
IN CONTEXT
Physics
1860 The distribution of so-called black-body radiation fails to match predictions made by theoretical models.
1870s Austrian physicist Ludwig Boltzmann’s analysis of entropy (disorder) introduces a probabilistic interpretation of quantum mechanics.
1905 Albert Einstein proposes that the quantum is a real entity, using Planck’s concept of quantized light to introduce the idea of the photon.
1924 Louis de Broglie proves that matter behaves both as a particle and as a wave.
1926 Erwin Schrödinger formulates an equation for the wave behaviour of particles.
In December 1900, the German theoretical physicist Max Planck presented a paper setting out his method for resolving a long-standing theoretical conflict. In doing so, he made one of the most important conceptual leaps in the history of physics. Planck’s paper marked the turning point between the classical mechanics of Newton and quantum mechanics. The certainty and precision of Newtonian mechanics was to give way to an uncertain, probabilistic description of the Universe.
Quantum theory has its roots in the study of thermal radiation, the phenomenon that explains why we feel heat from a fire, even when the air in between it and us is cold. Every object absorbs and emits electromagnetic radiation. If its temperature rises, the wavelength of the radiation it emits decreases while its frequency increases. For example, a lump of coal at room temperature emits energy below the frequency of visible light, in the infrared spectrum. We cannot see the emission, so the coal appears black. Once we set the coal alight, however, it emits higher-frequency radiation, glowing a dull red as the emissions break into the visible spectrum, then white-hot and finally a brilliant blue. Extremely hot objects, such as stars, radiate even shorter-wavelength ultraviolet light and X-rays, which again we cannot see. Meanwhile, in addition to producing radiation, a body also reflects radiation, and it is this reflected light that gives objects colour even when they do not glow.
In 1860, German physicist Gustav Kirchhoff thought of an idealized concept he called a “perfect black body”. This is a theoretical surface that, when at thermal equilibrium (not heating up or cooling down), absorbs every frequency of electromagnetic radiation that falls on it, and does not itself reflect any radiation. The spectrum of thermal radiation coming off this body is “pure”, since it is not mixed with any reflections – it will only be the result of the body’s own temperature. Kirchhoff believed that such “black-body radiation” is fundamental in nature – the Sun, for example, comes close to being a black-body object whose emitted spectrum is almost entirely a result of its own temperature. Studying the distribution of a black body’s light would show that emission of radiation depended only on a body’s temperature, and not its physical shape or chemical composition. Kirchhoff’s hypothesis kick-started a new experimental programme aimed at finding a theoretical framework that would describe black-body radiation.
Entropy and black bodies
Planck arrived at his new quantum theory through the failure of classical physics to explain the experimental results of black-body radiation distribution. Much of Planck’s work focussed on the second law of thermodynamics, which he had identified as an “absolute”. This law states that isolated systems move over time towards a state of thermodynamic equilibrium (meaning that all parts of the system are at the same temperature). Planck attempted to explain the thermal radiation pattern of a black body by working out the entropy of the system. Entropy is a measure of disorder, though more strictly it is defined as a count of the number of ways a system can organize itself. The higher the entropy of a system, the more ways the system has of organizing and producing the same overall pattern. For instance, imagine a room where all the molecules of air start off bunched up in the top corner. There are far more ways for the molecules to organize themselves so that there is roughly the same number of them in each cubic centimetre of the room than there are for them all to remain in the top corner. Over time, they distribute themselves equally throughout the room as the entropy of the system rises. A cornerstone of the second law of thermodynamics is that entropy works in one direction only. En route to thermal equilibrium, the entropy of a system always increases or remains constant. Planck reasoned that this principle ought to be evident in any theoretical black-body model.
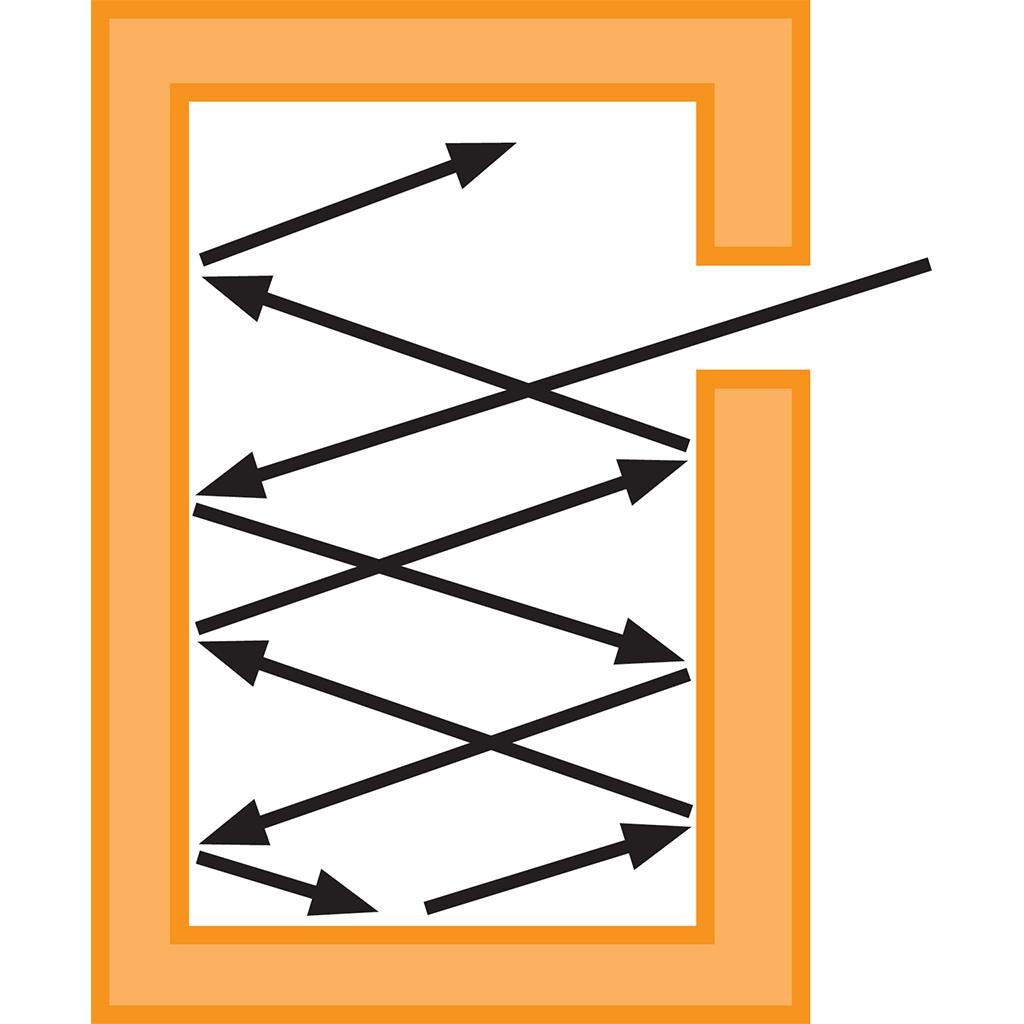
A cavity with a small hole will trap most of the radiation that enters through the hole, making it a good approximation of an ideal black body.
The Wien–Planck Law
By the 1890s, experiments in Berlin came close to Kirchhoff’s perfect black-body, using so-called “cavity radiation”. A small hole in a box kept at a constant temperature is a good approximation of a black body, as any radiation entering the box gets trapped inside, and the body’s emissions are purely a result of its temperature.
The experimental results proved bothersome for Planck’s colleague Wilhelm Wien, as the low-frequency emissions recorded did not fit at all with his equations for radiation. Something had gone wrong. In 1899, Planck arrived at a revised equation – the Wien–Planck law – that attempted a better description of the spectrum of thermal radiation from a black body.
"A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because…a new generation grows up that is familiar with it."
Max Planck
Ultraviolet catastrophe
A further challenge came a year later, when British physicists Lord Rayleigh and Sir James Jeans showed how classical physics predicts an absurd distribution of energy in black-body emission. The Rayleigh–Jeans Law predicted that, as the frequency of the radiation increased, the power it emitted would grow exponentially. This “ultraviolet catastrophe” was so radically at odds with experimental findings that the classical theory must have been seriously awry. If it were correct, a lethal dose of ultraviolet radiation would be emitted whenever a light bulb was turned on.
Planck was not too troubled by the Rayleigh–Jeans Law. He was more concerned about the Wien–Planck Law, which, even in its revised form, was not matching the data – it could accurately describe the short-wavelength (high-frequency) spectrum of thermal emission from objects, but not the long-wavelength (low-frequency emissions). This is the point at which Planck broke with his conservatism and resorted to Ludwig Boltzmann’s probabilistic approach to arrive at a new expression for his radiation law.
Boltzmann had formulated a new way to look at entropy by regarding a system as a large collection of independent atoms and molecules. While the second law of thermodynamics remained valid, Boltzmann’s reading gave it a probabilistic, rather than an absolute, truth. Thus, we observe entropy simply because it is overwhelmingly more likely than the alternative. A plate breaks but does not remake itself, but there is no absolute law preventing a plate from putting itself back together – it is just exceedingly unlikely to happen.
"Science cannot solve the ultimate mystery of nature. And that is because, in the last analysis, we ourselves are a part of the mystery that we are trying to solve."
Max Planck

No real-world object is a perfect black body, but the Sun, black velvet, and surfaces coated with lamp-black, such as coal tar, come close.
Quantum of action
Planck used Boltzmann’s statistical interpretation of entropy to arrive at a new expression for the radiation law. Imagining thermal radiation as being produced by individual “oscillators”, he needed to count the ways in which a given energy could be distributed between them.
To do this, he divided the total energy into a finite number of discrete energy chunks – a process called “quantization”. Planck was a gifted cellist and pianist and might have imagined these “quanta” in the same way that a fixed number of harmonics is available to the vibrating string of an instrument. The resulting equation was simple, and it fitted the experimental data. Introducing “quanta” of energy reduced the number of states of energy available to the system, and in doing this (although it wasn’t his aim), Planck solved the ultraviolet catastrophe. He thought of his quanta as a mathematical necessity – as a “trick” – rather than something that was real. But when Albert Einstein used the concept to explain the photoelectric effect in 1905, he insisted that quanta were a real property of light.
As with many of the pioneers of quantum mechanics, Planck spent the rest of his life struggling to come to terms with the consequences of his own work. While he was never in any doubt about the revolutionary impact of what he had done, he was – according to historian James Franck – “a revolutionary against his own will”. He found the consequences of his equations not to his taste since they often gave descriptions of physical reality that clashed with our everyday experience of the world. But for better or worse, after Max Planck, the world of physics has never been the same.
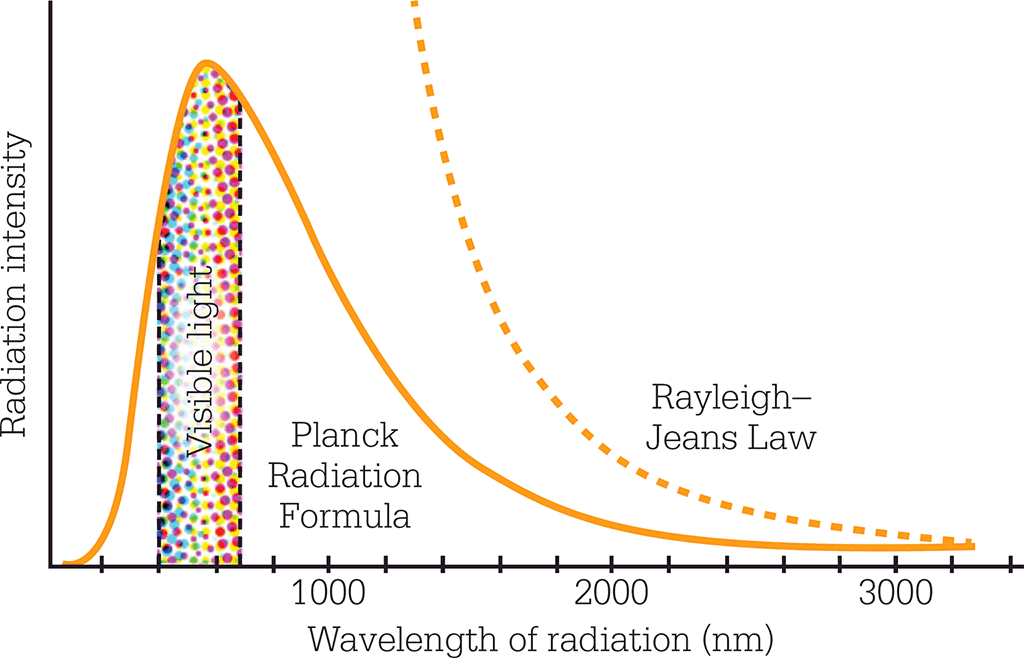
The ultraviolet catastrophe was a nonsense result predicted by classical physics (shown here as the Raleigh– Jeans Law) in which black-body radiation increased exponentially as its wavelength shortened. By quantizing radiation, Planck produced a formula that fitted with the experimental data.
MAX PLANCK

Born in Kiel in northern Germany in 1858, Planck was an able pupil at school and graduated early, aged 17. He chose to study physics at the University of Munich, where he soon became a pioneer of quantum physics. He received the Nobel Prize in Physics in 1918 for his discovery of energy quanta, although he never was able to satisfactorily describe the phenomena as a physical reality.
Planck’s personal life was beset by tragedy. His first wife died in 1909, and his eldest son was killed in World War I. Both of his twin daughters died giving birth to their children. During World War II, an Allied bomb destroyed his house in Berlin and his papers, and in the closing stages of the war, his remaining son was caught up in the plot to assassinate Hitler and was executed. Planck himself died soon after the war.
Key works
1900 Entropy and Temperature of Radiant Heat
1901 On the Law of Distribution of Energy in the Normal Spectrum
See also: Ludwig Boltzmann • Albert Einstein • Erwin Schrödinger