significant differences in safety limits for the different groups of EMF known as ionizing and non-ionizing radiation. Whereas ionizing radiation (the high-energy frequencies of EMF in the part of the EM spectrum above visible light) is widely regarded as hazardous to humans, low-energy non-ionizing radiation (those frequencies of EMF below visible light) has been viewed as much less harmful. This focus on energy has obscured the real biological measures of harmful responses, such as reactions with DNA. The biological studies to be discussed in the coming chapters have shown that such reactions of cells are stimulated at very low energy levels and very low power levels of EM radiation. Ignoring these potentially harmful biological reactions has led to unrealistic safety standards, especially in the non-ionizing ranges.
IONIZING RADIATION
So what is it about ionizing radiation that everyone is so afraid of? As mentioned earlier, all matter is composed of atoms, which have positively charged particles (protons), neutral particles (neutrons), and negatively charged particles (electrons). The protons and neutrons are clumped together in a nucleus, and the electrons move rapidly around the nucleus like planets around the sun. By default, in a stable atom, you find equal numbers of protons and electrons—meaning that the atom is neutral and has no net charge. Which brings us to ions.
You may recall from high school physics that an ion is a particular form of an atom (any atom) that has a charge. An ionized atom has a charge because the atom has gained or lost electrons. If the atom loses electrons, that atom is a positively charged ion; if the atom gains electrons, that atom is a negatively charged ion.
Why do ions matter in regards to EMF?
As I’ve explained, there are different frequencies of electromagnetic radiation. Those frequencies of EM radiation at the top of the spectrum are ionizing forms of radiation. Ionizing radiation vibrates at a very high frequency, with a tremendous amount of energy. So much energy, in fact, that when ionizing radiation comes into contact with an atom, it can knock an electron free from its orbit around the nucleus, and the atom becomes a positively charged ion. (The electron can then attach to another stable atom, resulting in a negatively charged ion). In this way, ionizing radiation causes neutral atoms to become charged ions.
Ionizing radiation has long been regarded as extremely dangerous to biological beings—to humans, like you, and all other living creatures. Ionizing radiation causes chemical reactions that, in turn, cause damage to biological systems (like the molecules in your body). So, for example, it has long been acknowledged that prolonged exposure to ultraviolet radiation can lead to skin cancer—this is why you put on sunblock when you go to the beach. Similarly, it is generally acknowledged that you should minimize the number of X-rays to which you are exposed because of their potential to cause damage to your body. And, of course, everyone recognizes the dangers of ionizing EM radiation leaks (in addition to the leaks of radioactive substances) from nuclear fusion reactor failures such as Chernobyl and Fukushima Daiichi.
The scientific community and the public at large recognize the risks of ionizing radiation due to this power to alter the electric charge of atoms and create ions. Forms of non-ionizing EMF, with frequencies lower than that of visible light, do not contain enough energy to force electrons loose—non-ionizing EM radiation cannot cause atoms to become ions. However, as we will see in subsequent chapters, non-ionizing EMF can cause significant chemical changes in important molecules such as DNA.
NON-IONIZING RADIATION
All of the technology and science that are discussed in this book deal with non-ionizing EMF. Cell phones, smart phones, wireless devices, and home cordless phones all generate non-ionizing RF (3 kHz to 300 gigahertz, or GHz) and MW radiation (in the range of 300 MHz to 300 GHz; microwave radiation and radio frequency radiation are often grouped together as RF/MW). Other home appliances and the power lines that feed them generate ELF (from 3 to 300 Hz), which is also non-ionizing.
It has been assumed that non-ionizing radiation and the devices that generate it are biologically safe at levels insufficient to heat human tissue. But this is not the case. As I will discuss in the coming chapters, there is a significant body of peer-reviewed, high-quality science that directly and clearly demonstrates that all forms of electromagnetic radiation—including non-ionizing radiation—have observable effects on biological systems. Biological reactions can be affected by exposure to all parts of the spectrum—even in the very low frequency ELF range. All EMF is bioactive.
OLD NEWS
As we begin our investigation of the known science linking electromagnetic radiation and negative health outcomes, I wish to emphasize that these ideas are not new. In 1891, Jacques-Arsène d’Arsonval (a French doctor and inventor of the moving-coil galvanometer, which measures electric current) and Nikola Tesla demonstrated effects of electromagnetic radiation on entire biological systems—documenting changes in bodily characteristics including perspiration, respiration, and body weight—resulting from exposure to EMF. And in 1900, V. J. Dani-lewsky (a Russian clinical investigator) wrote of the effect of “electricity at a distance,” arguing that such “long-range” electricity likely affects entire organisms (not just individual biological systems). “Dozens of monographs and thousands of articles devoted to the biological effect” of EMF followed the process of electrification in the United States.2 You will note that many studies cited in this book date from the 1960s to 1990s.
And yet we find ourselves today, more than 120 years after d’Arsonval’s 1891 paper, still debating this fundamental question: whether non-ionizing electromagnetic radiation can cause disease and other negative health effects in humans. As you will see throughout this book, science clearly demonstrates that the answer is yes. Before discussing what science tells us about the biological and health effects of exposure to electromagnetic radiation, it can be useful to examine the scope of the EMF issue. As we’ll see in the next chapter, since Thomas Edison’s invention of the mass-produced lightbulb, we have been increasing the amount of our exposure to non-ionizing EMF, to the point where today residents of industrialized nations are exposed to multiple frequencies of EMF on a near-continuous basis.