Chapter 2
The fundamentals
Carbon: an elemental socialite
In Chapter 1, it was stated that the number of possible carbon-based compounds is so vast that it can never be achieved. This huge number of structures is given the rather unusual term chemical space. In a sense, there is an analogy between exploring the universe and exploring the synthesis of new organic compounds. In both cases, it represents an endless task, but one which is full of exciting discoveries. In this section, we investigate why the element carbon, rather than any other element, is so suited for the generation of so many different compounds.
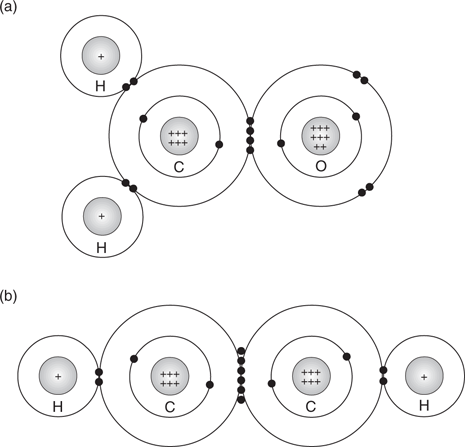
Carbon has atomic number 6, which means that it has six protons located in its nucleus. For a neutral carbon atom, there are six electrons occupying the space around the nucleus (Figure 3).
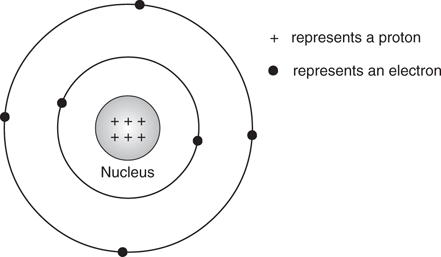
3. The carbon atom.
These electrons occupy two different shells (or orbits) around the nucleus. The first inner shell contains two electrons, which is the maximum number of electrons that it can accommodate, while the second shell (the outer shell) takes the remaining four electrons. The electrons in the outer shell are defined as the valence electrons and these determine the chemical properties of the atom. The valence electrons are easily ‘accessible’ compared to the two electrons in the first shell. The inner-shell electrons are closer to the nucleus, and hidden by the second shell of electrons.
There is great significance in carbon being in the middle of the periodic table. Elements which are close to the left-hand side of the periodic table can lose their valence electrons to form positive ions. For example, lithium can lose its only valence electron to form a positive ion 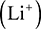 , magnesium can lose its two valence electrons to form a magnesium ion
, magnesium can lose its two valence electrons to form a magnesium ion 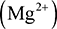 , while aluminium can lose its three valence electrons to form an aluminium ion
, while aluminium can lose its three valence electrons to form an aluminium ion 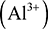 . Elements on the right-hand side of the table can gain electrons to form negatively charged ions. For example, fluorine can gain one valence electron to give a fluoride ion
. Elements on the right-hand side of the table can gain electrons to form negatively charged ions. For example, fluorine can gain one valence electron to give a fluoride ion 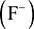 , while oxygen can gain two electrons to form an oxide ion
, while oxygen can gain two electrons to form an oxide ion 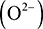 . The impetus for elements to form ions is the stability that is gained by having a full outer shell of electrons. For example, a fluoride ion has a full complement of eight electrons in its outer shell. Similarly, when a lithium ion loses its single valence shell electron, it is left with a full inner shell of electrons.
. The impetus for elements to form ions is the stability that is gained by having a full outer shell of electrons. For example, a fluoride ion has a full complement of eight electrons in its outer shell. Similarly, when a lithium ion loses its single valence shell electron, it is left with a full inner shell of electrons.
Ion formation is feasible for elements situated to the left or the right of the periodic table, but it is less feasible for elements in the middle of the table. For carbon to gain a full outer shell of electrons, it would have to lose or gain four valence electrons, but this would require far too much energy. Therefore, carbon achieves a stable, full outer shell of electrons by another method. It shares electrons with other elements to form bonds. Carbon excels in this and can be considered chemistry’s ultimate elemental socialite. Instead of living a solitary existence as an atom or an ion, a carbon atom forms bonds with other atoms to form atomic networks called molecules. The atoms are linked together by covalent bonds, with each bond containing two electrons shared between the two atoms involved.
One of the simplest organic molecules is methane, where a carbon atom shares its four valence electrons with four hydrogen atoms. Similarly, each of the hydrogen atoms shares its single valence electron with the carbon atom. Each bond is made up of two electrons—one from each atom involved in the bond. By sharing valence electrons, each atom in the molecule has a full outer shell of electrons (Figure 4). Unlike an ion, the molecule has no charge.
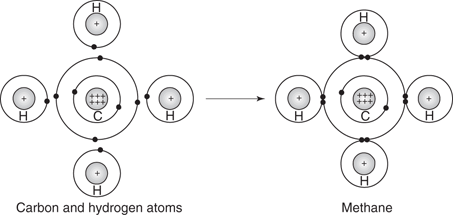
4. Methane.
A covalent bond can also be formed between two carbon atoms. For example, ethane has a covalent bond between its two carbon atoms, as well as six covalent bonds between the carbon and hydrogen atoms (Figure 5).
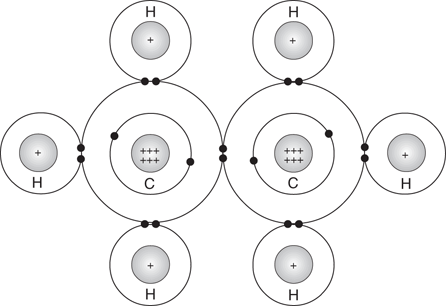
5. Ethane.
Carbon’s ability to form covalent bonds with other carbon atoms is one of the principle reasons why so many organic molecules are possible. Carbon atoms can be linked together in an almost limitless way to form a mind-blowing variety of carbon skeletons. These include linear chains, branched chains, rings, and combinations of all three. However, the variety does not stop there. Carbon can form covalent bonds to a large range of other elements. We have already seen that carbon can form a bond to hydrogen, but it can also form bonds to atoms such as nitrogen, phosphorus, oxygen, sulphur, fluorine, chlorine, bromine, and iodine. As a result, organic molecules can contain a variety of different elements. Further variety can arise because it is possible for carbon to form double bonds or triple bonds to a variety of other atoms. The most common double bonds are formed between carbon and oxygen, carbon and nitrogen, or between two carbon atoms. One example is methanal (formaldehyde) (Figure 6a). The most common triple bonds are found between carbon and nitrogen, or between two carbon atoms. An example is ethyne (acetylene) (Figure 6b).
6. (a) Methanal and (b) ethyne.
Naming compounds and identifying their structure
Each organic compound is given a specific name that accurately defines its structure using nomenclature rules determined by the International Union of Pure and Applied Chemistry (IUPAC). The more complex the structure, the more complex the name. For example, the IUPAC name for a well-known steroid hormone is (8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol. This is quite a mouthful, and so well-known compounds having fairly complex names are often identified by more user-friendly names. For example, the steroid hormone with the long IUPAC name is more commonly known as estradiol. A lot of biologically important compounds are generally identified by their common name; for example morphine, haemoglobin, and adrenaline.
Organic chemists are specifically interested in molecular structure. In the same way that an architect is interested in the structure of a building and uses plans to visualize that building, a chemist is interested in molecular architecture, and how the atoms present are linked together. Therefore, a structural representation of a molecule often means more to a chemist than its name. We have already seen a method of drawing structures in Figures 3–6, but diagrams like this take a long time to draw. A simpler method involves using a line to represent each bond, and the element symbol to represent each atom. For example, structural representations of methane, ethane, and estradiol are shown in Figure 7.
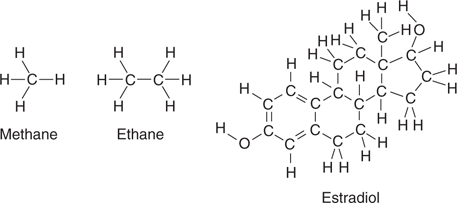
7. Structures of methane, ethane, and estradiol.
These simplified diagrams identify all the atoms that are present and the way in which they are linked. However, this approach is still unwieldy when trying to represent complex molecules such as estradiol. An even simpler ‘shorthand’ approach is to omit the carbon atom labels, and to leave out the hydrogen atoms and their bonds. This method cannot be used for methane, since the structure would end up being represented by a dot, but it can be used for ethane and estradiol (Figure 8). In structures such as these, a carbon atom is understood to be present at the end of each line, as well as at each corner. The exception to this rule is when an elemental symbol is indicated, as in the two hydroxide (OH) groups present in estradiol. The number of hydrogen atoms attached to each carbon atom can be worked out by appreciating that each carbon atom must have four bonds. If it has fewer than four, the missing bonds are assumed to be to hydrogen atoms. This can be demonstrated by comparing the structures of ethane and estradiol in Figures 7 and 8.
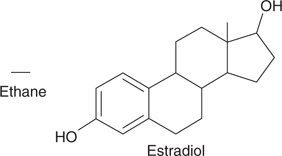
8. ‘Shorthand’ structural diagrams for ethane and estradiol.
There are several advantages in representing molecules in this way. First, they are much quicker to draw. Second, it is easier to identify the molecular skeleton. As an analogy, the skeleton of a tree is difficult to pick out in the summer because of its leaves, but easily discernible in winter when the leaves have fallen. As far as molecules are concerned, the hydrogen atoms correspond to the leaves. A third advantage of drawing molecules in this way is the ease with which functional groups (discussed later on in this chapter) can be identified.
Stereochemistry
Molecules are three-dimensional objects with particular shapes. Carbon atoms in a molecule can be described as tetrahedral, trigonal, or digonal, but this can be a bit misleading since it is not the carbon atoms themselves that have these shapes. Instead, the shapes refer to the arrangement of the bonds around the carbon atoms. Thus, methane has a central carbon atom with its four bonds pointing to the corners of a tetrahedron (Figure 9). When drawing methane, simple lines indicate the orientation of bonds that are in the plane of the paper. Solid wedge-shaped bonds represent bonds that are pointing out of the page towards the viewer. Hatched wedge-shaped bonds represent bonds that are pointing behind the page away from the viewer. In general, carbon atoms having four single bonds are described as tetrahedral carbons and the bond angles are approximately 109°.
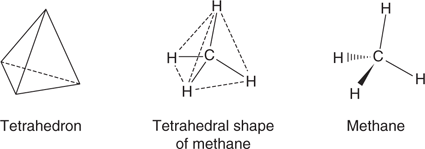
9. The tetrahedral shape of methane.
When a carbon atom is part of a double bond, it is described as trigonal, and the bonds around it are in the same plane. For example, both carbon atoms in ethene are trigonal planar, making the overall molecule planar in shape (Figure 10). The bond angles are larger than those of a tetrahedral carbon at 120°. Carbon atoms involved in a triple bond are described as digonal. Here, the bond angle is 180°, and so ethyne (also known as acetylene) is linear in shape.
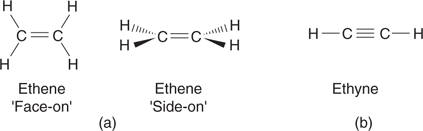
10. Shapes of ethene and ethyne.
The double bond in ethene is rigid and cannot rotate. This has important consequences in terms of stereochemistry if there are substituents at each end of the double bond. For example, two different structures are possible for 2-butene (Figure 11). These are known as the cis and the trans isomers. In the cis isomer, the methyl substituents are on the same side of the double bond, whereas in the trans isomer, they are on opposite sides. The two isomers cannot interconvert because of the rigid double bond and are different compounds with different chemical and physical properties.
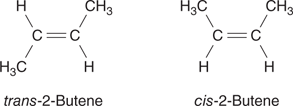
11. Cis and trans isomers of 2-butene.
It is possible to get organic molecules where the carbon atoms are linked together to form rings. These, too, can have distinctive shapes. For example, benzene and cyclohexane are both 6-membered rings (Figure 12). The carbon framework for cyclohexane is made up of six single bonds, whereas the carbon framework for benzene appears to be made up of alternating single and double bonds. In Figure 12a, the two rings look identical in shape, but if they are viewed ‘side on’, as in Figure 12b, the benzene ring is seen to be planar and the cyclohexane ring is puckered into what is termed a chair shape. This is a consequence of the different bond angles of the carbon atoms involved. The carbon atoms in cyclohexane have bond angles of 109° as in methane and ethane. In benzene, the bond angles are 120° as in ethene.
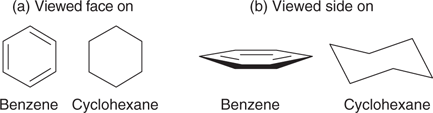
12. Benzene and cyclohexane viewed (a) face on and (b) side on.
The difference in shape between the two molecules is further emphasized if the hydrogen atoms are shown (Figure 13). In benzene, the hydrogen atoms are in the same plane as the ring, whereas in cyclohexane they are pointing in different directions. This means that cyclohexane is a much bulkier molecule.
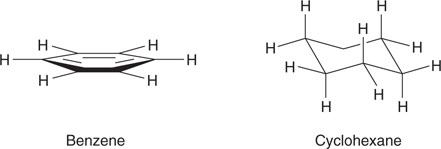
13. Side-on views of benzene and cyclohexane with hydrogen atoms included.
The carbon–carbon bond lengths in cyclohexane are identical, which is to be expected since they are all single carbon–carbon bonds. Curiously, the carbon–carbon bond lengths in benzene are also identical. This would not be expected if the ring was made up of alternating single and double bonds, since double bonds are known to be shorter than single bonds. This is evidence that there is more to benzene than meets the eye. In fact, six of the electrons involved in these double bonds are shared round the ring as a whole—a process known as delocalization. This gives the benzene ring greater stability than a molecule containing three distinct double bonds. The delocalization of the electrons in benzene is sometimes indicated by representing the benzene ring with a circle in the middle to show that the six electrons have mobility round the ring (Figure 14).
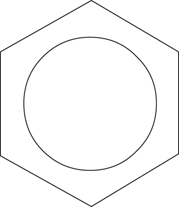
14. Representation of benzene indicating delocalization of electrons.
The stability of the benzene ring means that it is found in many natural products, and, whenever it is present, it indicates a planar region of the molecule. For example, Figure 15 illustrates a three-dimensional structure of the hormone estradiol from two different perspectives. It is clear that the region of the molecule containing the benzene ring is planar, whereas the rest of the molecule is much bulkier. The planar region has a crucial role in the biological activity of estradiol. Estradiol has to bind to a protein in the body in order to have its hormonal activity. This is only possible because the benzene ring fits into a narrow slot in the protein, something that would be impossible with a bulkier ring.
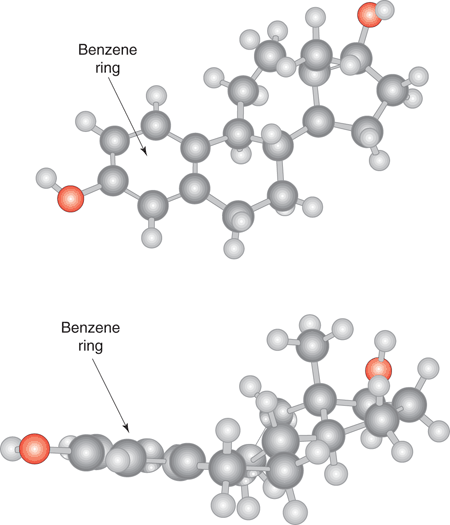
15. The shape of estradiol from two different perspectives.
Wedge-shaped bonds are often used to give an impression of a molecule’s 3D shape (Figures 9, 10, 12, and 13). They are also important in defining the relative orientation of bonds where there might be some ambiguity. For example, the structure of estradiol is often represented as shown in Figure 16. The wedge-shaped bonds define the shape (or stereochemistry) at key positions known as chiral centres.
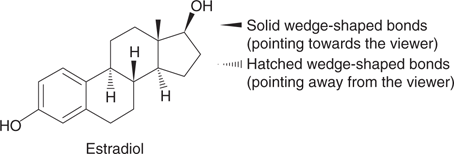
16. Structure of estradiol including wedge-shaped bonds.
A chiral carbon centre is a tetrahedral carbon atom with four single bonds to four different substituents. For example, the amino acid alanine has a chiral centre indicated by the star in Figure 17.
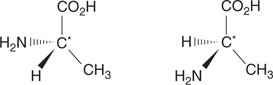
17. The two enantiomers of alanine.
Any molecule with a chiral centre is defined as being chiral or asymmetric. In other words, it lacks symmetry. There are two possible structures for such molecules, each one being a non-superimposable mirror image of the other. An asymmetric molecule must be one mirror image or the other—it cannot be both. Neither can it switch from one mirror-image structure to the other. These mirror structures are called enantiomers, and the wedge-shaped bonds are essential in distinguishing one from the other. In terms of chemistry, two enantiomers behave identically in their reactions with common chemical reagents. They also have identical physical properties.
At first sight, this might suggest that chirality is of little consequence. In fact, chirality has huge importance. The two enantiomers of a chiral molecule behave differently when they interact with other chiral molecules, and this has important consequences in the chemistry of life. As an analogy, consider your left and right hands. These are asymmetric in shape and are non-superimposable mirror images. Similarly, a pair of gloves are non-superimposable mirror images. A left hand will fit snugly into a left-hand glove, but not into a right-hand glove. In the molecular world, a similar thing occurs. The proteins in our bodies are chiral molecules which can distinguish between the enantiomers of other molecules. For example, enzymes can distinguish between the two enantiomers of a chiral compound and catalyse a reaction with one of the enantiomers but not the other.
Functional groups
A key concept in organic chemistry is the functional group. A functional group is essentially a distinctive arrangement of atoms and bonds. There are hundreds of different types of functional groups, and some of the more common ones are shown in Figure 18.
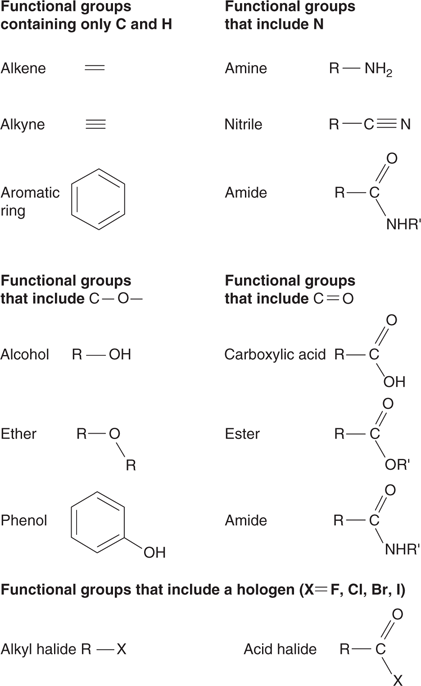
18. Examples of common functional groups (R indicates the rest of the molecule).
Functional groups react in particular ways, and so it is possible to predict how a molecule might react based on the functional groups that are present. For example, a carboxylic acid and a phenol both contain an acidic hydrogen that can be lost in the presence of a base to produce a negatively charged ion (Figure 19a and c). An amine functional group is basic in nature and can be protonated to give a positively charged ion (Figure 19b).
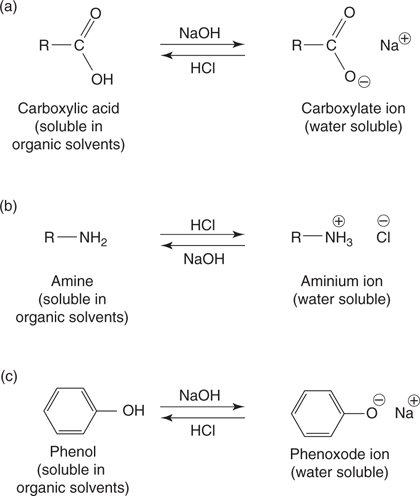
19. Ionization of (a) carboxylic acids, (b) amines, and (c) phenols.
These properties are extremely useful in practical organic chemistry as it is possible to separate compounds containing carboxylic acids, phenols, or amines from other types of organic compound in a process known as extraction (see also Figure 35). Compounds containing carboxylic acids or phenols will dissolve in aqueous base, whereas compounds containing amines will dissolve in aqueous acid. Organic compounds containing neither of these functional groups are generally insoluble in water.
By taking advantage of these properties, it is possible to extract compounds containing carboxylic acids, phenols, and amines from synthetic mixtures or from plant extracts.
Intermolecular and intramolecular interactions
The covalent bonds that link atoms within a molecule are strong and are not easily broken. However, weaker forms of bonds can exist between molecules. These are defined as intermolecular bonds. The main ones are hydrogen bonding, London dispersion forces (also known as van der Waals interactions), and ionic interactions. These interactions play an important role in the chemistry of life and in the properties of both natural and synthetic compounds. For example, the low molecular mass of water suggests that it should be a gas at room temperature. The fact that it is a liquid is due to the hydrogen bonds that exist between individual water molecules. This acts as a weak ‘glue’ between the molecules and results in a higher boiling pointthan expected since more energy is required to break the intermolecular hydrogen bonds (Figure 20). Similarly, carboxylic acids have a higher boiling point than might be expected because of hydrogen bonding.
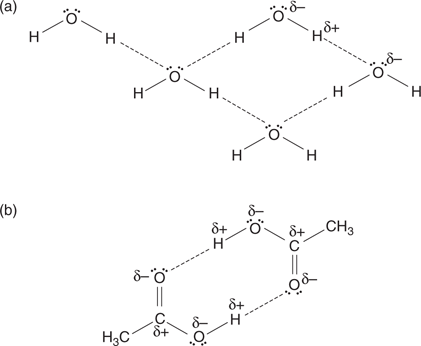
20. Hydrogen bonding between (a) water molecules and (b) carboxylic acids.
What is hydrogen bonding and how does it occur? Hydrogen bonding relies on the presence of partially charged atoms within a molecule. For example, the oxygen atom in water has a partial negative charge that is indicated with the symbol δ− in Figure 20, while the hydrogen atoms have a partial positive charge  . This partial charge results from the different electronegativities of the oxygen and hydrogen atoms making up a water molecule. Oxygen is to the right of the periodic table, and so it is more electronegative than hydrogen. As a result, oxygen has a greater pull on the electrons within each O–H bond. Since the electrons in the bond end up closer to oxygen, oxygen becomes slightly negative and hydrogen becomes slightly positive. Thus, the O–H bonds making up water are polar covalent in nature, rather than covalent.
. This partial charge results from the different electronegativities of the oxygen and hydrogen atoms making up a water molecule. Oxygen is to the right of the periodic table, and so it is more electronegative than hydrogen. As a result, oxygen has a greater pull on the electrons within each O–H bond. Since the electrons in the bond end up closer to oxygen, oxygen becomes slightly negative and hydrogen becomes slightly positive. Thus, the O–H bonds making up water are polar covalent in nature, rather than covalent.
Because of these partial charges, different molecules of water can interact with each other such that a partially charged oxygen atom in one molecule interacts with a partially charged hydrogen atom in another molecule. This is indicated by the dashed lines in Figure 20. Since the interaction is between a slightly negative charge and a slightly positive charge, the interaction can be viewed as a weak form of ionic interaction. However, the interaction is known as hydrogen bonding because a slightly positive hydrogen atom is involved. The hydrogen atom involved in the hydrogen bond is known as the hydrogen bond donor (HBD), whereas the slightly negative oxygen atom is known as the hydrogen bond acceptor (HBA).
Hydrogen bonding between molecules can occur whenever one molecule contains an electronegative atom with a partial negative charge (the HBA), and the other molecule contains a hydrogen atom with a partial positive charge (the HBD). Typically, the HBA is oxygen or nitrogen, while the HBD is a hydrogen atom linked to oxygen or nitrogen. Hydrogen bonding plays an important role in molecular recognition, such as the ability of an enzyme to recognize a substrate (Chapter 4), or the ability of a particular drug or pesticide to bind to a protein target (Chapters 5 and 6).
Intermolecular ionic interactions are possible if one molecule has a positively charged functional group, and the other molecule has a negatively charged functional group. This can occur if one molecule has an aminium group, and the other molecule has a carboxylate group (Figure 21). An ionic interaction is much stronger than a hydrogen bond.
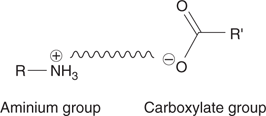
21. An ionic interaction between two opposite charges on different molecules.
London dispersion forces (or van der Waals interactions) typically occur between hydrocarbon regions of different molecules; in other words regions containing only carbon and hydrogen atoms. This interaction is much weaker than a hydrogen bond or an ionic bond, but should not be underestimated. There are often more van der Waals interactions between molecules than hydrogen bonds or ionic interactions, and so the cumulative effect of these interactions can be very significant. The interaction is possible because of the random movement of electrons around atoms and molecules. This can result in regions which are briefly electron rich or electron deficient. For any specific region, the effect is only brief and transient. Nevertheless, these transient regions of variable electron density can result in an attractive interaction between molecules, where a transient electron-rich region in one molecule interacts with a transient electron-deficient region in another.
Hydrogen bonding, ionic interactions, and dispersion forces can also take place between different regions of the same molecule. When this occurs, the interactions are defined as intramolecular rather than intermolecular. Such interactions play an important role in the way large molecules (macromolecules) such as proteins and nucleic acids fold up into particular shapes.