Chapter 6
Pesticides
Pesticides are organic chemicals produced by the agrochemical industry to improve agricultural yields and to fight crop disease. They include insecticides, fungicides, and herbicides, which have proved vital in increasing food production for a global population that is expected to increase by 33 per cent over the next thirty-five years. Without pesticides, food production could only be maintained by increasing the amount of land committed to crops, but this would mean turning over much of the world’s prairies, woodlands, grasslands, and meadows to agriculture—a strategy that would adversely affect biodiversity and cause unpredictable effects on the ecosystem.
Concerns over the effects of traditional pesticides has promoted research into designing safer and more environmentally friendly pesticides—a role that falls to organic chemists. It takes about ten years and £160 million to develop a new pesticide, and only large companies can afford that level of investment. Several companies specialize in agrochemical production and research, and there is a vast global market for agrochemicals. The volume of global sales increased 47 per cent in the ten-year period between 2002 and 2012, while, in 2012, total sales amounted to £31 billion. The biggest markets were in Brazil, the USA, and Japan.
In many respects, agrochemical research is similar to pharmaceutical research. The aim is to find pesticides that are toxic to ‘pests’, but relatively harmless to humans and beneficial life forms. The strategies used to achieve this goal are also similar. Selectivity can be achieved by designing agents that interact with molecular targets that are present in pests, but not other species. Another approach is to take advantage of any metabolic reactions that are unique to pests. An inactive prodrug could then be designed that is metabolized to a toxic compound in the pest, but remains harmless in other species. Finally, it might be possible to take advantage of pharmacokinetic differences between pests and other species, such that a pesticide reaches its target more easily in the pest.
Insecticides
Prior to World War II, only naturally occurring insecticides were available. For example, sulphur was used for pest control in ancient Greece, and is still used in some parts of the world today. In 1690, it was reported that tobacco extracts were effective in controlling insects, and in the early 1800s, other plant extracts were used for their insecticidal properties—namely pyrethrins from chrysanthemums and rotenone from derris roots. More recently, extracts from an Indian plant called the neem tree have proved effective. The agents responsible for the insecticidal activity of these extracts have been identified as nicotine from tobacco plants, pyrethrum from chrysanthemums, and azadirachtin from the neem tree.
Natural products are limited in their availability, selectivity, and effectiveness, and so it was not until the advent of synthetic insecticides that potent, selective, and affordable insecticides became available on an industrial scale. The early synthetic insecticides included organochlorines, organophosphates, methylcarbamates, and pyrethroids. In general, these agents were potent and showed selective toxicity against insects rather than mammals. However, their cumulative effects on the environment and other life forms were not fully anticipated at the time. They have now been largely replaced by insecticides that are more selective and environmentally friendly.
Insecticides: organochlorine agents
Organochlorine agents were the first synthetic insecticides to reach the market starting with DDT (Figure 58). DDT was first synthesized in 1874. However, its properties as an insecticide were not discovered until 1939, when it was found to be effective against mosquitoes, ticks, and locusts. This led to it being used by the military during World War II to combat malaria in South-East Asia, and typhus in Eastern Europe. After the war, DDT played a major role in eliminating malaria from Europe and North America, and, at one point, there were hopes that it might even eradicate malaria worldwide. Unfortunately, resistance to the chemical developed in more tropical areas.
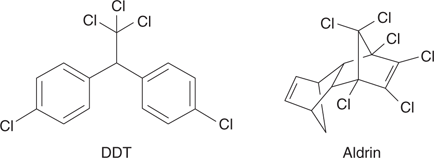
58. Examples of organochlorine insecticides.
Nevertheless, DDT has saved a vast number of lives from insect-borne diseases such as malaria, yellow fever, and sleeping sickness. One estimate puts the number of lives saved as 500 million between the 1940s and 1960s. As a result, the 1948 Nobel Prize in Medicine was awarded to Paul Muller—the man who discovered DDT’s insecticidal properties.
As well as combating disease, DDT found widespread use as an agricultural insecticide, with an average of 40,000 tons being produced each year. DDT is very toxic to insects, but has a much lower toxicity to mammals. In fact, the lethal dose for a human would be equivalent to the amount of DDT required to treat an acre of land. Unfortunately, despite the undoubted benefits of DDT in terms of lives saved and increased crop production, there was a heavy environmental cost. DDT is a relatively stable molecule, and so it accumulates in the environment. It is also hydrophobic in nature, which means that it is poorly soluble in water, but dissolves easily in the body fat of various forms of wildlife. Later research revealed that the concentration of DDT in wildlife increased the higher one goes up the animal food chain, and this proved particularly devastating for predatory birdlife. DDT was blamed for the near extinction of the bald eagle and the peregrine falcon in the USA, since it was found to cause egg shell thinning. These fragile eggs tended to break before hatching occurred, killing the embryos.
DDT was banned from agricultural use in the USA in 1972, with the UK following suit in 1984. A worldwide ban was introduced in 2004 by the Stockholm Convention, but DDT is still permitted for the eradication of insects if they are likely to be harmful to human health. For example, DDT is still used in India to control malaria.
Another example of an organochloride insecticide is aldrin (Figure 58). Like DDT, aldrin contains several chlorine atoms and is a hydrophobic molecule, but it has a totally different carbon skeleton in the form of a complex multi-ring system.
The organochlorine insecticides act on ion channels, resulting in the disruption of nerve transmission, spasms, and death. DDT acts on sodium ion channels, whereas aldrin and its analogues act on chloride ion channels. Because they act on different types of ion channels, resistance to DDT does not result in cross-resistance to aldrin. Resistance develops when mutations alter amino acids in the target ion channels. This, in turn, weakens binding interactions with insecticides.
Insecticides: methylcarbamates and organophosphates
The methylcarbamates and organophosphates were developed after the organochlorides. The design of methylcarbamates was based on a natural product called physostigmine (Figure 59), which is a poison found in calabar beans. Physostigmine inhibits an enzyme called acetylcholinesterase, which catalyses the hydrolysis of a neurotransmitter called acetylcholine (Figure 60). When the enzyme is inhibited, acetylcholine levels increase and over-stimulate protein receptors in the insect’s nervous system, leading to toxicity and death. The acetylcholinesterase enzyme is also present in humans, and so it is crucial that the methylcarbamate insecticides are selectively toxic to the insect version of the enzyme. Physostigmine itself does not have this selectivity, but carbaryl is an analogue that does.
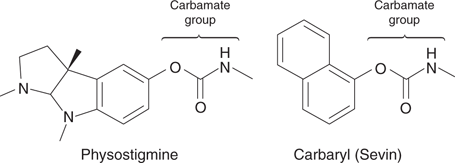
59. Physostigmine and the insecticide carbaryl.
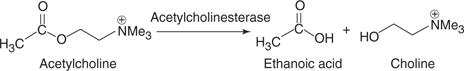
60. Hydrolysis of acetylcholine catalysed by acetylcholinesterase.
The organophosphates also target the acetylcholinesterase enzyme, and act as irreversible inhibitors. Several organophosphates are too toxic to be used as insecticides. Indeed, several have been used as nerve toxins in chemical warfare. These include sarin, tabun, soman, dyflos, and VX. In all these examples, the poison occupies the active site of the enzyme, then reacts with a serine residue. A phosphate group is transferred from the nerve agent to a serine residue and ‘caps’ it such that it can no longer catalyse the hydrolysis of acetylcholine (Figure 61).
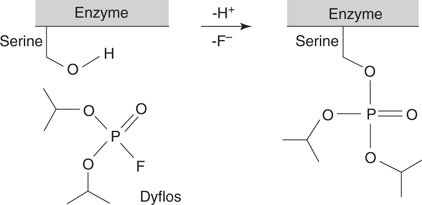
61. Reaction of dyflos with a serine residue in the active site of acetylcholinesterase.
Considering the toxicity of nerve agents, it seems a tall order to design a safe organophosphate insecticide. In fact, this was achieved by designing prodrugs that were only metabolized in insects to the active compound. Parathion, malathion, and chlorpyrifos (Figure 62) are insecticides that contain a P=S group. As such, they have no direct effect on the acetylcholinesterase enzyme. However, insects have a metabolic enzyme that alters the P=S group to a P=O group. The resulting nerve agent can then inhibit acetylcholinesterase.
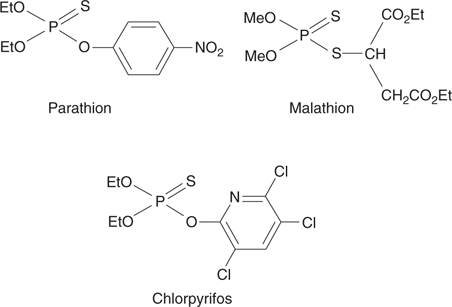
62. Organophosphate prodrugs used as insecticides.
In mammals, these insecticides are metabolized by different enzymes to give inactive compounds which are excreted. Despite this, organophosphate insecticides are not totally safe, and prolonged exposure can cause serious side effects if they are not handled with care. They also have a cumulative toxic effect on wildlife, and so alternative agents are now favoured.
Insecticides: pyrethrins and pyrethroids
Pyrethrum is a plant extract obtained by crushing chrysanthemums in water, and contains a mixture of natural products called pyrethrins. Such extracts have been used as insecticides and insect repellents for many years, and it is believed that the Chinese may have used them as early as 1000 bc. French soldiers reportedly used chrysanthemums to repel fleas and lice during the Napoleonic wars. Six pyrethrins with very similar structures have been identified so far (Figure 63). Like DDT, they bind to sodium ion channels in an insect’s nervous system, resulting in paralysis and death. A potential problem with pyrethrins is the fact that they act on the same target as DDT. This means that any pests that acquire resistance to DDT are often resistant to pyrethrins—an example of cross-resistance.
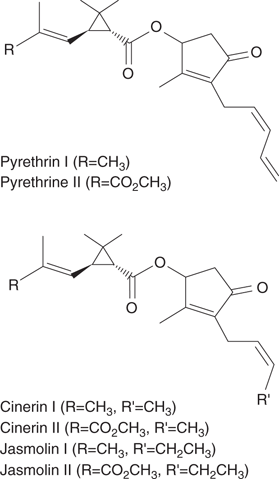
63. Structures of pyrethrins.
Combining pyrethrins with the synthetic additives piperonyl butoxide or sesamex (Figure 64) allows pyrethrins to be effective against a wider range of insects, including those that are normally resistant. This is because the synthetic additives inhibit those enzymes in the insect that normally metabolize and deactivate the pyrethrins. An agent that enhances the activity of another is called a synergist. One disadvantage with a synergist is that it could potentially inhibit mammalian metabolic enzymes and increase susceptibility to toxins.
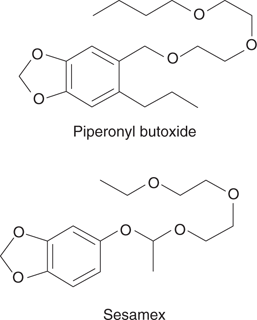
64. Examples of synergists.
The pyrethrins are considered to be among the safest insecticides on the market. Consequently, several household pesticides contain them. They are also biodegradable when exposed to light or oxygen (unlike DDT), and form harmless products. Unfortunately, pyrethrins are harmful to bees, and so they should be applied at night when bees are not pollinating.
The pyrethroids are synthetic analogues of pyrethrins and were introduced to replace organochlorines during the 1950s. They are not as biodegradable as pyrethrins, which makes them more effective as insecticides, but this also makes them more likely to accumulate in the environment. Some commercial insecticides and shampoos contain both pyrethrins and pyrethroids, and there is an element of risk in their use, especially in terms of allergies. Examples of synthetic pyrethroids include phenothrin and cypermethrin (Figure 65).
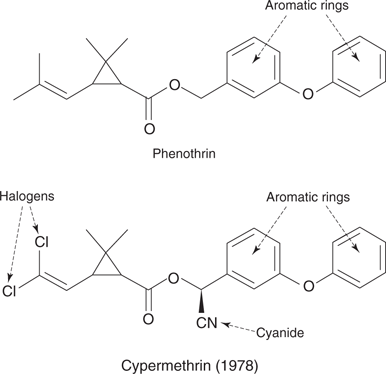
65. Examples of pyrethroids.
Insecticides: neonicotinoids
Nicotine (Figure 66) has insecticidal properties because it activates a type of cholinergic receptor called the nicotinic receptor. As a result, nerves are overstimulated, resulting in toxic effects. Although nicotine has been used as an insecticide in the form of tobacco extracts, it is not as potent as synthetic insecticides, and shows poor selectivity between the cholinergic receptors in insects and those in mammals. A large range of structurally related analogues were synthesized to try and find compounds with improved selectivity, but without success. It was not until a structurally unrelated lead compound was discovered that potent and selective agents could be developed. These also bind to the nicotinic receptor, and have been dubbed neonicotinoids.
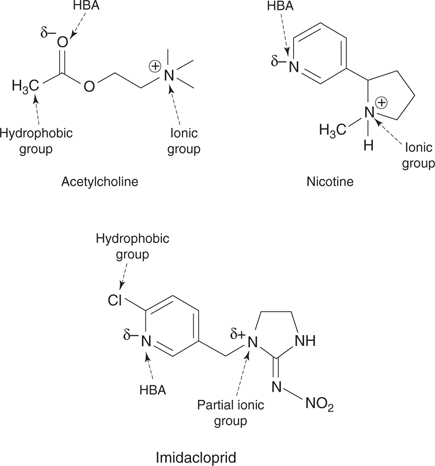
66. Important binding groups in acetylcholine, nicotine, and imidacloprid. HBA stands for hydrogen bond acceptor (see Chapter 2).
The development of the neonicotinoids began in 1970, and led ultimately to the development of imidacloprid (Figure 66) which proved 10,000 times more active than nicotine. It was patented in 1985 and introduced to the market in 1991. The compound proved extremely successful and it was the first insecticide to reach sales of a billion dollars per year. It soon became the most widely used insecticide in the world, and its development has been described as a milestone in insecticidal research. As well as its use as an agricultural insecticide, it is used in veterinary practice to control ticks and fleas. Several other neonicotinoids have since been developed and they have become established as the most important class of insecticides on the market.
Imidacloprid, nicotine, and acetylcholine share structural features that are important in binding these agents to the receptor binding site (Figure 66). They all contain a positively charged or partially positively charged (δ+) nitrogen that can interact with the receptor binding site through ionic interactions. In addition, they all contain an atom with a slight negative charge (δ−), which can form a hydrogen-bonding interaction with the binding site. Imidacloprid can form additional binding interactions because the hydrophobic chlorine substituent fits into a hydrophobic pocket in the binding site.
However, this does not explain why imidacloprid binds 1,000 times more strongly to insect receptors than human receptors—a key reason for its selectivity. A major reason for this selectivity is the presence of the nitro group, which can interact with an arginine residue that is present in the binding site of insect receptors but not mammalian receptors. A second reason is the lack of a fully positively charged nitrogen atom, which weakens the ionic interactions with the mammalian receptor. Pharmacokinetic factors also play a role in enhancing selectivity. Imidacloprid can cross the blood brain barrier of insects to attack their central nervous system, but it is unable to cross the blood brain barrier of mammals.
Neonicotinoids were originally thought to have low toxicity to bees, but many now feel that they have been responsible for a rapid decline in honey bee numbers since 2006—a phenomenon called colony collapse disorder. The situation became so serious that commercial bee-keepers in the USA had lost up to half their hives by 2012. The impact on agriculture was even greater since it is estimated that bees are responsible for pollinating US crops worth £9.8 billion. It has been suggested that neonicotinoids may affect the ability of bees to forage, learn, and remember navigation routes to and from food sources.
In 2013, the European Union decided to restrict the use of neonicotinoids until December 2015, and recommended that they be restricted to crops that did not attract bees. The US followed suit soon afterwards. This certainly marked a victory for environmentalists, but several scientists claim that the decision was taken as much for political reasons as scientific. The company Bayer Cropscience, which produces two of the three restricted products, stated that neonicotinoids are safe for bees, as long as they are used responsibly. They also argued that the decline in bee populations had begun before neonicotinoids were introduced and could be due to many other factors such as mites that carry viruses, fungal disease, and a reduction in flowering plants due to increasing agriculture. They also highlighted the fact that Australia has very healthy bees despite the widespread use of neonicotinoids. This could well be due to the lack of the varroa mite in Australia compared to its prevalence in Europe. In truth, it is difficult to be sure which specific factor is most important in causing the decline in bee populations, and it is perfectly possible that a combination of several factors are important.
Banning neonicotinoids brings its own risks. Farmers might be forced to revert back to older forms of insecticide which are more environmentally damaging. This could also increase the emergence of resistance towards these agents. Regardless of who is right or wrong, the demands for safer and more selective insecticides continue to challenge the ingenuity of research chemists. As a result, other insecticides have been developed or are under investigation. For example, a number of different structures act on the nicotinic cholinergic receptor. One of these involves a group of agents that are activated by insect metabolism to form nereistoxin—a naturally occurring neurotoxin produced by a marine annelid worm. Other compounds include the spinosyns, which are complex natural products produced by a bacterial strain called Saccharopolyspora spinosa, sulfoximines, and analogues of a natural product called stemofoline. One such structure is flupyradifurone, which has recently been approved (Figure 67).
67. Stemofoline and flupyradifurone.
Future insecticides
Insecticides are being developed that act on a range of different targets as a means of tackling resistance. If resistance should arise to an insecticide acting on one particular target, then one can switch to using an insecticide that acts on a different target. Ryanoids such as chlorantraniliprole, cyantraniliprole, and flubendiamide were marketed in 2006–7. They act by binding to calcium ion channels in muscle cells and cause paralysis and death. They show high selectivity for insects over mammals, and have very little toxicity towards birds or aquatic organisms. More recent research at the University of Newcastle has discovered a naturally occurring peptide that also targets calcium ion channels. The peptide is found in the venom of the Australian funnel web spider, and is toxic to aphids and caterpillars, but harmless to mammals and honey bees.
Several insecticides act as insect growth regulators (IGRs), and target the moulting process rather than the nervous system. In general, IGRs take longer to kill insects but are thought to cause less detrimental effects to beneficial insects. A juvenile insect grows a new exoskeleton under its old one, then sheds the old exoskeleton by moulting. This allows the new exoskeleton to swell and harden.
There are two major hormones involved in the moulting process—juvenile hormone and ecdysone. Eight varieties of juvenile hormone have been identified from different insect species, but they all contain a methyl ester at one end of the chain and an epoxide at the other end (Figure 68). Juvenile hormones must be absent if a pupa is to moult into an adult, and so several IGRs prevent insects maturing into adults by mimicking juvenile hormones. The IGRs used to mimic juvenile hormone are called juvenoids and are structural analogues of the juvenile hormone. Methoprene is the most successful of these and is considered sufficiently safe to add to drinking water cisterns in mosquito-prone areas. This has helped to control malaria and reduce the spreadof West Nile virus. It is also used to control fleas on domestic animals, and as a food additive in cattle feed to prevent fly-breeding in manure.
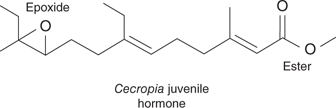
68. Example of a juvenile hormone.
The opposite approach is to prevent the production of juvenile hormones by inhibiting biosynthetic enzymes. By inhibiting the production of juvenile hormones, moulting takes place too soon and produces non-functional adults. IGRs that inhibit the enzymes JH acid methyltransferase and cytochrome P450 CYP15 are of particular interest. These enzymes are specific to insects, and so it should be feasible to design inhibitors that will have minimal side effects on mammals and other species.
IGRs that target the ecdysone receptor disrupt the transformation of larvae into adults at the pupal stage. Tebufenozide is an example of an ecdysone receptor agonist, and is effective in controlling caterpillars. The compound has high selectivity and low toxicity, earning the company that developed it (Rohm and Haas) a Presidential Green Chemistry Award.
Other IGRs inhibit the biosynthesis of chitin—an essential carbohydrate required for the exoskeleton—which means that insects are trapped in their old exoskeleton. These inhibitors are quicker acting and longer lasting than hormonal IGRs. One example is diflubenzuron, which was marketed in 1976 (Figure 69). Diflubenzuron is used primarily in forest management to control boll weevils and various types of moths. It is highly toxic to insects and relatively non-toxic to mammals.
69. Diflubenzuron.
The search for new insecticides often involves taking a leaf out of nature’s book. Several plants, fungi, and bacterial strains produce compounds that act as insecticides or repellents. For example, the bacterial strain Bacillus thuringiensis infects insects, and produces toxins that kill beetles, mosquitoes, and caterpillar larvae. Genetic engineering has been used to incorporate this bacterial toxin (Bt toxin) into plants.
Several plants emit volatile chemicals known as terpenes that act as insect repellents and could serve as the starting point for the design of new insecticides. One current research area involves synthesizing analogues of a natural product called germacrene D—a compound that repels aphids and other insect pests. A Japanese research team has also discovered that tomatoes exude a volatile chemical when they are under attack from caterpillars. This chemical acts as a chemical warning for neighbouring tomatoes, which then produce an insecticide to defend themselves against potential attack. Remarkably, this insecticide is produced from one of the alarm chemicals absorbed by the plant. It is possible that other plants have similar defence mechanisms and this could offer new approaches to the control of insects.
Fungicides
Fungicides kill fungal infections that are harmful to crops or farm animals. Some plants and organisms contain natural fungicides as a chemical defence against fungal disease. These fungicides include cinnamaldehyde, monocerin, cinnamon, citronella, jojoba, oregano, rosemary, and extracts from the tea tree and neem tree. The bacterium Bacillus subtilis and the fungus Ulocladium oudemansii can sometimes be used as fungicides, while kelp is fed to cattle to protect them from fungi in grass.
A number of synthetic fungicides produced in the laboratory have also proved effective. Examples of older fungicides include benomyl, vinclozolin, and metalaxyl. However, the more modern fungicides show better selectivity and potency. Examples of the latter include a class of compounds known as the ‘quinone outside inhibitors’ (QoI)—considered the most important development in fungicides in recent years. One example of this class of inhibitors is azoxystrobin (Figure 70), which was developed by Jealott’s Hill International Research Centre from natural antifungal agents produced by a species of European small mushroom. The key feature for antifungal activity (the toxophore) is the portion encircled. Another example are the triazole fungicides—so called because they contain a triazole ring in their structure. Prothioconazole (Figure 70) is an example of this class of fungicide and has the added bonus that it stimulates plant growth.
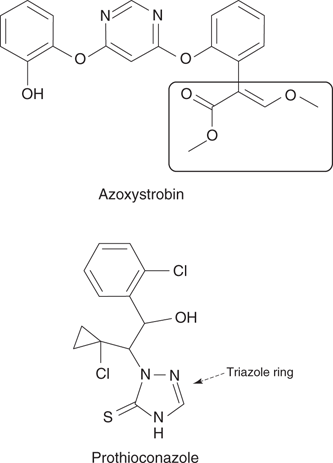
70. Examples of fungicides.
A co-formulation of fluoxastrobin (a strobilurin) and prothioconazole is commercially available under the trade name of Fandango and provides broader antifungal protection than using the two different antifungal agents on their own. Combining two fungicides that act on different targets lessens the chances of fungal strains gaining resistance. If resistance should arise to one of the fungicides, the fungal strain should still be susceptible to the other fungicide. For example, when metalaxyl was used to control potato blight in Ireland, resistance developed within one growing season. However, in the UK, resistance developed more slowly because metalaxyl was used in combination with other fungicides.
Resistance can arise due to mutations that alter key amino acids in the binding sites of target proteins. This often affects all the fungicides within a particular structural class—a property known as cross-resistance. For example, black sigatoka is a fungal disease of bananas that is resistant to all the QoI fungicides, and is caused by a mutation that replaces a glycine residue with alanine.
Herbicides
Herbicides control weeds that would otherwise compete with crops for water and soil nutrients. More is spent on herbicides than any other class of pesticide, with six billion dollars being spent in the USA alone. Common salt was used in historical times, while inorganic herbicides were used before World War II. However, these chemicals were not particularly selective and could damage crops.
Selective herbicides are necessary when treating crops, but non-selective herbicides are useful if the aim is to kill all plants on waste ground, industrial sites, and railways. Some plants produce natural herbicides that affect neighbouring plant life (a characteristic known as allelopathy). One example is the ‘tree of heaven’, which has been given less complimentary names such as the ‘stink tree’ or the ‘tree from Hell’. This is because of its foul smell and invasive properties. Leaves of the black walnut tree contain a herbicide called juglone which is toxic to apple trees and a number of plants. When the leaves fall to the ground, the chemical is released and prevents other plants from competing for available space and nutrients.
A number of synthetic herbicides have been designed that mimic the action of plant hormones called auxins (Figure 71). These plant hormones are generated in response to external environmental conditions and coordinate plant growth. Natural auxins, such as 4-chloroindole-3-acetic acid, contain a carboxylic acid and an aromatic ring.
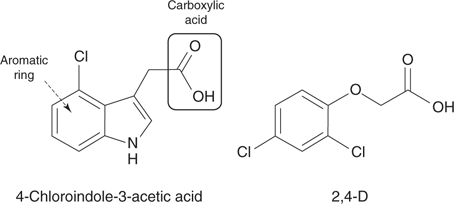
71. Examples of auxins.
The synthetic agent 2,4-D (Figure 71) contains these same functional groups and mimics the action of auxins. It was synthesized by ICI in 1940 as part of research carried out on biological weapons, and was found to kill broad-leaved weeds without harming narrow-leaf cereal crops. It was first used commercially in 1946 and proved highly successful in eradicating weeds in cereal grass crops such as wheat, maize, and rice. It is one hundred times more potent than inorganic herbicides and was largely responsible for the post-war expansion in agricultural output. The compound is easy and cheap to synthesize, and it is still the most widely used herbicide in the world.
An ester derivative of 2,4-D was one of the active constituents present in Agent Orange—the herbicide used by US forces during the Vietnam war. In the 1950s, triazine herbicides such as atrazine (Figure 72) were developed (triazine refers to the presence of a six-membered aromatic ring containing three nitrogen atoms). These agents kill weeds by inhibiting a protein that is important in photosynthesis.
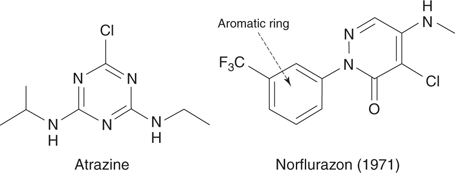
72. Miscellaneous herbicides.
During the 1970s, a group of herbicides described as bleaching herbicides were introduced to the market. These act as enzyme inhibitors that prevent the biosynthesis of photosynthetic pigments. The first of these to reach the market was norflurazon in 1971 (Figure 72).
Another useful enzyme target for herbicides is acetolactate synthase—a key enzyme in the biosynthesis of amino acids such as valine, leucine, and isoleucine. Two groups of herbicides inhibit this enzyme (Figure 73). The sulfurons were developed in the 1980s and 1990s, and include chlorsulfuron. These agents have proved extremely potent. For example, only one ounce of chlorsulfuron (Glean) is required to treat one acre of land. The carbazones were developed more recently and include propoxycarbazone-sodium.
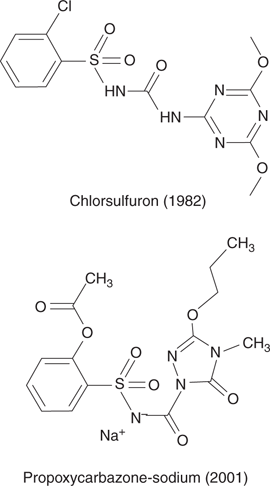
73. Examples of acetolactate synthase inhibitors.
There are several other commercially available herbicides that act on different targets. One example is glyphosate, which is used in the garden weedkiller Roundup (Figure 74). This agent inhibits an enzyme involved in the biosynthesis of phenylalanine. Glyphosate is selective for weeds, because people and animals obtain phenylalanine from their diet and do not synthesize it. In other words, the target enzyme is not present in mammalian cells.
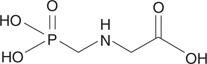
74. Glyphosate.