
It is not essential to learn about botany to garden well: it’s inevitable. Why is the science of plants relevant to the propagator? For the same reason that the physician needs to know about human physiology.
By observing the extraordinary truths and beauty of the plant kingdom, we can recognize where to go, how to get there, what to do, and when to do it. Gardeners can discover how to capitalize on plants’ primal goal: to perpetuate themselves, by either passing on genes through seeds or by regenerating tissue, sometimes creating an entire new plant from a single leaf.
The fact that many ancient organisms still exist today is proof that the reproductive strategies that evolved over time are extremely reliable. Well before the first flowers appeared for sexual procreation, fungi reproduced asexually via fruiting bodies —mushrooms —which release billions of spores into the air. A few of these spores would settle in comfortable spots, divide as cells do, and create new beings. The spores grew into exact copies of their single parents.
Evolution and natural selection favor chance: sexual propagation, with its exchange of genetic material, increases the odds for accidental improvements. Mosses and ferns, among the earliest plants, produce spores, but unlike fungi, these plants have sex. A fern spore grows both male and female organs, and a reproductive structure called a prothallus has an aqueous film in which a male gamete (fertile reproductive cell) travels to the female. On rare occasions, however, one of the sexual partners might arrive from a neighboring plant, carried perhaps in the splash of a raindrop. The resulting hybrid —containing genes from both parents —is evolution’s dividend. The new fern may prove better able to survive environmental changes and in time dominate the species.
Gardeners need to understand fern reproduction when sowing spores to grow more plants, but recognizing the impact of hybridization reveals the achievements of natural selection. The sexual plants that evolved after ferns —the gymnosperms, such as conifers, cycads, and the ginkgo —came up with a way to exchange genetic material through the dry medium of air. The gymnosperms encased their male gametes in pollen; and even more revolutionary, they introduced the seed.
Flowers fine-tuned the delivery system, but at a great cost. Intricate and elaborate blossoms enlist the help of animals in swapping chromosomes with like flowers in distant neighborhoods. But it takes a great deal of energy to produce a fancy flower. Adaptations to environmental circumstances must be made, as well as adjustments to the independent evolution of the specific animal partner.
Some of the most recent plants that have evolved returned to the strategy utilized by earlier plants. These plants have found that the energy conserved in producing modest flowers can be directed into building large colonies of individuals in close proximity.
It is probably no coincidence that the world’s most important food crops —rice, wheat, and corn —come from the grass family, whose barely visible flowers grow in vast numbers.
The next time you husk an ear of corn look at the withered silk. Each thread is actually a pistil leading to a single kernel.
Flowers of Stipa gigantea are the largest of all grasses.
Gymnosperms, the sexual plants that evolved after ferns, such as this fir with immature purple cones, use air as a vehicle to transport reproductive material. But the trees do not produce spores; they flood the air with male pollen in search of receptive female cones.
The great food plants of the world—rice, corn, and wheat (shown)—are members of the grass family. Grasses, the most recent flowering plants to evolve, found it efficient to produce unassuming flowers in huge colonies. Airborne pollen is transmitted and received over a relatively short distance.
Fungi were once thought to be part of the botanical kingdom, because they have spores like mosses and ferns. A mushroom produces many millions of spores that are set adrift on the wind in an effective, if not economical, method of asexual reproduction: only a few find the perfect spot to grow. If all of the spores of a single fungus grew, the progeny would soon cover the earth.

Thousands of fern spores are stored in sori, seen as golden dots beneath a frond. Ferns introduced sexual reproduction, while still relying on huge numbers of spores.

In order for an ear of corn to be filled with kernels, the tips of each of its pistils—the silk—must come in contact with a grain of pollen and be fertilized.
The innovation of flowers sped up plant evolution and adaptation, and as animals proliferated, plants began to change in ways that could exploit the creatures’ mobility. In order to attract animals such as insects, plants developed incentives, such as rewards of nectar, and advertised them with flowers. Refining this symbiotic relationship led to more intricate floral lures, guaranteeing that specific animals would visit specific flowers, easily pick up pollen, and make a special delivery to another flower of the same species. Our love of flowers —their colors, forms, fragrance —is purely coincidental. When we wish to participate in pollination —either to produce fruit or perhaps to create our own hybrid —we need to know what to look for.
The parts of a flower are arranged in concentric rings, or whorls. The innermost whorls comprise the male stamens (usually in multiples) and a singular female pistil. When one flower has both male and female organs, it is considered “perfect,” and if it has all parts —petals, sepals, stamens, pistils —it is “complete.” These flowers grow on plants that are monœcious (from the Greek for “one household”), which may be capable of self-pollination. Since mixing genes is the goal, many flowers stagger the ripening of their organs so that self-pollination does not occur. Some plants can even recognize their own pollen grains and reject them, while accepting pollen from another individual member of the same species.
To improve the odds for innovation, many other plants are diœcious (“two households”). These plants evolved male and female flowers on separate plants. Independent male plants bear only male flowers, and female plants with only female flowers bear the fruit that results from pollination. That is why at least one male holly bush, such as Ilex verticillata ‘Rhett Butler’, must be included in the garden to play stud to the female holly plants, for example, of the variety ‘Scarlett O’Hara’.
Exchanging genetic material is the goal, and although the Codonopsis flower is perfect, self-pollination is unlikely. The female pistil (upper flower) ripens a day before the male anthers (lower flower), by which time fertilization is under way.

The receptive stigma is at the top of the female pistil, and ovaries—containing immature ovules that will become seeds—are at the base. Pollen-bearing anthers are to the left and right.

A squash plant produces a separate male flower and female flowers that open at different times. Other plants, such as holly, bear flowers of one gender on one plant and the other gender on another. At least one male must be included among female shrubs for pollination and berries.

The cactus flower is “perfect,” having male and female organs.
The next step for plants was to disseminate their seeds. Many of the conifers produced winged seeds to use the wind just as the plants’ pollen did. There are seeds produced with tempting rewards; those with elaisomes—yew shrubs, for example—attract insects, which carry these seeds off to their burrows, where the tasty parts are consumed —leaving the seeds unharmed and in a perfect haven for germination.
The seed vessels that result from floral pollination and the swelling of ovaries or ovary-like structures are fruits—regardless of which side of the produce aisle fruits are found on —whether they are sweet and juicy, hard and dry, pea pods or luscious peaches —anything that contains seeds is a fruit. The containers have been thoughtfully designed by nature to protect the precious contents and, in many cases, to help disseminate the cargo. Moist fruits encase seeds in sweet flesh to entice, and colorful skins are used to publicize delicacies to animals that might eat them and help distribute the seeds.
Prickly dry fruits may enlist animals as well, with a barbed capsule to hitch onto a hiker’s pant leg. Other dry fruits may have seeds attached to fluff that can become airborne (like a dandelion’s parachute).
Buoyant pods float on streams and rivers, but the coconut is probably the champion long-distance traveler —Caribbean coconuts are occasionally seen sprouting on the coast of Scotland, delivered there by the waters of the Gulf Stream. Violets produce two kinds of fruit: ones at the base of the plant drop seeds on the soil; others face skyward and, when ripe, explode. Harvesting even a few seeds presents a challenge when violets shoot their seeds 6 to 7 feet away to test uncharted territory.
Fruits and seeds take many forms, and it is important for the gardener to know about their guises —for harvesting, cleaning, storing, or sowing at just the right time.
If the product of a pollinated flower has seeds, it’s a fruit, moist or dry. Fruits may protect seeds, delay germination, or help seeds find a new or hospitable place for germination, which is what burdock fruits do, using hooks to hitch a ride on unsuspecting passersby. Kapok seeds are encased in airborne fluff. Water arum plunges its ripening fruits into the surrounding muck.



Behind the familiar star-shaped sepal of a rose, berrylike hips form around seeds called “achenes.”

A single strawberry flower has many pistils and each swells and fuses into a pulpy fruit. But strawberries are not classified as moist fruits; they are aggregates of achenes with dry seeds on the outside.

One defense against a seed’s premature sprouting is its coat. This outer layer, often covered by water-resistant wax or shellaclike resins, protects the seed while it lies in wait. In nature, microorganisms may eat through the seed coat. An animal may snag a fruit, and the seed might emerge having passed through the animal’s gut with the coat scarred by digestive acids. For the propagator, drying, chilling, nicking and filing, hot-water baths, or a simple overnight soak may be needed to compromise the coat’s integrity. Once there’s a breach in the coat, moisture can be imbibed. But moisture is not the only agent effecting germination.
Seeds don’t sprout at the wrong time in the wrong place. If moisture and warmth were all that was needed for a seed to sprout, nicotiana seeds would germinate when their goblet-fruits filled with water from late-summer rains; the seeds would die as soon as they dried again. If pumpkin seeds sprouted inside their vine-ripened fruits, the seedlings would die without light. Given the complexity of nature, mechanisms more elaborate than the seed’s coat can be suspected of delaying germination.
Scientists conducting research on the elements that affect plant growth have discovered that the hormonal compound abscisic acid (ABA) —the same chemical that is responsible for winter dormancy of mature plants —accumulates in seeds as they ripen. But it isn’t accurate to think of seeds as experiencing a dormant cycle. Seeds can remain in suspended animation for months or years. ABA stops the clock, and in order for seeds to move to the next phase in their development, this chemical inhibitor must be removed or destroyed.
The key to this process lies in the origin of the individual seed: the type of plant that produced it and the environment of its homeland. One can speculate that when a bird consumes a fruit and expels its seed, the flesh may have contained the inhibitor. For a plant indigenous to an alpine meadow, exposure to frigid winds and snow could be necessary to prepare its seed. The seeds of annuals grown in our gardens must be harvested when ripe and stored in a frost-free place until it is time to sow the seeds indoors or out. This dry storage may be just what these seeds need —perhaps sitting in a packet on the garden-center shelf provides a coincidental advantage. The seeds of hardy plants are subjected to fluctuations in temperature, and it is likely that this contributes to the gradual destruction of inhibitors.
Gardeners often have to discover how to turn off the inhibitors to “condition” seeds in preparation for germination. The seeds of hardy plants can be sown outdoors, but there are many reasons why pretreating them indoors —perhaps by subjecting the seeds to periods of warmth, cold, and warmth again in the home nursery —may be advantageous. Conditioning could be called for to get a head start on the growing season, to make up for lost time when seeds arrive in winter —months after natural conditioning would have begun outdoors —or to achieve a higher success rate with precious seeds under the gardener’s watchful eye and controlled surroundings.
A chestnut seed’s nearly impenetrable coat helps it stay fresh and viable until the right time and place for germination—perhaps where it was buried and forgotten by a squirrel.

Although depicted with a bird in an eighteenth-century illustration by naturalist Mark Catesby, the North American native sweet bay magnolia (Magnolia virginiana) is not an aggressive spreader. But keep in mind that birds eat fruits, clean away the pulp, sometimes etching a seed’s hard coat with digestive acids, and deposit seeds away from their sources with a nutritional bonus. This method of preparation and dissemination should be a warning against planting potentially invasive bird-attracting plants, such as barberry and autumn olive.

Once the inhibitor is destroyed, moisture and warmth will initiate germination. Seeing the inside of a seed is like viewing a human embryo with ultrasound. To glimpse the next steps, consider a large seed such as the fast-sprouting lima bean (Phaseolus lunatus). Soaking the bean in warm water will begin the process.
When the seed coat (testa) of the bean is wet, it will loosen and slip off to reveal the cotyledon (seed leaf). The bean has two cotyledons, and they can be pried apart to reveal the embryo attached to one part. The miracle of nature is breathtakingly apparent, displayed as a plant in miniature with tiny leaves and an appendage called a radicle. After imbibing water, the radicle elongates, emerges from the seed, and becomes the plant’s first root. The emergence of the radicle is an indication that growth and life are under way, but the sprouting of the cotyledons is considered the sign of germination. These cotyledons grow to become the seed leaves —predecessors of the plant’s first true leaves. This version of the process is epigeal germination.
Some plants, however, do not act so simply, or swiftly. In hypogeal germination, the cotyledons remain underground and nourish the seedling without emerging. Sometimes, as with “two-step germinators” (see Two-Step Germination), several seasons pass before the process is completed.
Plants such as the epigeal lima bean that produce two seed leaves are classified as dicotyledons. Plants whose seeds send up a single seed leaf, like a blade of grass, are monocotyledons. When sowing seeds, it is helpful to note the distinction: some seedlings will emerge with two semicircular sections joined at the stem, and others with a solitary shoot. The second set of leaves to appear are the true leaves, which resemble the leaves of the plant-to-be.
The dicots again produce a pair of leaves, and the monocots bear a second single leaf. The true leaves of the dicots have veins that branch; monocots have parallel veins. When the plants are mature, the distinctions persist —consider the dicotyledonous maple tree and the monocotyledonous palm. Recognizing the differences between these types of plants affects options for vegetative propagation.
Inside a bean seed are the embryonic makings of an entire new plant. Visible on the right half is the immature root called the “radicle” with tiny “true leaves” above it. The remainder of the bean, the nutritional endo-carp, becomes the cotyledons, or seed leaves. When germination begins, the radicle elongates. The process is complete when the cotyledons emerge.

Plants that produce seeds are either dicotyledons with two seed leaves, like the bean, or monocotyledons with one. As they grow, leaves of dicots develop a network of branching veins that can be seen in the skeletal remains of a linden leaf.

Leaves of monocots, such as Canna ‘Tropicana Phaison’ and other members of the lily family, have parallel veins.
Plants reproduce sexually via seeds, but the mechanisms that allow them to repair themselves after injuries also enable us to reproduce them asexually, or vegetatively. Buds, which are nestled in the nodes where leaves and lateral shoots emerge from stems, produce hormones that prompt cells to grow and make necessary repairs. Latent buds, or “eyes,” are signaled in the event of an emergency, such as defoliation by insects. A gardener can pinch terminal growth, causing the plant to develop new branches or leaves. A young tree damaged in a snow storm may grow a straight and tall new leader. The newest cells of the bud’s growing point, or meristem, can transform into fresh growth because of an ability called totipotency, a term that derives from the Latin for “all powerful.”
Totipotency makes reproduction from cuttings possible as well. The cambium layer of meristem tissue, which contains cells in a formative stage, lies beneath the corky bark of woody plants and the epidermis of soft-tissue ones. The cells of the cambium can differentiate to become any part of a plant, from leafy growth to protective calluses to new roots. When a large branch breaks off a tree, the cells around the wound divide wildly to form a callus that seals off the exposed tissue. When a woody stem cutting is made, a callus forms through which tiny roots will emerge.
It is possible for plants to reproduce without sex through various mechanisms. One explanation can be found beneath the bark of a dicot. A thin layer of cambium cells has an undetermined destiny. Under particular circumstances, these cells can grow to become stems, leaves, or even roots.

A cutting taken from a mature plant, one that is making flowers and fruit, is using its energy for sexual reproduction, not vegetative propagation. For example, a stem taken from an herbaceous perennial in spring will root easily. If taken later, when flowering, the cutting will be difficult, if not impossible, to root. Likewise, cuttings from young woody plants root more easily than ones from mature, aged plants. Trees, being the longest-lived plants, are often the most difficult to root, although they readily grow from seed. If there is a special cultivated variety of tree that cannot be replicated from sown seed, however, it has to be reproduced asexually through vegetative propagation. Stem sections taken from older trees (known as scions) can be grafted onto young plants (called understocks), grown from seeds of similar species. The cells of the cambium layer divide to knit one plant to the other. The sapling imparts its youth and vigor to the scion.
Mature plants, such as trees, and ones that are in flower or fruit are not eager to return to a juvenile state when they were actively adding leafy tissue and easier to propagate. Transformations may begin when the cambium produces a gnarled mass of cells through which roots will grow. Adventitious buds, seen as pale bumps on a Dieffenbachia cane, may grow into new stems and leaves or roots. Dormant buds on hardy plants can be “switched on” by hormones when there is trauma to the terminal growth and sprout stems and leaves. The eyes of a potato can grow roots and shoots.





The water lily multiplies by producing new plants from eyes on rhizomes that creep freely through the earth bottom of a shallow pond.
Many plants exhibit adventitious growths in nature. The mother of thousands (Kalanchoe pinnata) produces baby plants along the margins of its leaves.
Some plants are so eager to reproduce vegetatively that they don’t wait for gardeners to propagate them. They produce adventitious growths, known as propagules or baby plants. Many Kalanchoe species, collectively known as “mother of thousands,” produce plantlets along the edges of their leaves. When large enough, the offspring drop to the ground and grow. Tropical water lilies may produce new plants that grow in a bit of captured water in the mature plant’s leaf, and new flowers may even bloom there. Aloe vera produces brood after brood of offsets around a mature plant. Some orchids do the same; their progeny are called “keikis.” One of the most familiar plants to bear live young is the spider plant (Chlorophytum comosum). Its babies grow from the ends of the flower stalks, and they are easily rooted for new plants.
A few plants that are monocarpic (they fruit only once), such as bromeliads, die after they flower and set seed, but they may prepare for their demise by producing compact plantlets around the central parent. Hardy succulent hen-and-chicks (Sem-pervivum) follow this pattern.
Suckers are sometimes produced from adventitious buds on shallow roots, leading to sprouts around shrub clumps of plants such as lilac or sumac. The same action causes straight shoots, called water sprouts, from the trunk and branches of trees or shrubs if they are damaged, severely pruned, or attacked by disease.
Grass plants, such as those in a traditional lawn, also reproduce vegetatively, from rhizomes —underground stems just below the surface of the soil. New roots, stems, and leaves push out from nodes along the subterranean stem. While that’s good for a lawn, consider the grasses’ cousin, the bamboo, on the rampage across your garden, having escaped from the property next door.
Latent buds, or eyes, on roots are virtually invisible, but in many cases, roots are capable of reproducing entire new plants from cut tissue. These buds are not unlike the eyes of tubers.
The familiar spider plant (Chlorophytum comosum) grows offspring at the ends of its flower stalks.
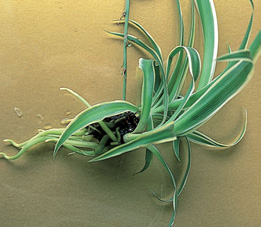
The viviparous piggyback plant (Tolmiea menziesii) sprouts a new plant from an old leaf.

The mother fern (Asplenium bulbiferum) bears its bright green young from the veins of a frond.

Bromeliads, such as Aechmea chantinii ‘Samurai’, produce offsets called “pups” from the base of the plant that can be removed and grown into independent plants.

Geophytes can be propagated asexually in several ways. The prolific tiger lily (Lilium lancifolium) produces black bulbils at its leaf axils that roll to the ground, root, and grow.

Some plants produce stems and leaves that swell with stored carbohydrates and sugars. We know these organs as bulbs, tubers, corms, and fleshy rhizomes —collectively, the plants are called geophytes.
The scales of flowering bulbs may either be arranged in concentric layers, as those of an onion, or be separate, as in lily bulbs. The lily’s scales are arrayed like the bracts of an artichoke. Tulips and daffodils —tunicate bulbs with papery coverings —split to form new bulbs. Tunicate bulbs can be cut into sections for propagation. Lilies —nontunicate bulbs —can be propagated from individual scales.
A few lilies—the tiger lily, for instance—produce miniature black “bulbils” at their leaf axils. These bulbils roll off the plant, fall onto the ground, sprout roots, and push up stems and leaves. In about two years, the new plants flower and form bulbils of their own.
Other geophytes arise from tubers. As is easily seen with a potato, tubers have dormant buds —eyes —with cells that have the ability to transmogrify into roots, shoots, and all other plant organs. (Similar buds can be found at ground level at the crown of dormant herbaceous perennials in winter.) Some tuberous plants form tubercles similar to lily bulbils. A few tuberous vines produce their tubercles in midair, such as the true yam and the aptly named rosary vine.
Gladioli grow from corms, which resemble bulbs but do not have scales, and reproduce by growing whole new corms beneath the shriveled ones of the season before or by making tiny cormels around the base of old ones.
There is one other geophyte: a subterranean rhizome with a growing point and the ability to store nutrients and moisture during times of drought or cold. During the summer, a tiny chunk of a canna rhizome with a single eye will produce leafy growth above ground that is reflected below the soil by an elongating, thickening structure, which by season’s end, might sport a dozen dormant buds. Some canna rhizomes also produce appendages—2-inch spherical nodules nicknamed “toes.” The rhizome buds bear the “meristem” or newest cells addressed in the anomaly of the Sansevieria clone that follows and here.
A narcissus bulb not only contains everything necessary for next year’s flower show (including incipient blossoms with immature anthers), it can also be propagation material propagated from sections of the bulb and basal plate below.

Potatoes can be cut into pieces that include at least one growing “eye.”

New plants that are produced vegetatively are called clones—identical in every way to their parents. A flowering maple (Abutilon) cultivar, for instance, may exhibit variegation caused by a virus, and it will pass on this characteristic through cuttings. However, certain traits, harbored only in the new cells of the most terminal growth, will not be passed on. In the case of certain variegated plants, for example, the genetic instructions for producing the colorful trait are carried only in these “meristem” cells and will not appear on new plants produced from stem or root cuttings. Immature meristem cells have not fully differentiated into the tissues of organs they are to become.
Early in the twentieth century, scientists began to experiment with these undetermined cells, growing entire new plants from the emerging new growth of leaf buds or slices from a nugget or callus. This micropropagation, like all forms of vegetative propagation, is a testament to a plant’s determination to survive. Glimpses into the science of plant reproduction —the botany of propagation —may or may not be proof of evolution’s divinity, but they are evidence of nature’s mastery.
Plants propagated vegetatively produce clones—genetically identical replicas of their parents. Variegation is reproduced asexually; however, in the case of the flowering maple (Abutilon pictum ‘Thomp-sonii’), the mottling is caused by a virus that is transmitted to cuttings.

But in a variegated snake plant (Sansevieria trifasciata ‘Bantel’s Sensation’), the characteristic is developed by mutating meristem cells at the tip of the new growth. Cuttings grow roots and a new rhizome from their bases, so the result reverts to the species form.
