
IN CONTEXT
Chemistry
c.400 BCE Democritus proposes that the world is made of indivisible particles.
8th century CE Persian polymath Jabir ibn Hayyan (or Geber) classifies elements into metals and non-metals.
1794 Joseph Proust shows that compounds are always made of elements combined in the same proportions.
1811 Amedeo Avogadro shows that equal volumes of different gases contain equal numbers of molecules.
1869 Dmitri Mendeleev draws up a periodic table, displaying elements by atomic weight.
1897 Through his discovery of the electron, J J Thomson shows that atoms are not the smallest possible particle.
Towards the end of the 18th century, scientists had begun to realize that the world is made up of a range of basic substances, or chemical elements. But no one was certain what an element was. It was John Dalton, an English meteorologist, who, through his study of weather, saw that each element is made wholly of its own unique, identical atoms, and it is this special atom that distinguishes and defines an element. In developing the atomic theory of elements, Dalton established the basis of chemistry. The idea of atoms dates back to ancient Greece, but it had always been assumed that all atoms were identical. Dalton agreed with Isaac Newton who, a century earlier, had described the atoms that made up the elements as “solid, massy, hard, impenetrable, moveable particles”. Dalton’s breakthrough was to understand that each element is made from different atoms.
Dalton’s ideas originated in his study of the way in which air pressure affected how much water could be absorbed by air. He became convinced that air is a mixture of different gases. As he experimented, he observed that a given quantity of pure oxygen will take up less water vapour than the same amount of pure nitrogen, and he jumped to the remarkable conclusion that this is because oxygen atoms are bigger and heavier than nitrogen atoms.
"An inquiry into the relative weight of the ultimate particles of bodies is a subject, as far as I know, entirely new."
John Dalton
Weighty matters
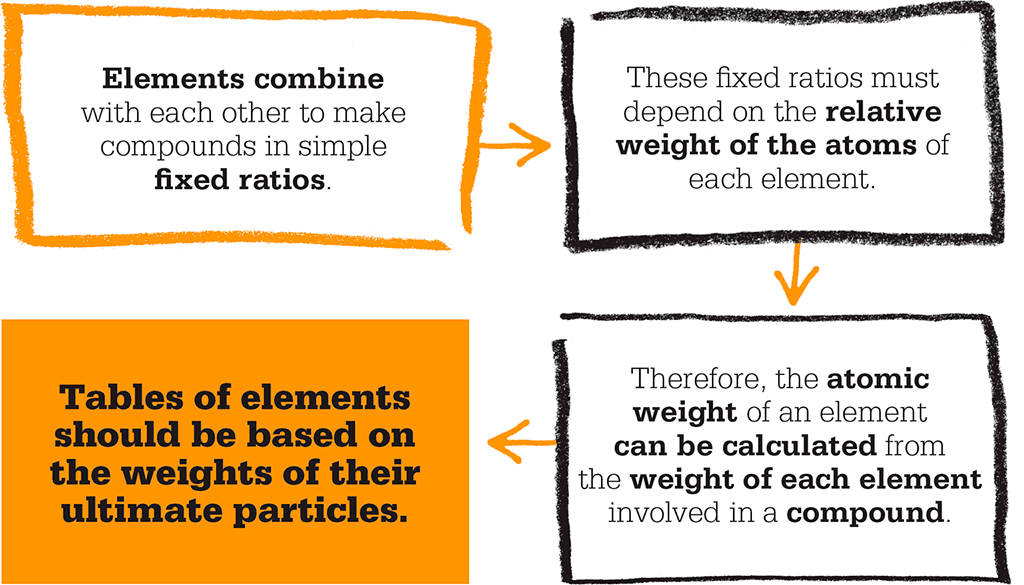
In a flash of insight, Dalton realized that atoms of different elements could be distinguished by differences in their weights. He saw that the atoms, or “ultimate particles”, of two or more elements combined to make compounds in very simple ratios, so he could work out the weight of each atom by the weight of each element involved in a compound. Very quickly, he worked out the atomic weight of each element then known.
Hydrogen, Dalton realized, was the lightest gas, so he assigned it an atomic weight of 1. Because of the weight of oxygen that combined with hydrogen in water, he assigned oxygen an atomic weight of 7. However, there was a flaw in Dalton’s method, because he did not realize that atoms of the same element can combine. He always assumed that a compound of atoms – a molecule – had only one atom of each element. But Dalton’s work had put scientists on the right track, and within a decade Italian physicist Amedeo Avogadro had devised a system of molecular proportions to calculate atomic weights correctly. Yet the basic idea of Dalton’s theory – that each element has its own unique-sized atoms – has proved to be true.

Dalton’s table shows symbols and atomic weights of different elements. Dalton was drawn to atomic theory through meteorology, when he asked himself why air and water particles could mix.
JOHN DALTON

Born into a Quaker family in England’s Lake District in 1766, John Dalton made regular observations of the weather from the age of 15. These provided many key insights, such as that atmospheric moisture turns to rain when the air cools. In addition to his meteorological studies, Dalton became fascinated by a condition he and his brother shared: colour blindness. His scientific paper on the subject gained him admission to the Manchester Literary and Philosophical Society, of which he was elected president in 1817. He wrote hundreds of scientific papers for the Society, including those about his atomic theory. The atomic theory was quickly accepted, and Dalton became a celebrity in his own lifetime – more than 40,000 people attended his funeral in Manchester in 1844.
Key works
1805 Experimental Enquiry into the Proportion of the Several Gases or Elastic Fluids, Constituting the Atmosphere
1808–27 New System of Chemical Philosophy
See also: Joseph Proust • Dmitri Mendeleev