
IN CONTEXT
Chemistry
1803 John Dalton introduces the idea of atomic weights.
1828 Johann Döbereiner attempts first classification.
1860 Stanislao Cannizzaro publishes an extensive table of atomic and molecular weights.
1913 Lothar Meyer shows the periodic relationship between elements by plotting atomic weight against volume.
1913 Henry Moseley redefines the periodic table using atomic numbers – the number of protons in an atom’s nucleus.
1913 Niels Bohr suggests a model for the structure of the atom. It includes shells of electrons that explain the relative reactivity of the different groups of elements.
In 1661, Anglo-Irish physicist Robert Boyle defined elements as “certain primitive and simple, or perfectly unmingled bodies; which not being made of any other bodies, or of one another, are the ingredients of which all those called perfectly mixt bodies are immediately compounded, and into which they are ultimately resolved”. In other words, an element cannot be broken down by chemical means into simpler substances. In 1803, British chemist John Dalton introduced the idea of atomic weights (now called relative atomic masses) for these elements. Hydrogen is the lightest element, and he gave it the value 1, which we still use today.

Law of eight
In the first half of the 19th century, chemists gradually isolated more elements, and it became clear that certain groups of elements had similar properties. For example, sodium and potassium are silvery solids (alkali metals) that react violently with water, liberating hydrogen gas. Indeed, they are so similar that British chemist Humphry Davy did not distinguish between them when he first discovered them. Similarly, the halogen elements chlorine and bromine are both pungent, poisonous oxidizing agents, even though chlorine is a gas and bromine a liquid. British chemist John Newlands noticed that when the known elements were listed in order of increasing atomic weight, similar elements occurred every eighth place. He published his findings in 1864.
In the journal Chemical News Newlands wrote: “Elements belonging to the same group appear in the same horizontal line. Also the numbers of similar elements differ by seven or multiples of seven…This peculiar relationship I propose to call The Law of Octaves.” The patterns in his table make sense as far as calcium, but then go haywire. On 1 March 1865, Newlands was ridiculed by the Chemical Society, who said that he might as well list the elements in alphabetical order, and refused to publish his paper. The significance of Newlands’ achievement would not be recognized for more than 20 years. Meanwhile, French mineralogist Alexandre-Émile Béguyer de Chancourtois had also noticed the patterns, publishing his ideas in 1862, but few people noticed.
The first to attempt a classification of the elements was German chemist Johann Döbereiner. By 1828, he had found that some elements formed groups of three with related properties.
Card puzzle
Around the same time, Dmitri Mendeleev was struggling with the same problem as he wrote his book Principles of Chemistry in St Petersburg, Russia. In 1863, there were 56 known elements, and new ones were being discovered at a rate of about one a year. Mendeleev was convinced that there must be a pattern to them. In an effort to solve the puzzle, he made a set of 56 playing cards, each labelled with the name and major properties of one element.
Mendeleev is said to have made his breakthrough as he was about to embark on a winter journey in 1868. Before setting out, he laid out his cards on the table and began to ponder the puzzle, as though playing a game of Patience. When his coachman came to the door for the luggage, Mendeleev waved him away, saying he was busy. To and fro he moved the cards until finally he managed to arrange all 56 elements to his satisfaction, with the similar groups running vertically. The following year, Mendeleev read out a paper at the Russian Chemical Society stating that: “The elements, if arranged according to their atomic weight, exhibit an apparent periodicity of properties.” He explained that elements with similar chemical properties have atomic weights that are either of nearly the same value (such as potassium, iridium, and osmium) or that increase regularly (such as potassium, rubidium, and caesium). He further explained that the arrangement of the elements into groups in the order of their atomic weights corresponds to their valency, which is the number of bonds the atoms can form with other atoms.
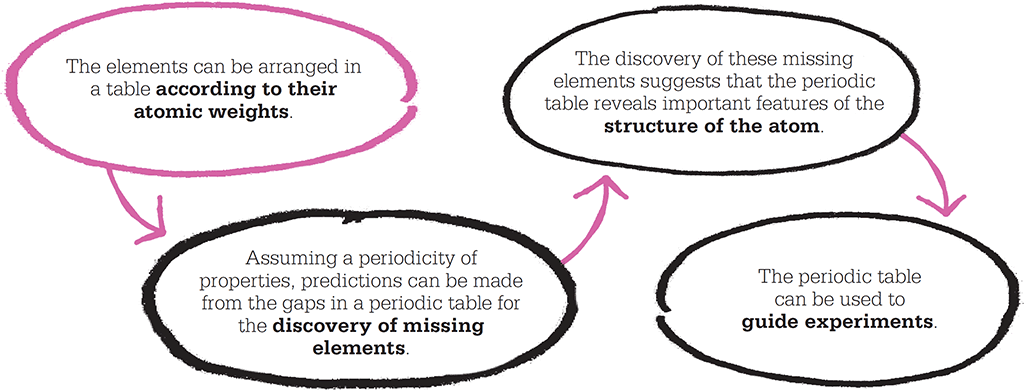
Mendeleev’s periodic table was the precursor of the modern table, shown here. He left gaps in his table where the corresponding element had not yet been discovered, and used these to predict the properties of the missing elements.
"It is the function of science to discover the existence of a general reign of order in nature and to find the causes governing this order."
Dmitri Mendeleev
Predicting new elements
In his paper, Mendeleev made a bold prediction: “We must expect the discovery of many yet unknown elements – for example, two elements, analogous to aluminium and silicon, whose atomic weights would be between 65 and 75.”
Mendeleev’s arrangement included crucial improvements over Newlands’ Octaves. Below boron and aluminium, Newlands had placed chromium, which made little sense. Mendeleev reasoned that there must exist an as-yet undiscovered element, and predicted that one would be found with an atomic weight of about 68. It would form an oxide (a compound formed by an element with oxygen) with a chemical formula of M2O3, where “M” is the symbol for the new element. This formula meant that two atoms of the new element would combine with three oxygen atoms to make the oxide. He predicted two more elements to fill other spaces: one with an atomic weight of about 45, forming the oxide M2O3, and the other with an atomic weight of 72, forming the oxide MO2.
Critics were sceptical, but Mendeleev had made very specific claims, and one of the most powerful ways to support a scientific theory is to make predictions that are proved true. In this case, the element gallium (atomic weight 70, forming the oxide Ga2O3) was discovered in 1875; scandium (wt 45, Sc2O3) in 1879; and germanium (wt 73, GeO2) in 1886. These discoveries made Mendeleev’s reputation.

The six alkali metals are all soft, highly reactive metals. The outer layer of this lump of pure sodium has reacted with the oxygen in the air to give it a coating of sodium oxide.
Mistakes in the table
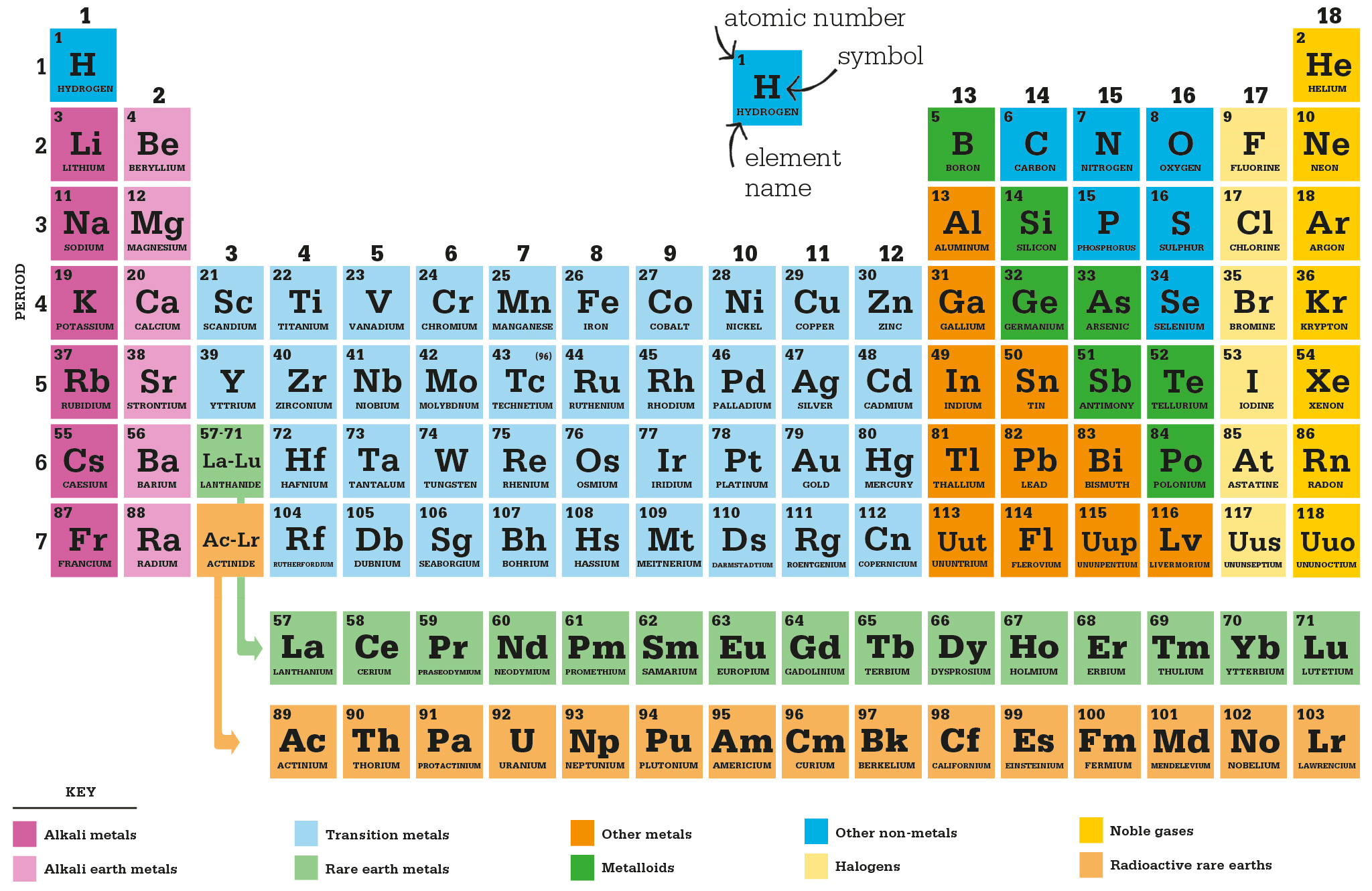
Mendeleev did make some mistakes. In his 1869 paper, he asserted that the atomic weight of tellurium must be incorrect: it should lie between 123 and 126, because the atomic weight of iodine is 127, and iodine should clearly follow tellurium in the table, according to its properties. He was wrong – the relative atomic weight of tellurium is in fact 127.6; it is greater than that of iodine. A similar anomaly occurs between potassium (wt 39) and argon (wt 40), where argon clearly precedes potassium in the table – but Mendeleev was not aware of these problems in 1869, because argon was not discovered until 1894. Argon is one of the noble gases, which are colourless, odourless, and hardly react with other elements. Difficult to detect, none of the noble gases were known at that time, so there were no spaces for them in Mendeleev’s table. Once argon had turned up, however, there were several more holes to fill, and by 1898, Scottish chemist William Ramsay had isolated helium, neon, krypton, and xenon. In 1902, Mendeleev incorporated the noble gases into his table as Group 18, and this version of the table forms the basis of the periodic table we use today.
The anomaly of the “wrong” atomic weights was solved in 1913 by British physicist Henry Moseley, who used X-rays to determine the number of protons in the nucleus of each atom of a particular element. This came to be called the atomic number of the element, and it is this number that determines the element’s position in the periodic table. The fact that atomic weights had given a close approximation followed from the fact that for the lighter elements, the atomic weight is roughly (though not exactly) twice the atomic number.
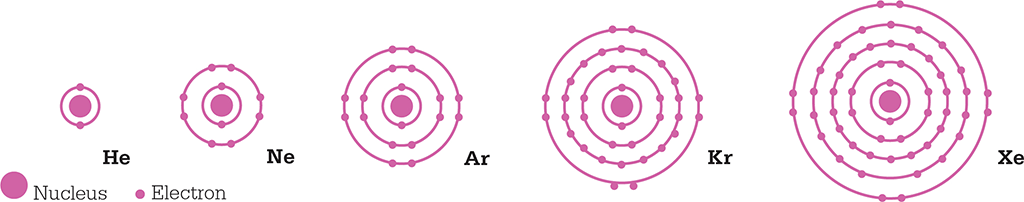
The six noble gases that occur naturally (listed in group 18 of the table) are helium, neon, argon, krypton, xenon, and radon. They have very low chemical reactivity because they each have a full valence shell – a shell of electrons surrounding the atom’s nucleus. Helium has just one shell containing two electrons, while the other elements have outer shells of eight electrons. Radioactive radon is unstable.
"We must expect the discovery of elements analogous to aluminium and silicon – whose atomic weight would be between 65 and 75."
Dmitri Mendeleev
Using the table
The periodic table of the elements may look like just a cataloguing system – a neat way of ordering the elements – but it has far greater importance in both chemistry and physics. It allows chemists to predict the properties of an element, and to try variations in processes; for example, if a particular reaction does not work with chromium, perhaps it works with molybdenum, the element below chromium in the table.
The table was also crucial in the search for the structure of the atom. Why did the properties of elements repeat in these patterns? Why were the Group 18 elements so unreactive, while the elements in the groups on either side were the most reactive of all? Such questions led directly to the picture of the structure of the atom that has been accepted ever since.
Mendeleev was to some extent lucky to have been credited for his table. Not only did he publish his ideas after Béguyer and Newlands, but also German chemist Lothar Meyer, who plotted atomic weight against atomic volume to show the periodic relationship between elements, was ahead of him, too, publishing in 1870. As so often in science, the time had been ripe for a particular discovery, and several people had reached the same conclusion independently, without knowing about each other’s work.
DMITRI MENDELEEV

The youngest of at least 12 children, Dmitri Mendeleev was born in 1834 in a village in Siberia. When his father went blind and lost his teaching post, Mendeleev’s mother supported the family with a glass factory business. When that burned down, she took her 15-year-old son across Russia to St Petersburg to receive a higher education.
In 1862, Mendeleev married Feozva Nikitichna Leshcheva, but in 1876 he became obsessed with Anna Ivanova Popova, and married her before his divorce from his first wife was final.
In the 1890s, Mendeleev organized new standards for producing vodka. He investigated the chemistry of oil, and helped to set up Russia’s first oil refinery. In 1905, he was elected a member of the Royal Swedish Academy of Science, who recommended him for a Nobel Prize, but his candidacy was blocked, possibly due to his bigamy. The radioactive element 101 mendelevium is named in his honour.
Key work
1870 Principles of Chemistry
See also: Robert Boyle • John Dalton • Humphry Davy • Marie Curie • Ernest Rutherford • Linus Pauling