10
The Neural Dark Ages
FROM 420 TO 375 million years ago, oceans became filled with progressively more diverse predatory fish of many shapes and sizes. What would have resembled the sharks and stingrays of today were common sightings. Twenty-foot-long placoderms, fish with armored head plates and thick bone-crushing teeth, found themselves at the top of this food chain.
Arthropods and other invertebrates were relegated to various niches. Some got smaller. Some evolved thicker shells. Some even took a cue from early vertebrates and survived by getting smarter—it was during this period that the cephalopods emerged, the ancestors of today’s squids and octopuses. Under severe pressure to survive their mass hunting by fish, cephalopods became impressively intelligent down an independent lineage with brains that work very differently than our own.
The most radical invertebrate survival strategy was to escape the sea all together. The arthropods, driven from their homeland by relentless predation, were the first animals to walk out of the oceans and populate the land. They found respite among the small leafless land-faring plants that had sparsely sprouted along the seashores.
The period between 420 to 375 million years ago is called the Devonian period, and it was here when land plants first evolved leaves for better absorption of sunlight and seeds for spreading, both of which enabled plants to propagate to previously inhospitable areas. Plants that resembled today’s trees first developed, growing thick roots and creating stable soils for nearby arthropods to live. In the early Devonian period, plants on land were no more than thirty centimeters tall, but by the end of it, they were thirty meters tall. It was only at this point that our planet began to appear green from above, as land plants spread across Earth’s surface.
While life for arthropods was horrifying in the sea, it was heavenly on land. Arthropods developed new tricks to meet the needs of life on land, diversifying into what resembled today’s spiders and insects. Unfortunately, as we have seen with today’s problem of climate change, Earth’s biosphere is unforgiving to those who proliferate rapidly and unsustainably. What began as a small oasis for arthropod refugees eventually became an overextended orgy of plant life, triggering a global extinction event that would eradicate close to half of all life.
A Story of Two Great Deaths
History repeats itself.
One and a half billion years ago, the explosion of cyanobacteria suffocated the Earth with carbon dioxide and polluted it with oxygen. Over a billion years later, the explosion of plants on land seems to have committed a similar crime.
The inland march of plants was too rapid for evolution to accommodate and rebalance carbon dioxide levels through the expansion of more CO2-producing animals. Carbon dioxide levels plummeted, which caused the climate to cool. The oceans froze over and gradually became inhospitable to life. This was the Late Devonian Extinction, the first great death of this era. There are competing theories of what caused it; some argue that it was not overproliferation of plants but some other natural disaster. In any case, it was from the icy graves of this tragedy that our ancestors emerged from the sea.
Extinction events create opportunities for small niches to transform into dominant strategies. Before the Late Devonian Extinction, our ancestors had found such a niche. Most fish stayed far away from the shore to avoid deadly beaching, a situation where a fish becomes stuck on land as tides recede. Although the risk of beaching made it dangerous to pursue, there was a big nutritional prize to be found close to the shore: the warm earthy puddles were full of small insects and vegetation.
Our ancestors were the first fish to evolve the ability to survive out of water. They developed a pair of lungs that augmented their gills, enabling them to extract oxygen from both water and air. And so our ancestors would use their fins both for swimming in water and for wading themselves short distances on land, traveling from puddle to puddle in search of insects.
When the Late Devonian Extinction Event began to freeze over the oceans, our air-breathing and land-walking ancestors were one of the few warm-water fish to survive. As the food supply in warm waters began to die, our ancestors spent more of their time living in the inland puddles. They lost their gills (and thus their ability to breath underwater), and their webbed fins gave way to fingered hands and feet. They became the first tetrapods (tetra for “four” and pods for “feet”), most closely resembling a modern amphibian such as a salamander.
One evolutionary lineage of tetrapods, who were lucky enough to live in parts of the Earth that still supported these warmer puddles, would maintain this lifestyle for hundreds of millions of years—they would become the amphibians of today. Another lineage abandoned the dying shores and wandered farther inland in search of food. This was the lineage of amniotes—the creatures that developed the ability to lay leathery eggs that could survive out of the water.
The first amniotes probably best resembled a lizard of today. Amniotes found an inland ecosystem abundant with food—insects and plants were everywhere for the feasting. Eventually, the Devonian ice age faded and amniotes spread and diversified to all corners of the Earth. The Carboniferous and Permian eras, which collectively lasted from 350 million years ago to 250 million years ago, saw an explosion of amniotes on land.
Living on land presented unique challenges to the amniotes that their fish cousins never faced. One such challenge was temperature fluctuations. Cycles of the day and season create only muted temperature changes deep in the oceans. In contrast, temperatures can fluctuate dramatically on the surface. Amniotes, like fish, were cold-blooded—their only strategy for regulating their body temperature was to physically relocate to warmer places.
One amniote lineage were the reptiles, who would eventually diversify into dinosaurs, lizards, snakes, and turtles. Most of these reptiles dealt with daily temperature fluctuations by becoming immobile at night. Temperatures were too low for their muscles and metabolisms to function properly, so they simply shut down. The fact that reptiles were shut down for a third of their lives presented an opportunity—creatures that could hunt in the night would reap an incredible feast of motionless lizards.
The other lineage of amniotes were our ancestors: the therapsids. The therapsids differed from reptiles at the time in one important way: they developed warm-bloodedness. Therapsids were the first vertebrates to evolve the ability to use energy to generate their own internal heat.* This was a gamble. They would require far more food to survive, but in return they had the ability to hunt at any time, including the cold nights when their reptile cousins lay immobile—an easy feast offered on a Permian platter.
In the Permian era, when the land was full of edible reptiles and arthropods, this gamble paid off. During the period from 300 to 250 million years ago, therapsids became the most successful land animals. They grew to the size of a modern tiger and began to grow hair to further maintain their heat. These therapsids would have looked like large hairy lizards.
Perhaps you can already see a trend emerging from the evolutionary history of life on Earth: all reigns come to an end. The therapsid reign on Earth was no different: the Permian-Triassic mass extinction event, which occurred around 250 million years ago, was the deadliest of all extinction events in Earth’s history. It was the second great death in this era. This extinction event was the most severe and perhaps the most enigmatic. Within five to ten million years, 96 percent of all marine life died, and 70 percent of all land life died. There is still controversy over what caused this—theories include asteroids, volcanic explosions, and methane-producing microbes. Some suggest that it was no single reason but rather a perfect storm of multiple unlucky occurrences. Regardless of the cause, we know the effects.
The large therapsids went almost entirely extinct. The gamble of warm-bloodedness that originally facilitated their rise was also the cause of their downfall. During a period of reduced access to food, the therapsids, with their need for huge amounts of calories, died first. The reptiles and their comparatively scant diets were much better suited to weather this storm.
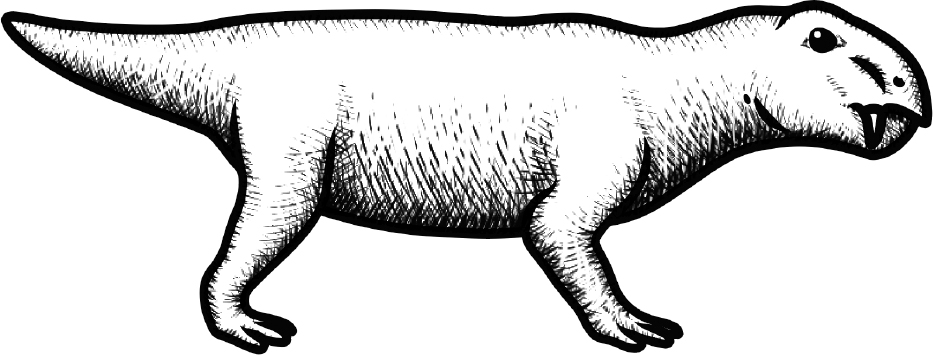
Figure 10.1: The first therapsid
Original art by Rebecca Gelernter
For about five million years, life survived only in tiny pockets of the world. The only therapsids that survived were the small plant-eating ones, such as the burrowing cynodonts. The cynodonts originally evolved into the niche of burrowing underground to hide from the larger and more predatory therapsids that dominated the world. As food supply went away and all those bigger animals died off, these small cynodonts were among the few surviving therapsids to emerge on the other side of the Permian-Triassic extinction.
Although the therapsid lineage was just barely preserved by the small cynodont, the world they found themselves in was different. On the other side of this extinction event, with 70 percent of land life extinguished, reptiles emerged numerous, diverse, and big. The eradication of the large therapsids handed the animal kingdom to their scaly reptilian cousins. From the end of this extinction event and for the next one hundred fifty million years, reptiles would rule.
Small lizards of the Permian evolved into twenty-foot-long predatory archosaurs with massive teeth and claws, resembling a smaller Tyrannosaurus. It was also during this period that vertebrates took to the skies—the pterosaur, a flying archosaur, was the first to grow wings and hunt from above.
In order to survive this ravenous era of predatory dinosaurs, pterosaurs, and other massive reptilian beasts, cynodonts got smaller and smaller until they were no more than four inches long. Equipped with warm-bloodedness and miniaturization, they survived by hiding in burrows during the day and emerging during the cold night when archosaurs were relatively blind and immobile. They made their homes in dug-out burrowed mazes or in the thick bark of trees. They hunted by quietly wandering the twilight forest floors and tree branches in search of insects. They became the first mammals.
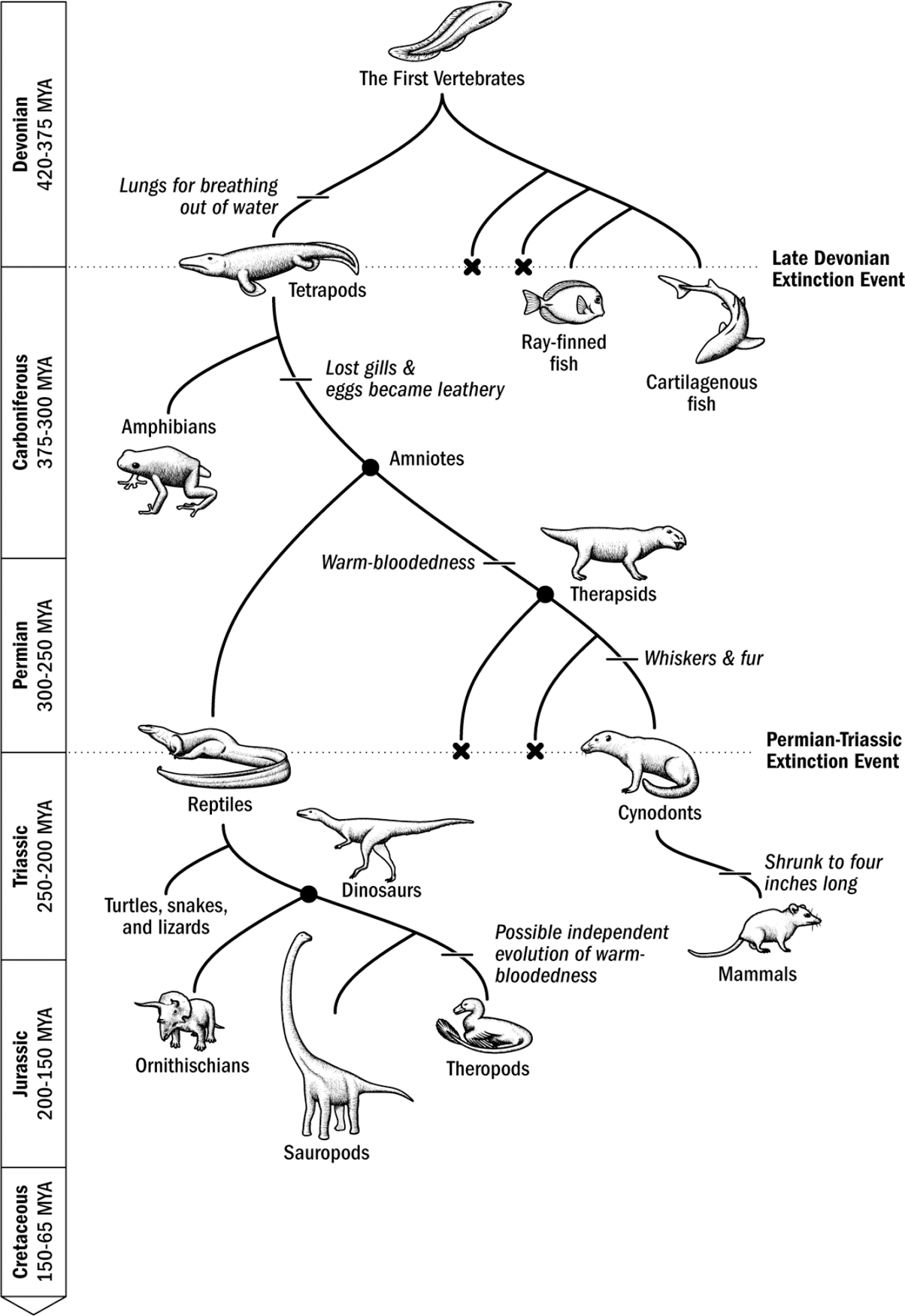
Figure 10.2: The evolutionary tree from the first vertebrates to the first mammals. MYA = million years ago.
Original art by Rebecca Gelernter
At some point in this hundred-million-year reign of dinosaurs, as these small mammals survived tucked away in nooks and crannies of the world, they added one more survival trick to their repertoire. They evolved a new cognitive ability, the biggest neural innovation since the Cambrian fish.
Surviving by Simulating
This early four-inch-long mammal, likely resembling a mouse or squirrel of today, was not stronger than dinosaurs or birds and surely unable to fight its way out of a predatory assault. It was probably also slower, or at least no faster, than an archosaur or a pterosaur swooping down from the sky. But the burrowing and arboreal lifestyle did indeed give early mammals a singular advantage: they got to make the first move. From an underground burrow or from behind a tree branch, they got to look around, spot a faraway bird and a tasty insect, and decide whether to make a run for it. This gift of the first move was left unexploited for hundreds of millions of years. But eventually a neural innovation emerged to exploit it: a region of the cortex transformed, through a currently unknown series of events, into a new region called the neocortex (neo for “new”).
The neocortex gave this small mouse a superpower—the ability to simulate actions before they occurred. It could look out at a web of branches leading from its hole to a tasty insect. It could see the faraway eyes of a nearby predatory bird. The mouse could simulate going down different paths, simulate the bird chasing it and the insects hopping away, then pick the best path—the one that, in its simulation, it found itself both alive and well fed. If the reinforcement-learning early vertebrates got the power of learning by doing, then early mammals got the even more impressive power of learning before doing—of learning by imagining.
Many creatures had previously found themselves in positions of having the first move—crabs hide under sand and small fish weave between the leaves of coral plants. So then why was it only with mammals that simulating emerged?
It has been speculated that there were two requirements for simulating to evolve. First, you need far-ranging vision—you need to be able to see a lot of your surroundings in order for simulating paths to be fruitful. On land, even at night, you can see up to one hundred times farther than you can underwater. Thus, fish opted not to simulate and plan their movements but instead to respond quickly whenever something came at them (hence their large midbrain and hindbrain, and comparatively smaller cortex).
The second speculated requirement is warm-bloodedness. For reasons we will see in the next few chapters, simulating actions is astronomically more computationally expensive and time-consuming than the reinforcement-learning mechanisms in the cortex-basal-ganglia system. The electrical signaling of neurons is highly sensitive to temperature—at lower temperatures, neurons fire much more slowly than at warmer temperatures. This meant that a side effect of warm-bloodedness was that mammal brains could operate much faster than fish or reptile brains. This made it possible to perform substantially more complex computations. This is why reptiles, despite their long-range vision on land, were never endowed with the gift of simulating. The only nonmammals that have shown evidence of the ability to simulate actions and plan are birds. And birds are, conspicuously, the only nonmammal species alive today that independently evolved warm-bloodedness.
Inside the Brain of the First Mammals
Throughout this several-hundred-million-year-long story, from the emergence of fish onto land to the rise of dinosaurs, there was an expansive diversification of animal shapes, sizes, and organs. And yet, there was one thing that was surprisingly unchanged: brains.
From the early vertebrates to the first tetrapods to reptiles and therapsids, brains were largely stuck in a neural dark age. Evolution settled for, or at least was resigned to, the reinforcement-learning brain of the early vertebrates, and it shifted its focus toward tweaking other biological structures—creating jaws, armor, lungs, more ergonomic bodies, warm-bloodedness, scales, fur, and other such morphological modifications. This is why the brain of a modern fish and that of a modern reptile, despite hundreds of millions of years of evolutionary separation, are remarkably similar.*
It was only in early mammals that a spark of innovation emerged from the eternity of neural stagnation. The fish cortex split into four separate structures in early mammals, three of which were effectively the same as the subregions that had come before, only one of which, the neocortex, could truly be considered new. The ventral cortex of early vertebrates became the associative amygdala in mammals, containing similar circuitry and serving largely the same purpose: learning to recognize patterns across various modalities, especially those that were predictive of valence outcomes (e.g., predicting that sound A leads to good things and sound B leads to bad things). The smell-pattern detectors in the lateral cortex of early vertebrates became the olfactory cortex in mammals, working the same way—detecting smell patterns through auto-associative networks. The medial cortex of early vertebrates, where spatial maps were learned, became the hippocampus of mammals, performing a similar function using similar circuitry. But a fourth region of the cortex underwent a more meaningful change—it transformed into the neocortex, which contained completely different circuitry.
Other than the emergence of the neocortex, the brain of early mammals was largely the same as that of early vertebrates. The basal ganglia integrated input about the world from the olfactory cortex, hippocampus, amygdala, and now also the neocortex to learn to take actions that maximized dopamine release. The hypothalamus still triggered direct valence responses and modulated other structures through neuromodulators such as dopamine. Midbrain and hindbrain structures still implemented reflexive movement patterns, albeit now specialized for walking as opposed to swimming.

Figure 10.3: How the cortex changed in the transition from early vertebrates to early mammals
Original art by Rebecca Gelernter
The neocortex of this early mammal was small and took up only a small fraction of the brain. Most volume was given to the olfactory cortex (early mammals, like many modern mammals, had an incredible sense of smell). But despite the small size of the neocortex in early mammals, it was still the kernel from which human intelligence would arise. In the human brain, the neocortex takes up 70 percent of brain volume. In the breakthroughs that followed, this originally small structure would progressively expand from a clever trick to the epicenter of intelligence.