
IN CONTEXT
Chemistry
1800 Alessandro Volta lists metals in decreasing order of electropositivity.
1852 British chemist Edward Frankland states that atoms have definite combining power, which determines the formulae of compounds.
1858 August Kekulé shows that carbon has a valency of four – it forms four bonds with other atoms.
1916 American physical chemist Gilbert Lewis shows that a covalent bond is a pair of electrons shared by two atoms in a molecule.
1938 British mathematician Charles Coulson calculates an accurate molecular orbital wavefunction for hydrogen.
In the late 1920s and early 1930s, in a series of landmark papers, American chemist Linus Pauling worked out a quantum-mechanical explanation of the nature of chemical bonds. Pauling had studied quantum mechanics in Europe with the German physicist Arnold Sommerfeld in Munich, with Niels Bohr in Copenhagen, and with Erwin Schrödinger in Zurich. He had already decided that he wanted to investigate the bonding within molecules, and realized that quantum mechanics gave him the right tools to do so.
Hybridization of orbitals
When he returned to the USA, Pauling published about 50 papers, and, in 1929, he laid down a set of five rules for interpreting the X-ray diffraction patterns of complicated crystals, now known as Pauling’s rules. At the same time, he was turning his attention to the bonding between atoms in covalent molecules (molecules in which atoms are bonded by sharing two electrons with each other), especially of organic compounds – those based on carbon.
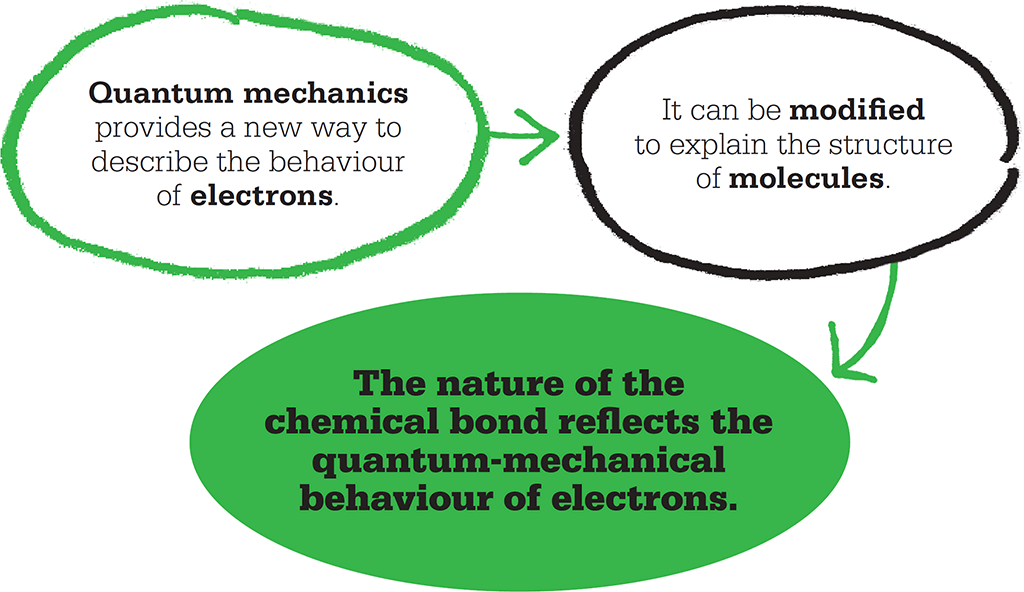
A carbon atom has six electrons in total. The European pioneers of quantum mechanics designated the first two as “1s-electrons”: these have a spherical orbital or shell around the carbon nucleus – like a balloon inflated around a golf ball in the centre. Outside the 1s shell is another shell containing two “2s-electrons”. The 2s shell is like another, bigger balloon outside the first. Lastly, there are “p-orbitals”, which have big lobes sticking out either side of the nucleus. The px orbital lies on the x-axis, the py on the y axis, and the pz orbital on the z-axis. The last two electrons of the carbon atom occupy two of these orbitals – perhaps one in px and one in py.
The new quantum-mechanical picture of electrons treated their orbits as “clouds” of probability densities. It was no longer quite right to think of the electrons as points moving around their orbits; rather, their existence was smeared across the orbits. This new non-local picture of reality allowed for some radical new ideas for chemical bonding. Bonds could either be strong “sigma” bonds, in which orbitals overlap head-on, or weaker, more diffuse “pi” bonds, in which orbitals are parallel to each other.
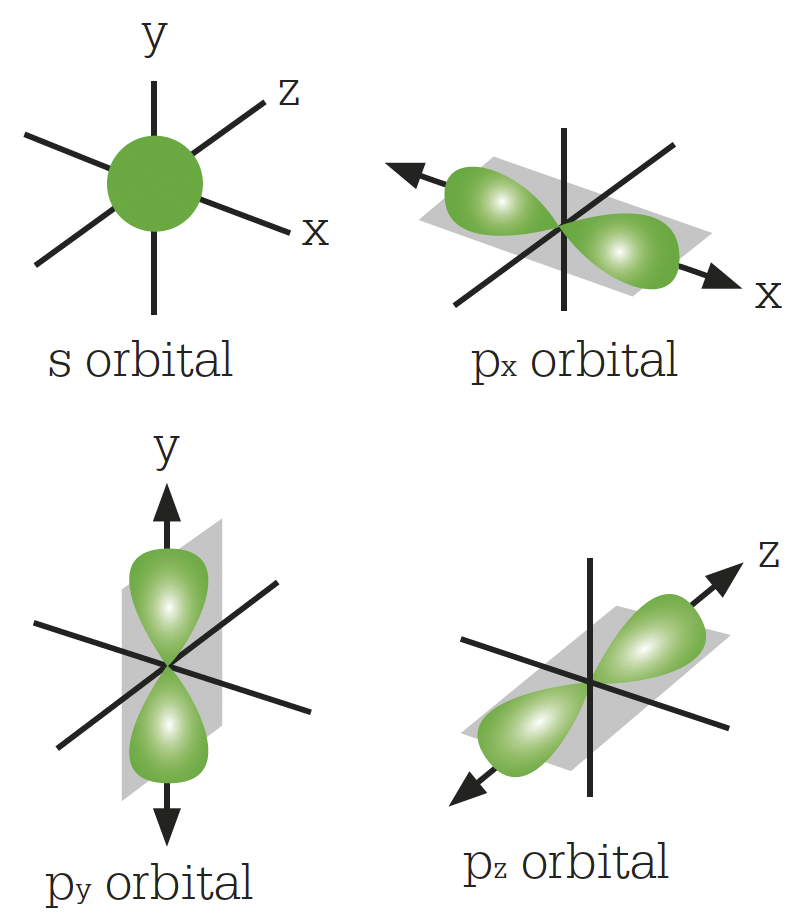
Pauling came up with the idea that in a molecule, as opposed to a bare atom, carbon’s atomic orbitals could combine, or “hybridize”, to give stronger bonds to other atoms. He showed that the s and p orbitals could hybridize to form four sp3 hybrids, which would all be equivalent, and would project from the nucleus towards the corners of a tetrahedron, with inter-bond angles of 109.5°. Each sp3 orbital can form a sigma bond with another atom. This is consistent with the fact that all the hydrogen atoms in methane (CH4), and all the chlorine atoms in carbon tetrachloride (CCl4), behave the same way.
As the structures of various carbon compounds were studied, the four nearest neighbouring atoms were often found in a tetrahedral arrangement. The crystal structure of diamond was among the first structures to be resolved by X-ray crystallography, in 1914. Diamond is pure carbon, and in the crystal each carbon atom is bonded to four others by sigma bonds at the corners of a tetrahedron. This structure explains diamond’s hardness.
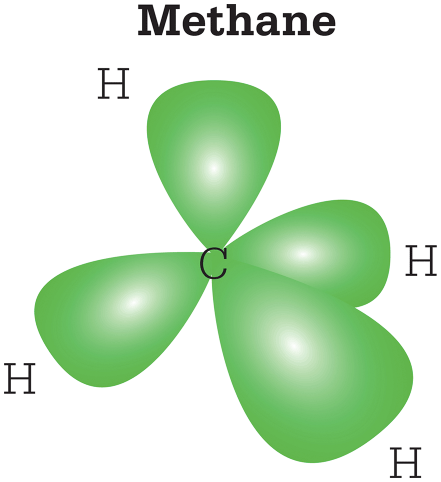
Another possible way for carbon atoms to bond to other atoms is for an s-orbital to mix with two p-orbitals to form three sp2 hybrids. These stick out from the nucleus in one plane, with angles of 120° between them. This is consistent with the geometry of molecules such as ethylene, which has the double-bond structure H2C=CH2. Here, a sigma bond is formed between the carbon atoms by one of the sp2 hybrids, and a pi bond by the fourth, unhybridized orbital.
Lastly, an s-orbital can mix with one p-orbital to form two sp hybrids, whose lobes stick out in a straight line, 180° apart. This is consistent with the structure of carbon dioxide (CO2), where the sp hybrids each form a sigma bond with the oxygen, and a second pi bond is formed by the remaining two unhybridized orbitals.
Electrons orbit an atomic nucleus in various ways – in shells around the centre (s) or lobes along one axis (p).
"By 1935, I felt I had an essentially complete understanding of the nature of the chemical bond."
Linus Pauling
Four electrons in the carbon atom hybridize to form four sp3 orbitals.
Three electrons in the carbon atoms hybridize to form three sp2 orbitals. The remaining unhybridized orbitals form a second pi bond between the carbon atoms.
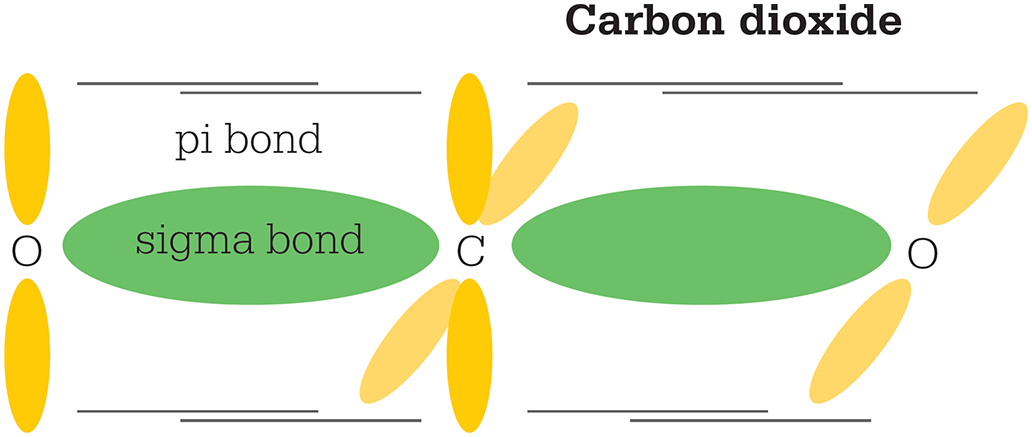
Two electrons in the carbon atom form two sp orbitals, each of which bonds with an oxygen atom. The remaining two orbitals bond to the oxygen in a pi bond.
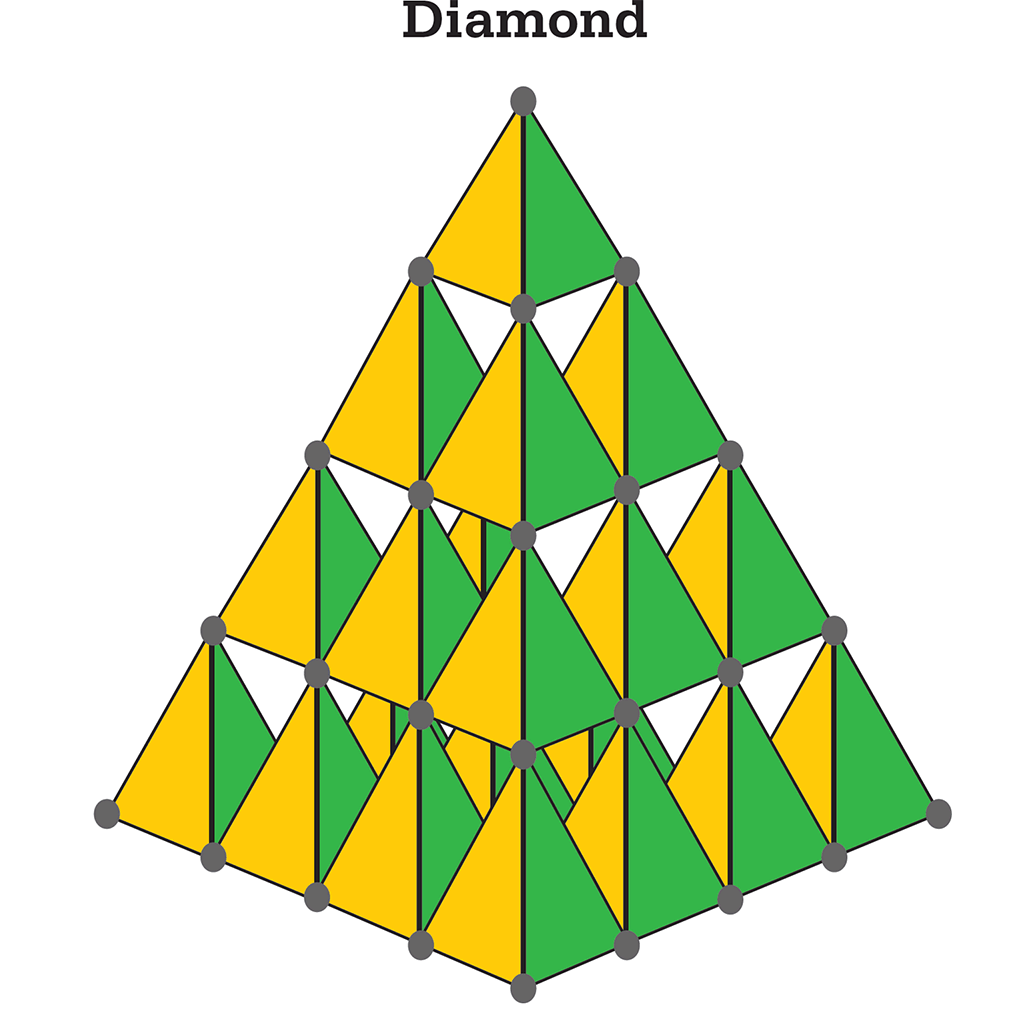
Each carbon atom in a diamond is bonded by sp3 hybrids to four other atoms to form the corner of a tetrahedron. The result is an infinite lattice held together by covalent carbon–carbon bonds, which are immensely strong.
A new structure of benzene
The structure of benzene, C6H6, had worried August Kekulé when he first proposed that it was a ring, more than 60 years earlier. He eventually suggested that the carbon atoms must be connected with alternate single and double bonds, and that the molecule oscillated between the two equivalent structures.
Pauling’s alternative solution was elegant. He said that the carbon atoms were all sp2 hybridized, so that the bonds between them and the hydrogen atoms all lie in the same xy plane and form an angle of 120° with each other. Each carbon atom has one remaining electron in a pz orbital. These electrons combine to form a bond connecting all six carbon atoms. This is a pi bond, and, in it, the electrons remain above and below the ring, and away from the carbon nuclei.
Ionic bonding
Methane and ethylene are gases at room temperature. Benzene and many other organic compounds based on carbon are liquids. They have small, lightweight molecules that can easily move around in the gas or liquid state. Salts such as calcium carbonate and potassium nitrate, by contrast, are almost invariably solids, and melt only at high temperatures. And yet a unit of sodium chloride (NaCl) has a molecular weight of 62, while benzene has a molecular weight of 78. The difference in their behaviour is explained not by their weight, but by their structure. Benzene is held together in single molecules by covalent bonds between the atoms; that is, each bond comprises one pair of electrons shared between two specific atoms.
Sodium chloride has quite different properties. The silvery metal sodium burns energetically in the greenish gas chlorine to produce the white solid sodium chloride. The sodium atom has a stable complete shell of electrons around the nucleus, plus one spare electron outside that. The chlorine atom is one electron short of a stable complete shell. When they react, an electron is transferred from the sodium atom to the chlorine atom, and both acquire stable complete shells of electrons, but now the sodium has become a sodium ion Na+, and chlorine has become the chloride ion Cl-. They have no spare electrons to form covalent bonds, but the ions are now charged: the sodium atom has lost a negatively charged electron so now has a positive overall charge; the chlorine atom has gained an electron and has a negative charge. The ions are held together by electrostatic attraction, plus to minus – a strong bond.
Sodium chloride was the first compound to be analysed by X-ray crystallography. It was found that in reality there is no such thing as a molecule of NaCl. The structure comprises an infinite array of alternating sodium and chloride ions. Each sodium ion is surrounded by six chloride ions, and each chloride is surrounded by six sodiums. Many other salts have similar structures: infinite lattices of one type of ion with different ions filling all the gaps.
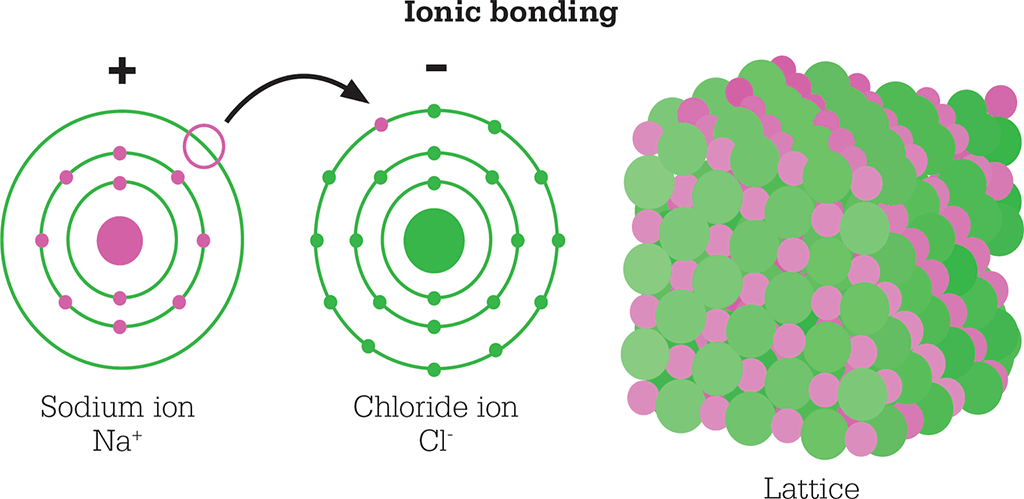
In sodium chloride, an electron in the sodium atom moves into a chlorine atom to form two charged, stable ions. The ions are held together by electrostatic attraction to form a stable lattice.
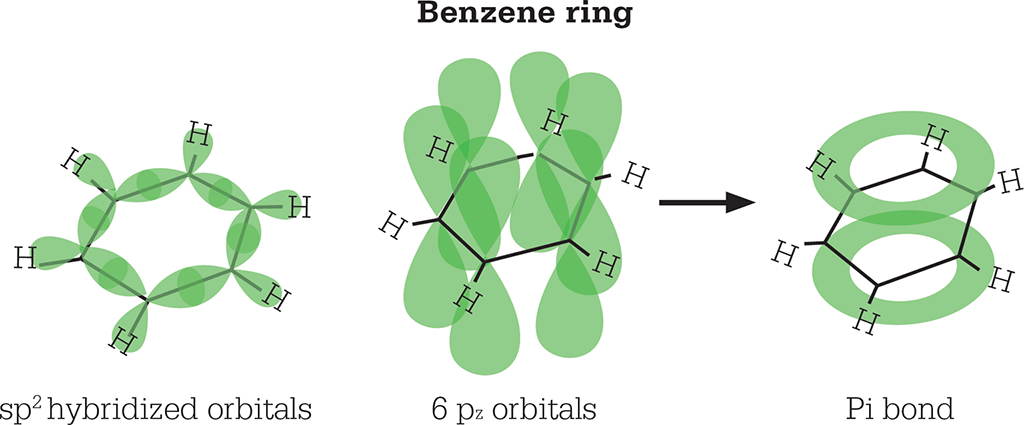
In a benzene ring, the carbon atoms are bonded to each other and a hydrogen atom by sp2 hybridized orbitals. The rings are bonded to each other by a non-localized pi-bond formed from the six pz orbitals.
"There is no area of the world that should not be investigated by scientists. There will always remain some questions that have not been answered. In general, these are the questions that have not yet been posed."
Linus Pauling
Electronegativity
Pauling explained ionic bonding in compounds such as sodium chloride, which is purely ionic, and also compounds in which the bonding is neither purely ionic nor purely covalent but somewhere in between. This work led him to develop the concept of electronegativity, which to some extent echoed the list of metals in decreasing order of electropositivity first introduced by Alessandro Volta in 1800. Pauling discovered that the covalent bond formed between atoms of two different elements (e.g. C–O) is stronger than might be expected from the average of the strengths of C–C bonds and O–O bonds. He thought that there must be some electrical factor that strengthened the bond, and set out to calculate values for this factor. The scale is now known as the Pauling scale.
The electronegativity of an element (strictly speaking in a particular compound) is a measure of how strongly an atom of the element attracts electrons towards itself. The most electronegative element is fluorine; the least electronegative (or the most electropositive) of the well-known elements is caesium. In the compound caesium fluoride, each fluorine atom pulls an electron entirely away from a caesium atom, resulting in an ionic compound Cs+F-.
In a covalent compound such as water (H2O), there are no ions, but oxygen is much more electronegative than hydrogen, and the result is that the water molecule is polar, with a small negative charge on the oxygen atom and a small positive charge on the hydrogen atoms. The charges make the water molecules stick together strongly. This explains why water has so much surface tension and such a high boiling point.
Pauling first proposed a scale of electronegativity in 1932, and he and others developed it further in subsequent years. For his work elucidating the nature of the chemical bond, he won the Nobel Prize in Chemistry in 1954.
LINUS PAULING

Linus Carl Pauling was born in Portland, Oregon, US. He first heard about quantum mechanics while still in Oregon, and won a scholarship to study the subject under some of the world experts in Europe in 1926. He returned to become assistant professor at California Institute of Technology, where he remained for most of his life.
Pauling took great interest in biological molecules, and he discovered that sickle-cell anaemia is a molecular disease. He was also a peace campaigner, and was awarded the Nobel Peace Prize in 1963 for attempting to mediate between the USA and Vietnam.
In later life, his reputation was damaged as a result of his enthusiasm for alternative medicine. He championed the use of high-dose vitamin C as a defence against the common cold, a treatment that has subsequently been shown to be ineffective.
Key work
1939 The Nature of the Chemical Bond and the Structure of Molecules and Crystals
See also: August Kekulé • Max Planck • Erwin Schrödinger • Harry Kroto