
IN CONTEXT
Chemistry
1852 Edward Frankland introduces the idea of valency – the number of bonds an atom can form with other atoms.
1858 Archibald Couper suggests that carbon atoms can link directly to one another, forming chains.
1858 Italian chemist Stanislao Cannizzaro explains the difference between atoms and molecules, and publishes atomic and molecular weights.
1869 Dmitri Mendeleev lays out the periodic table.
1931 Linus Pauling elucidates the structure of the chemical bond in general, and that of the benzene molecule in particular, using the ideas of quantum mechanics.
The early years of the 19th century saw huge developments in chemistry that fundamentally changed the scientific view of matter. In 1803, John Dalton suggested that each element was made of atoms that are unique to that element, and used the concept of atomic weight to explain how elements always combine with each other in whole-number proportions. Jöns Jakob Berzelius studied 2,000 compounds to investigate these proportions. He invented the naming system we use today – H for hydrogen, C for carbon, and so on – and compiled a list of atomic weights for all 40 elements that were then known. He also coined the term “organic chemistry” for the chemistry of living organisms – the term later came to mean most chemistry involving carbon. In 1809, French chemist Joseph Louis Gay-Lussac explained how gases combine in simple proportions by volume, and two years later the Italian Amedeo Avogadro suggested that equal volumes of gas contain equal numbers of molecules. It was clear that there were strict rules governing the combination of the elements. Atoms and molecules remained essentially theoretical concepts that nobody had seen directly, but they were concepts with growing explanatory power.
Valency
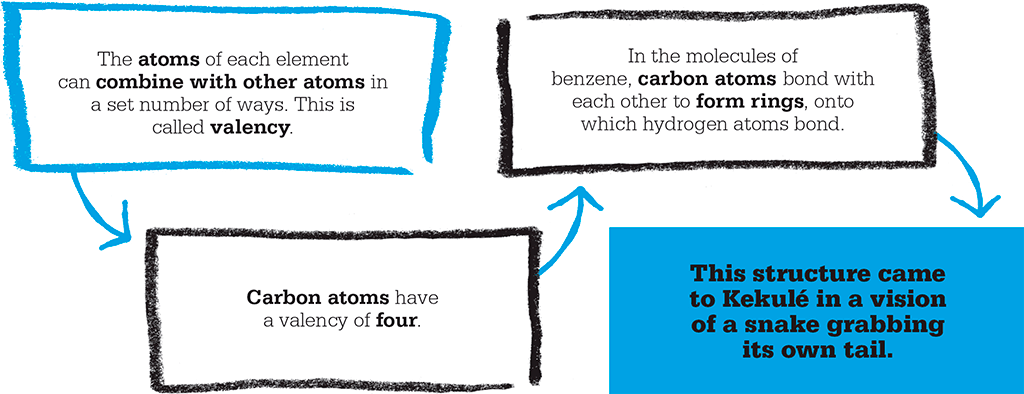
In 1852, the first step towards an understanding of how atoms combine with one another was taken by English chemist Edward Frankland, who introduced the idea of valency – which is the number of atoms each atom of an element can combine with. Hydrogen has a valency of one; oxygen has a valency of two. Then, in 1858, British chemist Archibald Couper suggested that bonds were formed between self-linking carbon atoms, and that molecules were chains of atoms bonded together. So water, which was known to consist of two parts of hydrogen to one of oxygen, could be represented as H2O, or H–O–H, where “–” signifies a bond. Carbon has a valency of four, making it tetravalent, so a carbon atom can form four bonds, as in methane (CH4), where the hydrogen atoms are arranged in a tetrahedron around the carbon. (Today, chemists think of a bond as representing a pair of electrons shared between the two atoms, and the symbols H, O, and C as representing the central part of the appropriate atom.)
Couper was working at the time at a laboratory in Paris. Meanwhile, in Heidelberg, Germany, August Kekulé had come up with the same idea, announcing in 1857 that carbon has a valency of four, and early in 1858 that carbon atoms can bond to one another. Publication of Couper’s paper had been delayed, allowing Kekulé to publish a month before him and claim priority for the idea of self-bonding carbon atoms. Kekulé called the bonds between atoms “affinities”, and explained his ideas in greater detail in his popular Textbook of Organic Chemistry, which first appeared in 1859.
"I spent a part of the night putting at least sketches of those musings down on paper. This is how the structural theory came into being."
Friedrich August Kekulé
Carbon compounds
Working out theoretical models based on evidence from chemical reactions, Kekulé declared that tetravalent carbon atoms could link together to form what he called a “carbon skeleton”, to which other atoms with other valencies (such as hydrogen, oxygen, and chlorine) could bond. Suddenly, organic chemistry began to make sense, and chemists assigned structural formulae to all sorts of molecules.
Simple hydrocarbons such as methane (CH4), ethane (C2H6), and propane (C3H8) were now seen to be chains of carbon atoms where the spare valencies were occupied by hydrogen atoms. Reacting such a compound with, say, chlorine (Cl2) produced compounds in which one or more of the hydrogen atoms were replaced by chlorine atoms, making compounds such as chloromethane or chloroethane. One feature of this substitution was that chloropropane came in two distinct forms, either 1-chloropropane or 2-chloropropane, depending on whether the chlorine was attached to the middle carbon atom or one of the end carbon atoms (see the diagram above). Some compounds need double bonds to satisfy the valencies of the atoms: the oxygen molecule (O2), for example, and the molecule of ethylene (C2H4). Ethylene reacts with chlorine, and the result is not substitution but addition. The chlorine adds across the double bond, to make 1,2 dichloroethane (C2H4Cl2). Some compounds even have triple bonds, including the nitrogen molecule (N2) and acetylene (C2H2), which is highly reactive, and used in oxyacetylene welding torches.
Benzene, however, remained a puzzle. It turned out to have the formula C6H6, but is much less reactive than acetylene, even though both compounds have equal numbers of carbon and hydrogen atoms. Devising a linear structure that was not highly reactive was a real conundrum. There clearly had to be double bonds, but how they were arranged was a mystery.
What was more, benzene reacts with chlorine not by addition (like ethylene) but by substitution: a chlorine atom replaces a hydrogen atom. When one of benzene’s hydrogen atoms is substituted by a chlorine atom, the result is only a single compound C6H5Cl, chlorobenzene. This seemed to show that all the carbon atoms were equivalent, since the chlorine atom might be attached to any one of them.

Kekulé used the concept of valency to describe the bonds that are formed between atoms to make various molecules. Here, each bond is represented by a line.
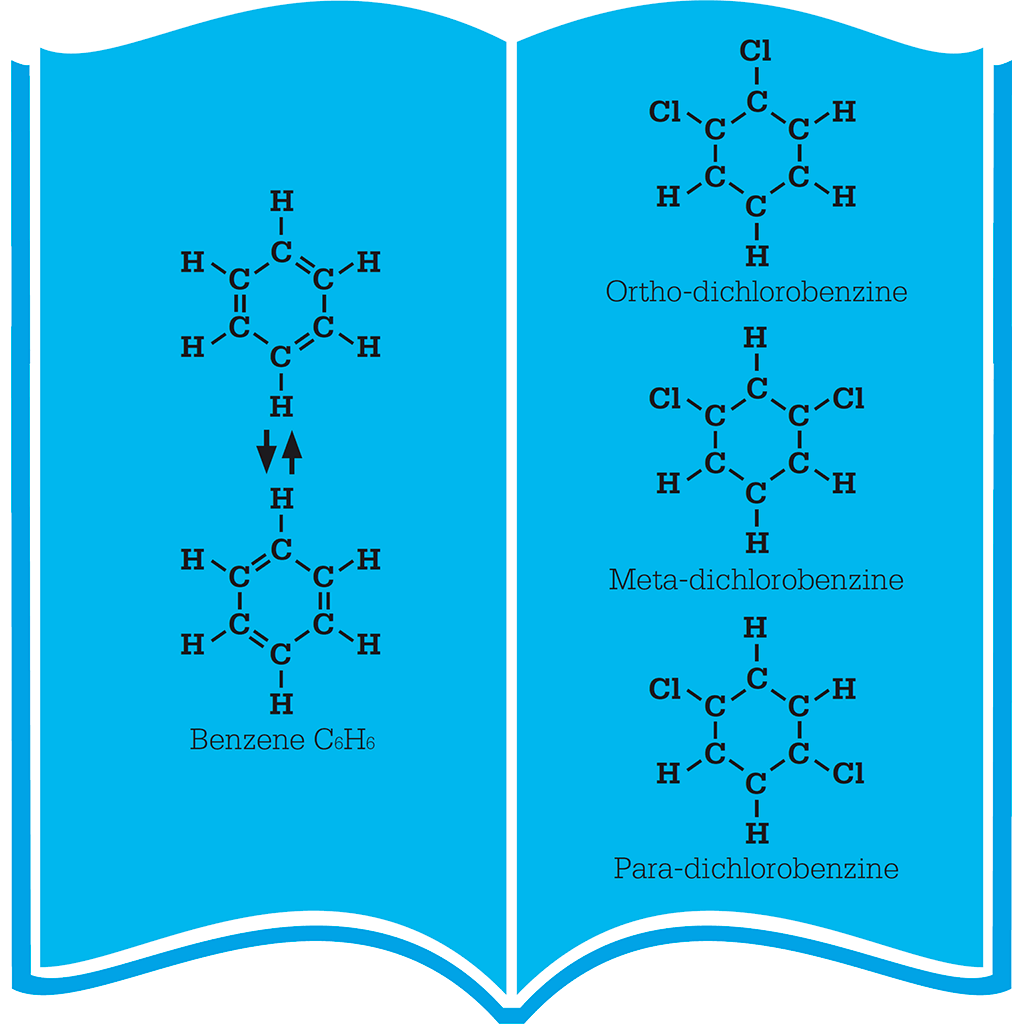
Kekulé suggested that double and single bonds between carbon atoms in a benzene ring alternated (left). Two chlorine atoms can substitute for two of the hydrogen atoms in three different ways (right).
Benzene rings
The solution to the puzzle of benzene’s structure came to Kekulé in 1865 in a dream. The answer was a ring of carbon atoms, a ring in which all six atoms were equal, with a hydrogen atom bonded to each one. This meant that the chlorine in chlorobenzene could be attached anywhere round the ring.
Further support for this theory came from substituting hydrogen twice, to make dichlorobenzene (C6H4Cl2). If benzene is a six-membered ring with all the carbon atoms equal, there should be three distinct forms, or “isomers”, of this compound – the two chlorine atoms could be on adjacent carbon atoms, on carbon atoms separated by one other carbon, or at opposite ends of the ring. This turned out to be the case, and the three isomers were named ortho-, meta-, and para-dichlorobenzene respectively.

This image of a hexabenzocoronene molecule was captured using an atomic force microscope. It is 1.4 nanometres in diameter and shows carbon–carbon bonds of different lengths.
Establishing symmetry
An unsolved mystery still remained over the observed symmetry of the benzene ring. To satisfy its tetravalency, each carbon atom should have four bonds to other atoms. This meant that they all had a “spare” bond. At first, Kekulé drew alternating single and double bonds around the ring, but when it became apparent that the ring had to be symmetrical, he suggested that the molecule oscillated between the two structures.
The electron was not discovered until 1896. The idea that bonds form through the sharing of electrons was first proposed by American chemist G N Wilson in 1916. In the 1930s, Linus Pauling then used quantum mechanics to explain that the six spare electrons in the benzene ring are not localized in double bonds, but are delocalized around the ring, and shared equally between the carbon atoms, so that the carbon-carbon bonds are neither single nor double, but 1.5. It would take these new ideas from physics to finally solve the puzzle of the structure of the benzene molecule.
Dream of inspiration
Kekulé’s report of his dream is the most cited personal account of a flash of inspiration in the whole of science. It seems that he was in a hypnagogic state – on the edge of going to sleep: that state where realities and imagination slide into one another. He described it as Halbschlaf, or half-sleep. In fact he describes two such reveries: the first, probably in 1855, on top of a bus in south London, heading for Clapham Road. “Atoms fluttered before my eyes. I had always seen these tiny particles in motion, but I had never succeeded in fathoming the manner of their motion. Today I saw how frequently two smaller ones merged into a pair; how larger ones engulfed two smaller ones, still larger ones bonded three and even four of the small ones.”
The second occasion was in his study in Ghent in Belgium, possibly inspired by the ancient ouroboros symbol of a snake biting its own tail: “The same thing happened with the benzene ring theory… I turned the chair to face the fireplace and slipped into a languorous state…atoms fluttered before my eyes.…Long rows, frequently linked more densely; everything in motion, winding and turning like snakes. And lo, what was that? One of the snakes grabbed its own tail and the image whirled mockingly before my eyes.”

Kekulé described the moment that he formulated his theory of benzene rings as a dream-like vision, in which he saw a snake biting its own tail as in the ancient symbol of the ouroboros, which is depicted here as a dragon.
AUGUST KEKULÉ

Friedrich August Kekulé, who called himself August, was born on 7 September 1829 in Darmstadt, now in the German state of Hesse. While at the University of Giessen, he abandoned the study of architecture and switched to chemistry after hearing the lectures of Justus von Liebig. He eventually became professor of chemistry at Bonn University.
In 1857 and the following years, Kekulé published a series of papers on the tetravalence of carbon, the bonding in simple organic molecules, and the structure of benzene, which made him the principal architect of the theory of molecular structure. In 1895, he was ennobled by Kaiser Wilhelm II, and became August Kekulé von Stradonitz. Three of the first five Nobel prizes in chemistry were won by his students.
Key works
1859 Textbook of Organic Chemistry
1887 The Chemistry of Benzene Derivatives or Aromatic Substances
See also: Robert Boyle • Joseph Black • Henry Cavendish • Joseph Priestley • Antoine Lavoisier • John Dalton • Humphry Davy • Linus Pauling • Harry Kroto