
IN CONTEXT
Physics
1895 Wilhelm Röntgen investigates the properties of X-rays.
1896 Henri Becquerel discovers that uranium salts emit penetrating radiation.
1897 J J Thomson discovers the electron while exploring the properties of cathode rays.
1904 Thomson proposes the “plum pudding” model of the atom.
1911 Ernest Rutherford and Ernest Marsden propose the “nuclear model” of the atom.
1932 British physicist James Chadwick discovers the neutron.
Like many major scientific discoveries, radiation was found by accident. In 1896, French physicist Henri Becquerel was investigating phosphorescence, which occurs when light falls on a substance that then emits light of a different colour. Becquerel wanted to know whether phosphorescent minerals also emitted X-rays, which had been discovered by Wilhelm Röntgen a year earlier. To find out, he placed one of these minerals on top of a photographic plate that was wrapped in thick black paper and exposed both to the Sun. The experiment worked – the plate darkened; the mineral appeared to have emitted X-rays. Becquerel also showed that metals would block the “rays” that caused the plate to darken. The next day was cloudy so he could not repeat the experiment. He left the mineral on a photographic plate in a drawer, but the plate still darkened, even without the sunshine. He realized that the mineral must have an internal source of energy, which turned out to be the result of the breakdown of atoms of uranium in the mineral he was using. He had detected radioactivity.
"It was necessary at this point to find a new para to define this new property of matter manifested by the elements of uranium and thorium. I proposed the word radioactivity."
Marie Curie
Rays produced by atoms
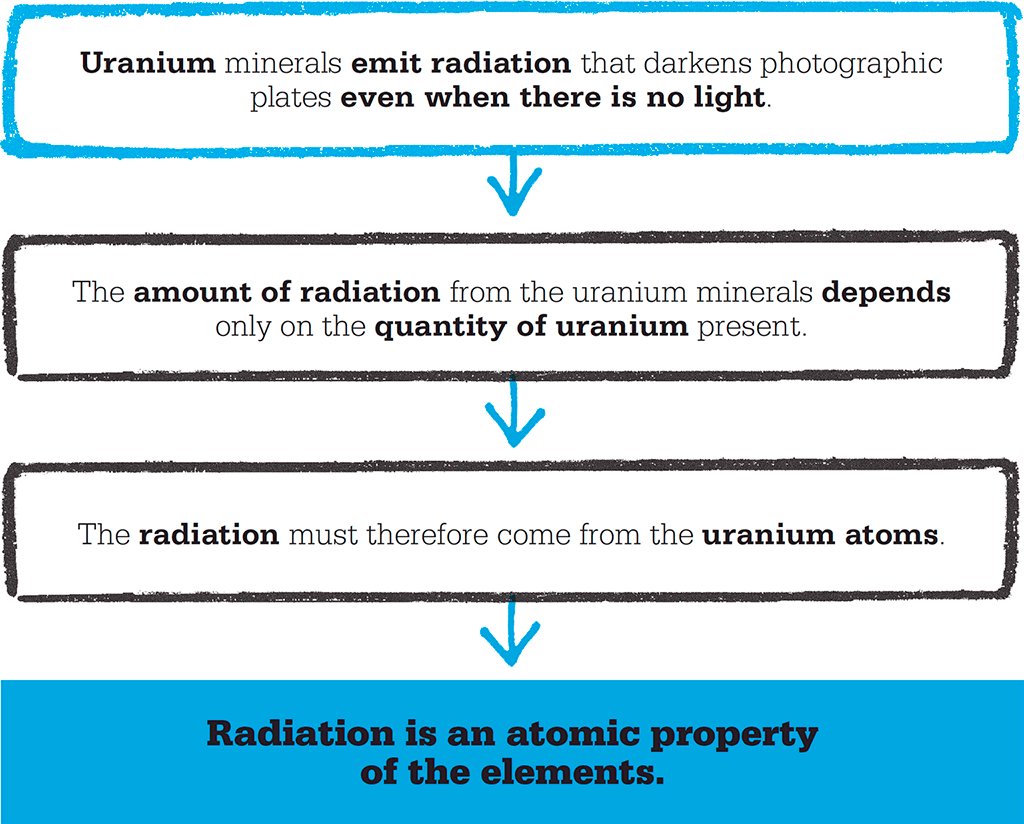
Following Becquerel’s discovery, his Polish doctoral student, Marie Curie, decided to investigate these new “rays”. Using an electrometer – a device for measuring electrical currents – she found that air around a sample of a uranium-containing mineral was conducting electricity. The level of electrical activity depended only on the amount of uranium present, not on the total mass of the mineral (which included elements other than uranium). This led her to the belief that the radioactivity came from the uranium atoms themselves, and not from any reactions between uranium and other elements.
Curie soon found that some minerals that contained uranium were more radioactive than uranium itself, and wondered whether these minerals contained another substance – one that was more active than uranium. By 1898, she had identified thorium as another radioactive element. She rushed to present her findings in a paper to the Académie des Sciences, but the discovery of thorium’s radioactive properties had already been published.
Science double
Curie and her husband Pierre worked together to discover the additional radioactive elements responsible for the high activity of the uranium-rich minerals pitchblende and chalcolite. By the end of 1898 they had announced the discovery of two new elements, which they called polonium (after her native country, Poland) and radium. They attempted to prove their discoveries by obtaining pure samples of the two elements, but it was not until 1902 that they obtained 0.1g (0.003oz) of radium chloride from a tonne of pitchblende.
During this time, the Curies published dozens of scientific papers, including one outlining their discovery that radium could help to destroy tumours. They did not patent these discoveries, but in 1903, they were jointly awarded the Nobel Prize in Physics, along with Becquerel. Marie continued her scientific work after her husband’s death in 1906, and succeeded in isolating a pure sample of radium in 1910. In 1911, she was awarded the Nobel Prize in Chemistry, becoming the first person to win or share in two prizes.

Marie and Pierre Curie did not have a dedicated laboratory. Most of their work was undertaken in a leaking shed next to the University of Paris’s School of Physics and Chemistry.
New model of the atom
The Curies’ discovery of radiation paved the way for the two New Zealand-born physicists Ernest Rutherford and Ernest Marsden to formulate their new model of the atom in 1911, but it was not until 1932 that English physicist James Chadwick discovered neutrons and the process of radiation could be fully explained. Neutrons and positively charged protons are subatomic particles that make up the nucleus of an atom, which also has negatively charged electrons whizzing around it. The protons and neutrons contribute almost all the mass of the atom. Atoms of a particular element always have the same number of protons but may have different numbers of neutrons. Atoms with different numbers of neutrons are called isotopes of the element. For example, an atom of uranium always has 92 protons in its nucleus, but may have between 140 and 146 neutrons. These isotopes are named after the total number of protons and neutrons, so the most common isotope of uranium, with 146 neutrons, is written as uranium-238 (i.e. 92 + 146).
Many heavy elements, such as uranium, have nuclei that are unstable, and this leads to spontaneous radioactive decay. Rutherford named the emissions from radioactive elements alpha, beta, and gamma rays. The nucleus becomes more stable by emitting an alpha particle, a beta particle, or gamma radiation. An alpha particle consists of two protons and two neutrons. Beta particles can be electrons or their opposites, positrons, emitted from the nucleus when a proton turns into a neutron or vice versa. Alpha and beta decay both change the number of protons in the nucleus of the decaying atom so that it becomes an atom of a different element. Gamma rays are a form of high-energy short-wave electromagnetic radiation and do not change the nature of the element.
Radioactive decay is different from the fission process that takes place inside nuclear reactors, and the fusion process that powers the Sun. In fission, unstable nuclei such as uranium-235 are bombarded with neutrons and break up to form much smaller atoms, releasing energy in the process. In fusion, two small nuclei combine to form a larger one. Fusion also releases energy, but the great temperatures and pressures required to start the process explain why scientists have only achieved fusion in the form of nuclear weapons. So far, attempts to use nuclear fusion to generate electricity consume more energy than is released.
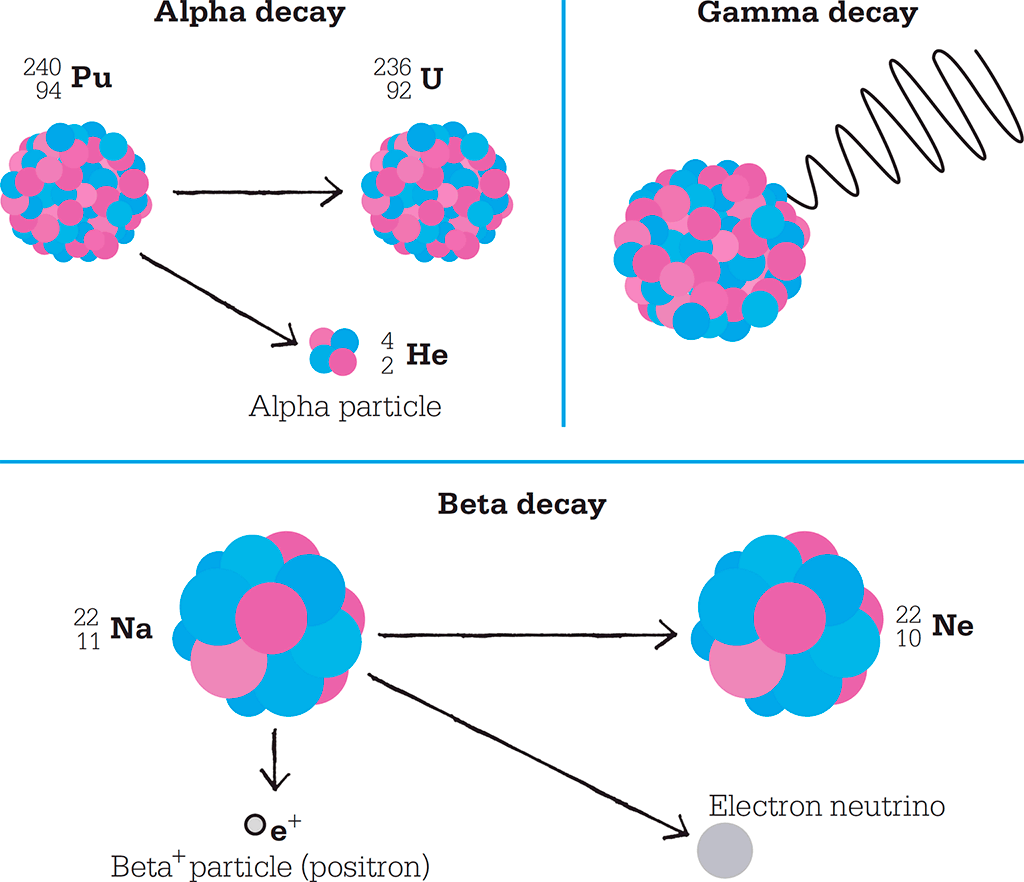
Radioactive decay can happen in three ways. Plutonium-240 (top left) decays to make uranium and an alpha particle. This is an example of alpha decay. During beta decay, sodium-22 decays to make neon, a beta particle (in this case a positron), and a neutrino. With gamma decay, a high-energy nucleus gives off gamma radiation but no particles.
Half-life
As a radioactive material decays, the atoms of the radioactive element change to other elements, and so the number of unstable atoms reduces with time. The fewer unstable atoms there are, the less radioactivity will be produced. The reduction in activity of a radioactive isotope is measured by its half-life. This is the time it takes for the activity to halve, which is the same as saying the time for the number of unstable atoms in a sample to halve. For example, the isotope technetium-99m is widely used in medicine, and has a half-life of 6 hours. This means that 6 hours after a dose is injected into a patient, the activity will be half of its original level; 12 hours after injection, the activity will be one quarter of the original level, and so on. By contrast, uranium-235 has a half-life of over 700 million years.
Radioactive dating
This idea of half-life can be used to date minerals or other materials. Many different radioactive elements with known half-lives can be used to do this, but one of the best known is carbon. The most common isotope of carbon is carbon-12, with 6 protons and 6 neutrons in each atom. Carbon-12 makes up 99 per cent of the carbon found on Earth, and has a stable nucleus. A tiny proportion of the carbon is carbon-14, which has two extra neutrons. This unstable isotope has a half-life of 5,730 years. Carbon-14 is constantly being produced in the upper atmosphere as nitrogen atoms are bombarded with cosmic rays. This means there is a relatively constant ratio of carbon-12 to carbon-14 in the atmosphere. As photosynthesizing plants take in carbon dioxide from the atmosphere, and our food consists of plants (or animals that have eaten plants), there is also a relatively constant proportion in plants and animals while they are alive, even though the carbon-14 is constantly decaying. When an organism dies, no more carbon-14 is taken into its body, while the carbon-14 already there continues to decay. By measuring the ratio of carbon-12 to carbon-14 in the body, scientists can work out how long ago the organism died.
This radiometric method is used to date wood, charcoal, bone, and shells. There are natural variations in the ratios of the carbon isotopes, but dates can be cross-checked with other dating methods such as tree-rings, and the corrections applied to objects of similar age.
"The Curie laboratory… was a cross between a stable and a potato-cellar, and, if I had not seen the worktable with the chemical apparatus, I would have thought it a practical joke."
Wilhelm Ostwald
A wonder treatment
Curie realized that radioactivity had medicinal uses. During World War I, she used the small amount of radium she had extracted to produce radon gas (a radioactive gas produced when radium decays). This was sealed into glass tubes and inserted into patients’ bodies to kill diseased tissue. It was seen as a wonder cure, and even marketed in beauty treatments to help firm up ageing skin. It was only later that the importance of using materials with a short half-life was recognized.
Radioactive isotopes are also widely used in medical imaging to diagnose disease, and in treatment of cancer. Gamma rays are used to sterilize surgical instruments, and even food, to increase its shelf-life. Gamma ray emitters can be used for the internal inspection of metal objects, to detect cracks, or to inspect the contents of cargo containers to identify contraband.

The erection of Ale’s stones in Sweden was dated to 600 CE by the radiometric dating of wooden tools found at the site. The actual stones are hundreds of millions of years older.
MARIE CURIE

Maria Salomea Skłodowska was born in Warsaw in 1867. At that time Poland was under Russian rule and women were not allowed into higher education. She worked to help finance her sister’s medical studies in Paris, France, and in 1891 moved there herself to study mathematics, physics, and chemistry. There, she married her colleague, Pierre Curie, in 1895. When her daughter was born in 1897, she began teaching to help support the family, but continued to research with Pierre in a converted shed. After Pierre’s death, she accepted his chair at the University of Paris, the first woman to hold this position. She was also the first woman to be awarded a Nobel Prize, and the first to be awarded a second Nobel. During World War I, she helped set up radiology centres. She died in 1934 of anaemia, probably caused by her long exposure to radiation.
Key works
1898 Emissions of Rays by Uranium and Thorium Compounds
1935 Radioactivity
See also: Wilhelm Röntgen • Ernest Rutherford • J Robert Oppenheimer