
IN CONTEXT
Chemistry
1966 British chemist David Jones predicts the creation of hollow carbon molecules.
1970 Scientists in Japan and Britain independently predict the existence of the carbon-60 (C60) molecule.
1988 C60 is found in soot from candles.
1993 German physicist Wolfgang Krätschmer and American physicist Don Huffman develop a method for synthesizing “fullerenes”.
1999 Austrian physicists Markus Arndt and Anton Zeilinger demonstrate that C60 has wave-like properties.
2010 The spectrum of C60 is seen in cosmic dust 6,500 light years from Earth.
For more than two centuries, scientists thought that elemental carbon (C) existed in only three forms, or allotropes: diamond, graphite, and amorphous carbon – the main constituent of soot and charcoal. That changed in 1985 with the work of British chemist Harry Kroto and his American colleagues Robert Curl and Richard Smalley. The chemists vaporized graphite with a laser beam to produce various carbon clusters, forming molecules with an even number of carbon atoms. The most abundant clusters had the formulae C60 and C70. These were molecules that had never been seen before.
C60 (or carbon-60) soon turned out to have remarkable properties. The chemists realized that it had a structure like a soccer ball – a complete spherical cage of carbon atoms, each bonded to three others in such a way that all the faces of the polyhedron are either pentagons or hexagons. C70 is more like a rugby ball; it has an extra ring of carbon atoms round its equator.
Both C70 and C60 reminded Kroto of the futuristic geodesic domes designed by American architect Buckminster Fuller, so he named the compounds buckminsterfullerene, but they are also called buckyballs, or fullerenes.
Properties of buckeyballs
The team found that the C60 compound was stable and could be heated to high temperatures without decomposing. It turned into a gas at about 650°C (1,202°F). It was odourless, and was insoluble in water, but slightly soluble in organic solvents. The buckyball is also one of the largest objects ever found to exhibit the properties both of a particle and of a wave. In 1999, Austrian researchers sent molecules of C60 through narrow slits and observed the interference pattern of wave-like behaviour.
Solid C60 is as soft as graphite, but when highly compressed, it changes into a super-hard form of diamond. The soccer ball, it seems, can withstand a lot of pressure.
Pure C60 is a semiconductor of electricity, meaning that its conductivity is between that of an insulator and a conductor. But when atoms of alkali metals such as sodium or potassium are added to it, it becomes a conductor, and even a superconductor at low temperatures, conducting electricity with no resistance at all.
C60 also undergoes a wide variety of chemical reactions, resulting in huge numbers of products (chemical substances) whose properties are still being investigated.
The new world of nano
Although C60 was the first of these molecules to be investigated, its discovery has led to an entire new branch of chemistry – the study of fullerenes. Nanotubes have been made – cylindrical fullerenes, only a few nanometres wide, but up to several millimetres long. They are good conductors of heat and electricity, chemically inactive, and enormously strong, which makes them hugely useful for engineering.
There are many others that are being investigated for everything from electrical properties to medical treatments for cancer to HIV. The latest spin-off from the fullerenes is graphene, a flat sheet of carbon atoms, like a single layer of graphite. This substance has remarkable properties that are being hotly studied.
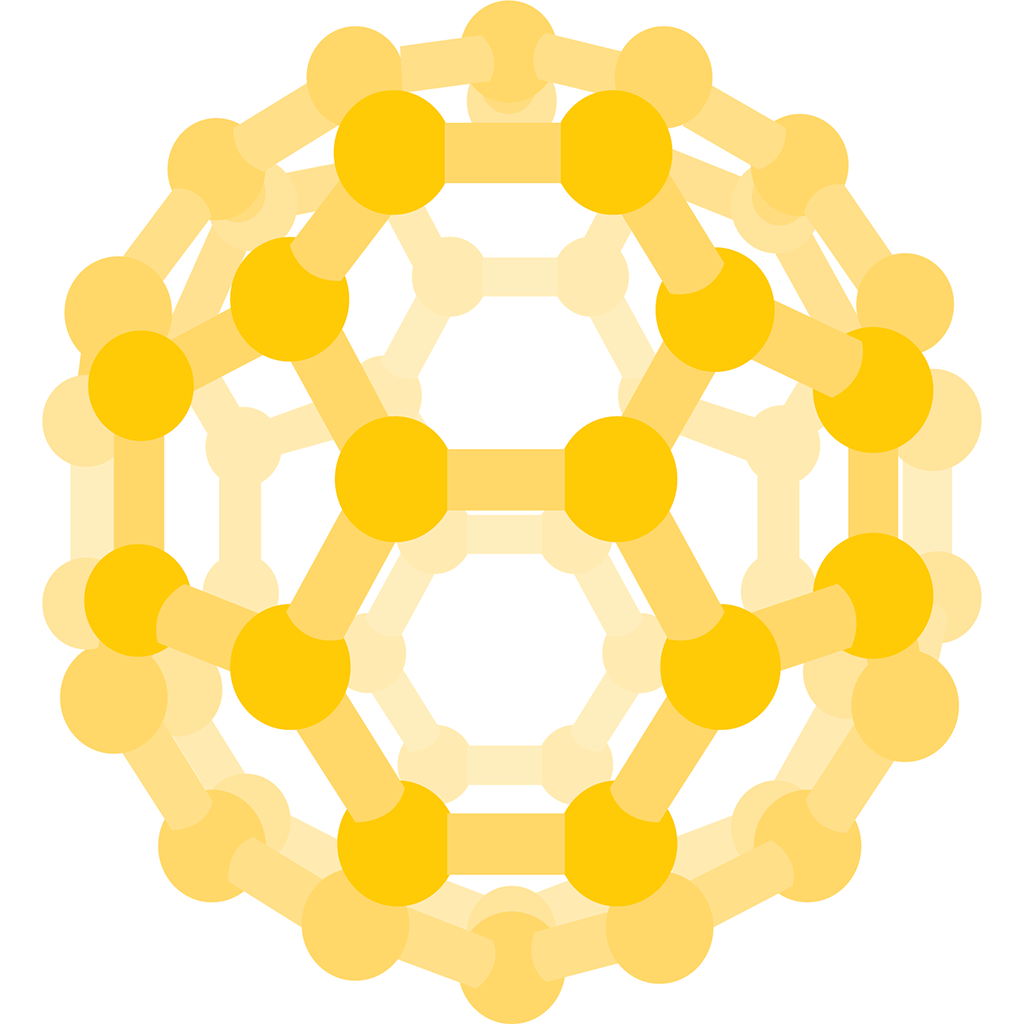
Each carbon atom of a C60 molecule bonds to three others. The molecule has 32 faces in total, 12 of which are pentagons and 20 hexagons, forming a distinctive, soccer-ball shape.
HARRY KROTO

Harold Walter Krotoschiner was born in Cambridgeshire, England, in 1939. Fascinated by the toy building set Meccano, he chose to study chemistry, and became a professor at Sussex University in 1975. He was interested in looking into space for compounds with multiple carbon-carbon bonds, such as H-C≡C-C≡C-C≡N, and found evidence using spectroscopy (studying the interaction between matter and radiated energy). When he heard of the laser spectroscopy work of Richard Smalley and Robert Curl at Rice University, he joined them in Texas, and together they discovered C60. Since 2004, Kroto has worked on nanotechnology at Florida State University.
In 1995, he set up the Vega Science Trust to make science films for education and training. They are freely available on the Internet at www.vega.org.uk.
Key works
1981 The Spectra of Interstellar Molecules
1985 60:Buckminsterfullerene (with Heath, O’Brien, Curl, and Smalley)
See also: August Kekulé • Linus Pauling