Of all the great transitions between major structural grades within vertebrates, the transition from basal amniotes to basal mammals is represented by the most complete and continuous fossil record, extending from the Middle Pennsylvanian to the Late Triassic and spanning some 75 to 100 million years.
JAMES HOPSON, “SYNAPSID EVOLUTION AND THE RADIATION OF NON-EUTHERIAN MAMMALS”
PROTO-MAMMALS
One of the most complete and best-documented transitions in the fossil record is the sequence that shows the evolution of mammals from the earliest amniotes (
figure 19.1). Literally hundreds of excellent specimens document almost every stage. The proper name of all these fossil “proto-mammals” is the Synapsida, a group that includes not only the ancestors of mammals, but also the mammals themselves. Paleontologists no longer use the obsolete term “mammal-like reptiles” because the mammal lineage (as represented by
Archaeothyris and
Protoclepsydrops from the Late Carboniferous) originated at the same time as, and evolved concurrently with, the earliest members of the reptile lineage (defining reptiles as turtles, snakes, lizards, and crocodiles and their relatives). At no time were the earliest ancestors of mammals part of the Reptilia. Unfortunately, obsolete terms that people learn early in their careers are hard to unlearn, so the mistaken “mammal-like reptiles” still appears widely in books and documentaries.
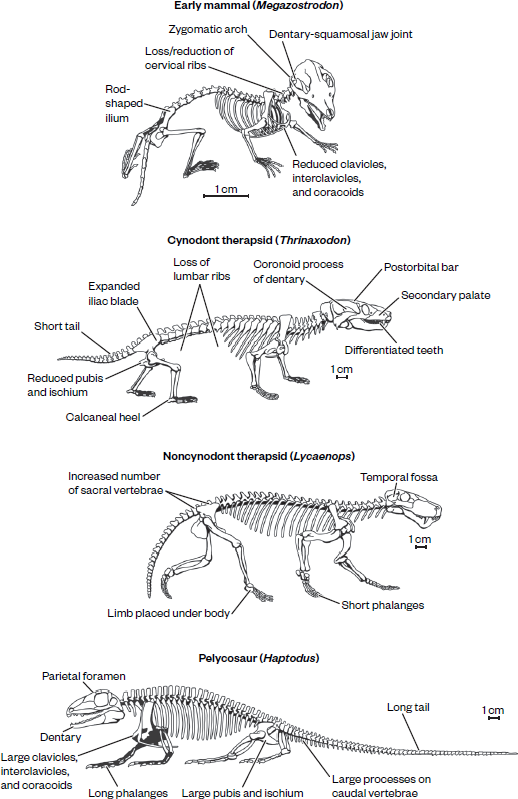
Figure 19.1
The evolution of the synapsid skeleton from that of primitive “pelycosaurs” like Haptodus, through those of noncynodont therapsids like Lycaenops and cynodonts like Thrinaxodon, to that of true mammals like Megazostrodon. (Drawing by Carl Buell; from Donald R. Prothero, Evolution: What the Fossils Say and Why It Matters [New York: Columbia University Press, 2007], fig. 13.4)
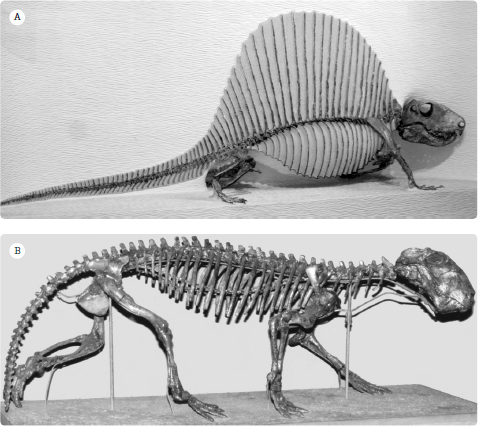
Figure 19.2
Skeletons of typical synapsids: (A) the finbacked “pelycosaur” Dimetrodon; (B) the wolf-like gorgonopsian Lycaenops. (Photographs courtesy R. Rothman)
The first well-known Synapsida are from the Early Permian red beds of northern Texas, site of the discovery of the “Frogamander” and many other important fossils (
chapter 11). The most spectacular of these synapsids are fin-backed creatures such as the huge predator
Dimetrodon (
figure 19.2; see
figure 19.1) and the herbivore
Edaphosaurus. Although these animals are often included in children’s dinosaur books and merchandise, and in plastic toy sets with dinosaurs, they have nothing to do with dinosaurs whatsoever—they are part of
our ancestry! (Sadly, much of the public thinks that if an animal is extinct, it was a dinosaur. Most merchandise of prehistoric animals contains a lot of non-dinosaurs labeled as dinosaurs, including mammoths and sabertooths; ichthyosaurs and plesiosaurs; and flying reptiles, or pterosaurs.) Being prehistoric or extinct does not make an animal into a dinosaur. Instead, being a dinosaur has to do with a specific set of unique anatomical features, including a hole through the hip socket, a distinctive hand with only three functional fingers (thumb, index, and middle finger) and reduced ring finger and pinkie; and , the joint in the middle of the ankle; among other characteristics.
Dimetrodon was the top predator in the Early Permian of Texas. It is known from many nearly complete skeletons and dozens of skulls and partial skeletons (see
figure 19.2A), since it is one of the most abundant fossils in these beds. Large individuals were more than 4.6 meters (15 feet) long, with a sail that reached about 1.7 meters (5 feet) above the ground, and they weighed up to 250 kilograms (550 pounds).
Dimetrodon had a narrow compressed skull, with strong curved jaws sporting a wicked set of conical stabbing teeth. They varied in size from the big canine-like teeth in the front of its jaw to the more simple conical teeth diminishing in size from front to back along the sides of its mouth. In fact, this feature led Edward Drinker Cope to name the genus
Dimetrodon (two-size teeth) in 1878. About the only mammalian feature in its skull besides the specialized teeth is the hole (temporal fenestra) low on the side of the head. The lower temporal fenestra is one of the defining features of the Synapsida and appears in modified form in all mammals. It probably served as an attachment point for stronger jaw muscles and allowed the muscles to bulge during chewing, a characteristic that is very important in later synapsids.
The reason for the amazing sail on Dimetrodon (and on the herbivore Edaphosaurus, which comes from the same beds) has long been controversial. The list of suggested functions is very long, but some paleontologists regard it as a device for warming or cooling its body, since Dimetrodon was cold blooded. When the sail was turned perpendicular to the sun, it would absorb heat rapidly; when it was turned parallel to the sun, it would release heat. However, since most other synapsids at that time did not have a sail for thermoregulation, other paleontologists argue that it was used for display—recognizing members of its own species and signaling its size and strength to other animals—just as large horns and antlers serve today in antelopes and deer.
In the heart of South Africa is a huge desert region called the Karoo. Like most deserts, it experiences extremes of both heat and cold, and both drought and flood. It receives an average of less than 25 centimeters (10 inches) of rain a year, most of it falling in a few huge flash floods during the limited wet season. For the South African settlers heading north out of Cape Town, it was a great barrier to cross in order to reach the grassy Highveld in the northern part of the country. The vegetation in the Karoo consists largely of succulents, such as the euphorbias, which mimic the appearance of cactuses in the New World, as well as aloes, desert ephemerals, and many other kinds of plants adapted to floods and droughts and extreme temperature change. Animals that can survive these conditions roam the Karoo, including many antelope (especially the springbok, a South African icon), wildebeest, ostriches, rare elephants, rhinos, and hippos, and at one time the half-striped species of zebra known as the quagga (now extinct). Lions, leopards, jackals, hyenas, and other carnivores preyed on them. But the introduction of irrigation has allowed sheep and cattle ranching to take hold on this poor forage, nearly wiping out the limited populations of wild animals.
The Karoo is also important in our study of life’s history. The beds of the Karoo Supergroup begin with the Dwyka Group, an Upper Carboniferous (310 million years old) unit with some of the earliest glacial deposits in Gondwana; continue through a thick sequence of Permian (300 to 250 million years old) beds of the Ecca and Beaufort groups that span the world’s greatest mass extinction (250 million years ago); and come to the end of the Beaufort Group in the Early Triassic (250 to 200 million years old). These Permian–Triassic red beds are capped by more Triassic rocks of the Stormberg Group and, finally, by Jurassic lava flows of the Drakensburg volcanics (about 180 million years old). The Beaufort Group is so rich in important Late Permian and Triassic fossils that it is the basis for telling time on land during these periods. In particular, the Beaufort has produced crucial fossils of synapsids and other Late Permian creatures that demonstrate the next phase of evolution to mammals. In some places, skulls and bones are weathering out in great abundance across the ground, and paleontologists must be selective and retrieve only the least broken and weathered skulls.
These incredible fossils were originally discovered by a Scotsman, Andrew Geddes Bain, at a road cut near Fort Beaufort in 1838. Some of the early specimens were sent to the British Museum, where pioneering paleontologist Sir Richard Owen described them. By the late nineteenth century, more and more fossils were arriving in Britain, where they caught the attention of another Scotsman, Robert Broom. As early as 1897, he realized that these fossils were not of reptiles, but of synapsids related to mammals.
Trained as a doctor and an anatomist in Glasgow, in 1903 Broom emigrated to South Africa, where he began collecting fossils as a hobby while performing his medical duties. Soon he had collected and described hundreds of specimens of Late Permian synapsids, as well as the bizarre reptiles of the Late Permian and gigantic amphibians. He became curator of vertebrate paleontology at the South African Museum in Cape Town, but the job paid very little and he was struggling to survive. His friend Raymond Dart (
chapter 24) wrote to Prime Minister Jan Smuts about this shameful situation. Consequently, Broom was hired in 1934 at the Transvaal Museum in Pretoria. There he shifted his focus to the Ice Age caves of northern South Africa, and he soon became famous for his discoveries of early hominids, including most of the specimens of
Australopithecus africanus and
Paranthropus robustus. In 1946, he received the Daniel Giraud Elliot Medal of the National Academy of Sciences, and late in life (he lived to the ripe old age of 84) he was honored for his pioneering contributions to both synapsid paleontology and paleoanthropology.
GORGON FACES, TERRIBLE HEADS, AND DOUBLE DOG TEETH
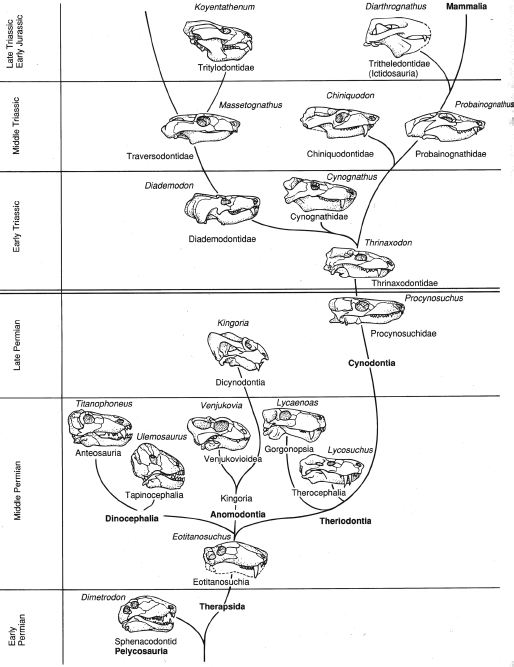
The Late Permian red beds have yielded an incredible diversity of synapsids and have demonstrated the evolution of this group over about 30 million years. Gone are the archaic fin-backed synapsids like
Dimetrodon, best known from the Early Permian of Texas (see
figure 19.2A). Instead, there are many types of more advanced and mammal-like synapsids, which have been lumped into a wastebasket group called Therapsida (
figure 19.3). Some were among the first herbivorous land animals. They included the squat creatures with a toothless beak and big canine tusks known as dicynodonts (Greek for “double dog teeth”), which reached 3.5 meters (11 feet) in length and weighed up to 1 metric ton (1.1 tons). The other herbivores were the dinocephalians (terrible heads), which sported an array of warts and bumps and thick bony battering rams on their heavily armored skulls. Some dinocephalians reached up to 4.5 meters (15 feet) in length and weighed up to 2 metric tons (2.2 tons).
Figure 19.3
The evolutionary radiation of synapsid skulls from the primitive pelycosaurs, through therapsids and cynodonts, to mammals. (From Kenneth V. Kardong, Vertebrates: Comparative Anatomy, Function, Evolution [Dubuque, Iowa: Brown, 1995]; reproduced by permission of the McGraw-Hill Companies)
Preying on these herbivores was a wide array of ferocious carnivorous therapsids, including the biarmosuchids, the therocephalians, and the bauriamorphs. The most impressive were the terrifying gorgonopsians (Greek for “Gorgon appearance”), which had huge skulls with impressive stabbing canine teeth, strong jaw muscles for chewing, and powerfully built bodies. The largest were bigger than bears, with a skull 45 centimeters (18 inches) long, saber teeth over 12 centimeters (4.7 inches) long, and a long sprawling crocodile-like body up to 3.5 meters (11 feet) long.
Throughout the evolution of these therapsids in the Late Permian, more and more mammal-like features appeared. The small opening on the side of the skull in Dimetrodon became a large expanded arch behind the eye into which strong jaw muscles could bulge and allow powerful bite forces and even some chewing. The original reptilian palate began to be covered by a secondary palate, which grew over it and enclosed the nasal passages. (You can feel it if you run your tongue over the roof of your mouth.) The secondary palate allowed therapsids that had it to chew a mouthful of food and breathe at the same time, essential to an animal with a fast metabolism. By contrast, a typical reptile (like a snake or lizard) must hold its breath until its prey is swallowed, but it has a slow metabolism.
Instead of a single ball joint on the back of the skull just below the spinal cord connecting to the neck, therapsids had a double ball joint, allowing for greater strength and flexibility in their neck muscles. Therapsids also showed many modifications of the skeleton (see
figure 19.1) that make them more mammalian in appearance than earlier synapsids, including a posture that which no longer sprawled on the belly like a crocodile, but held the body in a semi-sprawling to nearly upright position.
AN EARFUL OF JAWBONES
The most amazing transformation, however, occurred in the jaws and ear region. Primitive synapsids like
Dimetrodon had a jawbone composed of the primary tooth-bearing bone, the dentary, and a suite of other nondentary bones in the back of the jaw: the angular, surangular, splenials, articular, coronoids, and often more (
figure 19.4). The articular bone is particularly important, since it forms the jaw joint against the hinge of the quadrate bone of the skull. But all these extra bones and their sutures in the back of the jaw made the jaw apparatus complex and weaker than if it were a single bone, a disadvantage when the therapsids evolved complex chewing. Thus as therapsids became more and more specialized for chewing and other complex jaw motions, the dentary bone expanded backward and crowded out the nondentary bones in the back of the jaw. Eventually, these bones became tiny and eventually were lost as their function diminished.
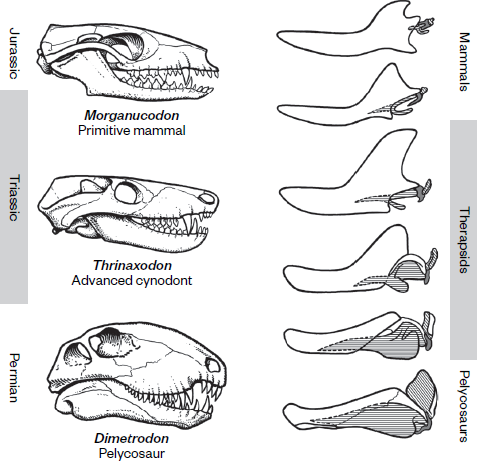
Figure 19.4
The gradual transformation of the jawbones during synapsid evolution, as the nondentary jaw elements (shaded) are reduced, while the dentary bone (unshaded) expands backward and crowds them out. All the nondentary jaw elements are lost in mammals except for the articular bone of the jaw, which joins with the quadrate bone of the skull to become the bones of the middle ear. (Drawing by Carl Buell; from Donald R. Prothero, Evolution: What the Fossils Say and Why It Matters [New York: Columbia University Press, 2007], fig. 13.5)
The exception was the articular bone, still attached to the quadrate bone of the skull and serving as the jaw joint. Eventually, the expanded dentary bone made contact with another skull bone, the squamosal, and a new jaw joint was born. In a few synapsids, such as
Diarthrognathus (Greek for “double jaw joint”),
both the dentary/squamosal jaw joint and the quadrate/articular jaw joint operated side-by-side, so this animal was literally double jointed on each side of its jaw.
What happened when the dentary/squamosal joint finally took over completely? Did the quadrate/articular joint vanish? No. Instead, in an amazing feat of evolutionary opportunism, it transformed into the bones of the middle ear! The quadrate is the incus, or “anvil,” and the articular is the malleus, or “hammer,” of the “hammer, anvil, stirrup” that carry vibrations from the eardrum to the inner ear. This may sound incredible, but the fossils prove it. It makes a lot of sense, since many reptiles hear only when their jaw picks up vibrations from the ground, since the quadrate/articular joint has the dual function of both ear bone and jaw joint.
If this still seems incredible, it has happened to you and to every other mammal in your own lifetime. When you were an early embryo, the cartilage predecessors of the quadrate and articular were in your embryonic jaw cartilage. As you developed embryonically, they moved to your middle ear—just as they had over the evolutionary history of synapsids.
THRINAXODON EVOLVING
Then the greatest extinction in Earth history occurred at the end of the Permian (about 250 million years ago), wiping out about 70 percent of the animals on land, including insects, and 95 percent of the animals in the ocean. The causes of the great Permian extinction (“the mother of all mass extinctions” in the words of Douglas Erwin) were complex, but the event was apparently triggered by huge volcanic lava flows pouring across most of northern Siberia. The lava injected large amounts of greenhouse gases (especially carbon dioxide) into the atmosphere and oceans. Earth became a “super-greenhouse” planet, and the oceans then became supersaturated in carbon dioxide, making them extremely hot and acidic and killing nearly everything that lived in them. The atmosphere became too low in oxygen and too loaded with carbon dioxide, so nearly all the terrestrial animals above a certain size vanished, and only a few smaller lineages of synapsids, reptiles, amphibians, and other land creatures made it through the hellish planet of the latest Permian and survived into the aftermath world of the earliest Triassic.
After the Late Permian therapsids nearly vanished in the mass extinction, the synapsids started all over again with a third great evolutionary radiation of much more mammal-like synapsids called cynodonts (Greek for “dog toothed”) (see
figure 19.3) . They included forms as big as a bear called
Cynognathus (dog jaw), which was 1 to 2 meters (3.3 to 6.6 feet) long, with a head over 60 centimeters (24 inches) in length, and many smaller species in the size range of raccoons and weasels. Most cynodonts had advanced postures, with their limbs completely under their body for rapid running (see
figure 19.1). Their nondentary jawbones were tiny and had been reduced to mere splints in the inside back part of the jaw near the hinge. They had secondary palates going all the way back to the throat, as the palate does in modern mammals, and many other indicators of active living and rapid metabolism. And many had multicusped cheek teeth instead of the simple conical pegs of the primitive synapsids, suggesting that they were capable of complex chewing motions, rather than gulping food whole, as do reptiles.
The transition from primitive amniotes to mammals is demonstrated by such a wealth of transitional fossils within the Synapsida that it is impossible to pick one specific fossil as the most crucial “missing link.” If we must pick one,
Thrinaxodon is as good as any (
figure 19.5; see
figure 19.1).
Thrinaxodon represents the start of the cynodont radiation of synapsids after the Early Permian finbacks and the Middle to Late Permian therapsids of the Karoo (see
figure 19.4).
Thrinaxodon was one of the earliest cynodonts, the first fossil to show many of the advanced features of the final phase of the evolution of synapsids into mammals. It was quite common in the Early Triassic (250 to 245 million years ago) of the Beaufort Group in South Africa, so many nearly complete specimens are available, and its anatomy and behavior are better known than are those of most other synapsids.
Figure 19.5
Thrinaxodon was an Early Triassic weasel-shaped advanced cynodont with many mammal-like features, including hair, a diaphragm, and advanced teeth that enabled chewing: (A) skull of a juvenile, showing the distinctive three-cusped molar teeth that gave the animal its name; (B) two individuals curled up together and buried in their burrow; (C) reconstruction of its appearance in life. ([A–B] courtesy Roger L. Smith, Iziko South African Museum, Cape Town; [C] courtesy Nobumichi Tamura)
There are two species of
Thrinaxodon, and both are about the size and shape of a weasel, with a long narrow snout and a long slender low-slung body with short legs. They were typically 30 to 50 centimeters (12 to 24 inches) in length. The dentary bone of
Thrinaxodon dominates its entire jaw, so the nondentary bones were tiny splints—although it still had the reptilian quadrate/articular jaw joint (see
figure 19.4).
Thrinaxodon had a complete secondary palate, so it could breathe and eat at the same time. It had large eyes (for seeing in the dark or in burrows) and a relatively large head. Like those of its descendants, its cheek teeth were not simple conical pegs, but had complex cusps and could be rightfully called molars and premolars. In fact,
Thrinaxodon is Greek for “trident tooth,” referring to the three-cusped molar teeth in its mouth (see
figure 19.5A). The temporal opening for the muscles on the side and top of its head was unusually large, allowing for complex chewing motions of the jaw. Yet unlike most mammals,
Thrinaxodon still had a bony bar that separated the temporal jaw opening from the eye socket.
On each side of its snout were tiny pits in the bone, suggesting that it had whiskers. If Thrinaxodon had hair on its snout, it’s a good bet that it had hair over its entire body. Hair normally does not fossilize, so this may be the first evidence of hair in the mammalian lineage.
Even though
Thrinaxodon had short legs, its posture placed its legs beneath its body in a semi-sprawling stance (see
figure 19.1). It had advanced shoulder bones and broad hip bones (especially the iliac blade, which attaches the hips to the spinal column and anchors the leg muscles), much like those of the more advanced cynodonts and mammals. Ribs are evident only in the chest region around the lungs; all the ribs of the lower back are lost, as in mammals. This allowed
Thrinaxodon to bend its back sharply, turn around in a small space, and curl up tightly (see
figure 19.5B). Even more revealing,
Thrinaxodon had broad flanges on its thoracic ribs that would have made the rib cage fairly solid and immobile, thus preventing the kind of rib-assisted breathing found in most reptiles (and apparently in primitive synapsids). Instead,
Thrinaxodon must have had a muscular wall between the lung cavity and the abdominal cavity, known as the diaphragm, which helps pump air into and out of the lungs. This muscle is found in all mammals. Putting all these clues together—complex cheek teeth, whiskers, diaphragm—suggests that
Thrinaxodon was extremely mammal-like, probably was covered in fur, and had a high metabolic rate and warm-blooded physiology.
In addition, a number of complete articulated
Thrinaxodon specimens have been found in what appear to be shallow burrows (see
figure 19.5B). Sometimes two or more individuals were trapped in a den, and fossils of a
Thrinaxodon and an amphibian,
Broomistega, were found together in a burrow. Whether the amphibian was prey for the cynodont, or both were seeking shelter and had crawled into the burrow for protection from the flash flood that buried them, or some other cause, it’s an odd association.
Thrinaxodon is the perfect transitional fossil between the reptilian features of most primitive synapsids and the more mammalian features of advanced cynodonts. It was extremely mammal-like in its small size, body hair, complex teeth and chewing capability, and high metabolism, yet it still had reptilian jawbones and jaw joint, reptilian bones in its shoulder, and some other primitive features. It lived in burrows as protection from the harsh world of the Triassic aftermath of the great Permian extinction, with its low level of atmospheric oxygen, thin ozone layer, and high level of atmospheric carbon dioxide. Burrows also would have provided protection against the much larger predators of the time and (together with the large eyes) suggest that Thrinaxodon emerged mostly at night to hunt. Given its size, it was probably a predator on small reptiles, or especially, insects and other arthropods, which would have been abundant in a world cleared of most of their predators.
Thrinaxodon had vanished by the Middle Triassic, but its more advanced cynodont descendants took over the world. They continued to dominate the Triassic, even as other groups of animals (especially the primitive relatives of crocodiles and the earliest dinosaurs) began to appear. By the latest Triassic, cynodonts were dying out, and the first unquestioned mammals (with a dentary/squamosal joint and complex molar teeth) had emerged (see
figures 19.1 and
19.3). They were only shrew-size creatures, but they were living in a world dominated by the rise of the huge dinosaurs. For the next 120 million years (two-thirds of the history of mammals), these Mesozoic mammals remained small (shrew- to rat-size) and evolved complex teeth and other features. They hid from the dinosaurs in the underbrush or came out mostly at night when the dinosaurs were asleep. Then 65 million years ago, the nonavian dinosaurs vanished, and mammals inherited the planet.
SEE IT FOR YOURSELF!
Many large museums display Dimetrodon and a number of other synapsids from the Early Permian red beds of Texas. They include the American Museum of Natural History, New York; Denver Museum of Nature and Science; Field Museum of Natural History, Chicago; Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts; and Sam Noble Oklahoma Museum of Natural History, University of Oklahoma, Norman.
Most of the Late Permian and Early Triassic synapsids are exhibited in museums in South Africa and in Russia, near where they were found, but the American Museum of Natural History does have some of these fossils as well.
FOR FURTHER READING
Chinsamy-Turan, Anusuya, ed. Forerunners of Mammals: Radiation, Histology, Biology. Bloomington: Indiana University Press, 2011.
Hopson, James A. “Synapsid Evolution and the Radiation of Non-Eutherian Mammals.” In Major Features of Vertebrate Evolution, edited by Donald R. Prothero and Robert M. Schoch, 190–219. Knoxville, Tenn.: Paleontological Society, 1994.
Hotton, Nicholas, III, Paul D. MacLean, Jan J. Roth, and E. Carol Roth, eds. The Ecology and Biology of Mammal-like Reptiles. Washington, D.C.: Smithsonian Institution Press, 1986.
Kemp, Thomas S. “Interrelationships of the Synapsida.” In The Phylogeny and Classification of the Tetrapods. Vol. 2. Mammals, edited by Michael J. Benton, 1–22. Oxford: Clarendon Press, 1988.
——. Mammal-Like Reptiles and the Origin of Mammals. London: Academic Press, 1982.
——. The Origin and Evolution of Mammals. Oxford: Oxford University Press, 2005.
Kielan-Jaworowska, Zofia, Richard L. Cifelli, and Xhe-Xi Luo. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. New York: Columbia University Press, 2004.
King, Gillian. The Dicynodonts: A Study in Palaeobiology. London: Chapman & Hall, 1990.
McLoughlin, John C. Synapsida: A New Look into the Origin of Mammals. New York: Viking, 1980.
Peters, David. From the Beginning: The Story of Human Evolution. New York: Morrow, 1991.