
IN CONTEXT
Physics
c.400 BCE Greek philosopher Democritus envisages atoms as solid, indestructible building blocks of matter.
1805 John Dalton’s atomic theory of matter marries chemical processes to physical reality and allows him to calculate atomic weights.
1896 Nuclear radiation is discovered by Henri Becquerel, and is used to reveal the internal structure of the atom.
1938 Otto Hahn, Fritz Strassman, and Lise Meitner split the atomic nucleus.
2014 Firing increasingly energetic particles at the nucleus continues to reveal a slew of new subatomic particles and antiparticles.
The discovery at the turn of the 20th century that the basic constituent of matter – the atom – could be broken into smaller fragments was a watershed moment for physics. This astonishing breakthrough revolutionized ideas about how matter is constructed and the forces that hold it and the Universe together. It revealed an entirely new world at the subatomic level – one that required a new physics to describe its interactions – and a slew of tiny particles that filled this infinitesimally small domain.
Atomic theories have a long history. The Greek philosopher Democritus developed the ideas of earlier thinkers that everything is composed of atoms. The Greek word átomos, which is credited to Democritus, means indivisible and referred to the basic units of matter. Democritus thought that the materials must reflect the atoms they are made of – so atoms of iron are solid and strong, while those of water are smooth and slippery.
At the turn of the 19th century, English natural philosopher John Dalton proposed a new atomic theory based on his “law of multiple proportions”, which explained how elements (simple, uncombined substances) always combine in simple, whole-number ratios. Dalton saw that this meant that a chemical reaction between two substances is no more than the fusing of individual small components, repeated countless times. This was the first modern atomic theory.
A stable science
A self-congratulatory mood was detectable in physics at the end of the 19th century. Certain eminent physicists made grandstanding, declarations to the effect that the subject was all but finished – that the principal discoveries had all been made and the programme going forwards was one of improving the accuracy of known quantities “to the sixth decimal place”. However, many research physicists of the time knew better. It was already clear that they were facing an entirely new and strange set of phenomena that defied explanation.
In 1896, Henri Becquerel, following a lead from Wilhelm Röntgen’s discovery of mysterious “X-rays” the previous year, had found an unexplained radiation. What were these new radiations and where were they coming from? Becquerel correctly surmised that this radiation was emanating from within uranium salts. When Pierre and Marie Curie studied the decay of radium, they discovered a constant and seemingly inexhaustible source of energy inside radioactive elements. If this were the case, it would break several fundamental laws of physics. Whatever these radiations were, it was clear that there were large gaps in current models.
J J Thomson is pictured here at work in his Cambridge laboratory. Thomson’s “plum pudding” model of the atom was the first to include the newly discovered electron.
Discovery of the electron
The following year, the British physicist Joseph John (J J) Thomson caused a sensation when he demonstrated that he could break lumps out of atoms. While investigating the “rays” emanating from high-voltage cathodes (negatively charged electrodes), he found that this particular kind of radiation was made of individual “corpuscles”, since it created momentary, point-like sparkles of light on hitting a phosphorescent screen; it was negatively charged, since a beam could be deflected by an electric field; and it was exceedingly light, weighing less than a thousandth of the lightest atom, hydrogen. Moreover, the weight of the corpuscle was the same, no matter which element was used as a source. Thomson had discovered the electron. These results were totally unanticipated theoretically. If an atom contains charged particles, why shouldn’t the opposing particles have equal mass? Previous atomic theories held that atoms were solid lumps. As befit their status as the most basic constituent of matter, they were entire, whole, and perfect. But when viewed in the light of Thomson’s discovery, they clearly were divisible. Put together, these new radiations raised the suspicion that science had failed to understand the vital components of matter and energy.
The plum-pudding model
Thomson’s discovery of the electron earned him the Nobel Prize for Physics in 1906. He was enough of a theoretician, however, to see that a radical new model of the atom was needed to adequately incorporate his findings. His answer, produced in 1904, was the “plum-pudding” model. Atoms have no overall electric charge and, since the mass of this new electron was small, Thomson postulated that a larger positively charged sphere contained most of the atom’s mass, and the electrons were embedded in it like plums in the dough of a Christmas pudding. With no evidence to suggest otherwise, it was sensible to assume that the point charges, like the plums in a pudding, were arbitrarily distributed across the atom.
Rutherford revolution
However, the positively charged parts of the atom steadfastly refused to reveal themselves, and the hunt was on to locate the missing member of the atomic pair. The quest resulted in a discovery that would produce a very different visualization of the internal structure of the basic unit of all elements.
At the Physical Laboratories at the University of Manchester, Ernest Rutherford devised and directed an experiment to test Thomson’s plum-pudding model. This charismatic New Zealander was a gifted experimentalist with a keen sense of which details to pursue. Rutherford had received the 1908 Nobel Prize in Physics for his “Theory of Atomic Disintegration”.
The theory proposed that the radiations emanating from radioactive elements were the result of their atoms breaking apart. With the chemist Frederick Soddy, Rutherford had demonstrated that radioactivity involved one element spontaneously changing into another. Their work was to suggest new ways to probe the inside of the atom and see what was there.
"All science is either physics or stamp collecting."
Ernest Rutherford
Radioactivity
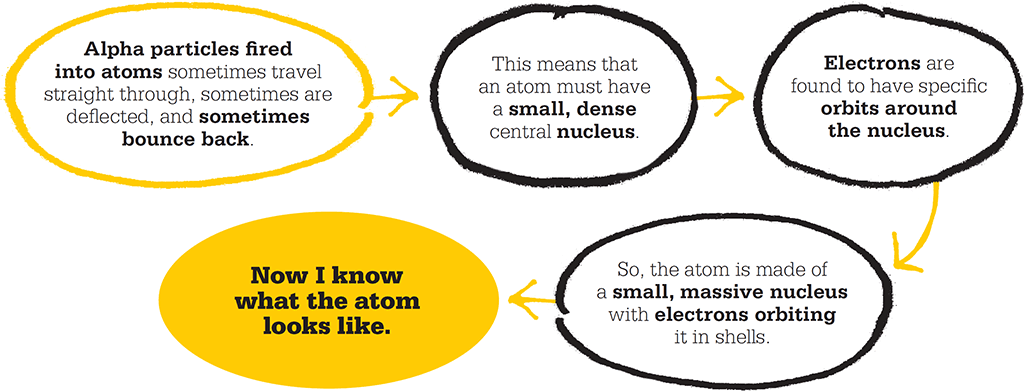
Although radioactivity was first encountered by Becquerel and the Curies, it was Rutherford who identified and named the three different types of what we would now call nuclear radiation. These are slow-moving, heavy, positively charged “alpha” particles; fast-moving, negatively charged “beta” particles; and highly energetic but uncharged “gamma” radiation. Rutherford classed these different forms of radiation by their penetrating power, from the least-penetrating alpha particles, which are blocked by thin paper, to gamma rays that require a thickness of lead to be stopped. He was the first to use alpha particles to explore the atomic realm. He was also the first to outline the notion of radioactive half-life and discover that “alpha particles” were helium nuclei – atoms stripped of their electrons.
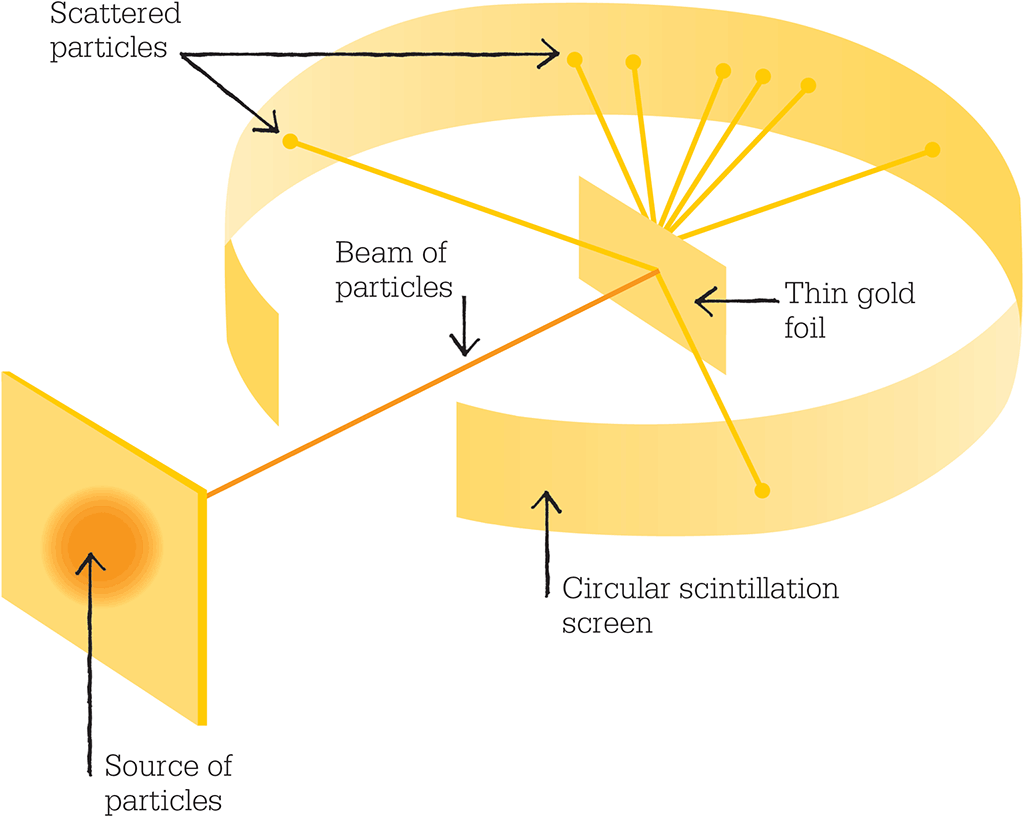
Geiger and Marsden aimed alpha particles from a radioactive source at an incredibly thin gold leaf. The scintillation screen could be spun around to detect particles rebounding at any given angle.
The gold foil experiment
In 1909, Rutherford set out to probe the structure of matter using alpha particles. The previous year, along with the German Hans Geiger, he had developed zinc sulphide “scintillation screens”, which enabled individual collisions of alpha particles to be counted as brief bright flashes, or scintillations. With the help of undergraduate student Ernest Marsden, Geiger would use these screens to determine whether matter was infinitely divisible or whether atoms contained fundamental building blocks.
They fired a beam of alpha particles from a radium source at an extremely thin strip of gold leaf, just a thousand or so atoms thick. If, as the plum-pudding model held, gold atoms consisted of a diffuse cloud of positive charge with point-like negative charges, then the massive, positively charged alpha particles would plough straight through the foil. Most of the particles would be deflected only slightly by interaction with the gold atoms and would be scattered across shallow angles.
Geiger and Marsden spent long hours sat in the darkened laboratory, peering down microscopes and counting the tiny flashes of light on the scintillation screens. Then, acting on a hunch, Rutherford instructed them to position screens that would catch any high-angle deflections as well as at the expected low-angle scintillations. With these new screens in place, they discovered that some of the alpha particles were being deflected by more than 90º, and others were rebounding off the foil back the way they came. Rutherford described the result as like firing a 15-inch shell at tissue paper and having it bounce back.
"It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
Ernest Rutherford
The nuclear atom
Halting heavy alpha particles in their tracks or deflecting them by high angles was possible only if the positive charge and mass of an atom were concentrated in small volume. In light of these results, in 1911, Rutherford published his conception of the structure of the atom. The “Rutherford Model” is a solar system in miniature, with electrons orbiting a small, dense, positively charged nucleus. The model’s major innovation was the infinitesimally small nucleus, which forced the uncomfortable conclusion that the atom is not at all solid. Matter at an atomic scale is mostly space, governed by energy and force. This was a definitive break from the atomic theories of the previous century.
While Thomson’s “plum-pudding” atom had been an instant hit, Rutherford’s model was largely ignored by the scientific community. Its failings were all too plain to see. It was well established that accelerating electric charges emit energy as electromagnetic radiation. Thus, as electrons swoop around the nucleus – experiencing circular acceleration that keeps them in their orbits – they ought to be continually emitting electromagnetic radiation. Steadily losing energy as they orbited, the electrons would spiral inexorably into the nucleus. According to Rutherford’s model, atoms ought to be unstable, but clearly they are not.
A quantum atom
Danish physicist Niels Bohr saved the Rutherford model of the atom from languishing in obscurity by applying new ideas about quantization to matter. The quantum revolution had begun in 1900 when Max Planck had proposed the quantization of radiation, but the field was still in its infancy in 1913 – it would have to wait until the 1920s for a formalized mathematical framework of quantum mechanics. At the time Bohr was working on this problem, quantum theory essentially consisted of no more than Einstein’s notion that light comes in tiny “quanta” (discrete packets of energy) that we now call photons. Bohr sought to explain the precise pattern of absorption and emission of light from atoms. He suggested that each electron is confined to fixed orbits within atomic “shells”, and that the energy levels of the orbits are “quantized” – that is, they can only take certain specific values.
In this orbital model, the energy of any individual electron is closely related to its proximity to the atom’s nucleus. The closer an electron is to the nucleus, the less energy it has, but it can be excited into higher energy levels by absorbing electromagnetic radiation of a certain wavelength. On absorbing light, an electron leaps to a “higher”, or outer, orbit. On attaining this higher state, the electron will promptly drop back into the lower-energy orbit, releasing a quantum of energy that precisely matches the energy gap between the two orbitals.
Bohr offered no explanation for what this meant or what it might look like – he simply stated that falling out of orbit into the nucleus was, for electrons, impossible. Bohr’s was a purely theoretical model of the atom. However, it agreed with experiment and solved many associated problems in an elegant stroke. The way in which electrons would have to fill up empty shells in a strict order, getting progressively farther from the nucleus, matched the march of the properties of the elements seen across the periodic table as atomic number increases. Even more convincing was the way in which the theoretical energy levels of the shells neatly fitted actual “spectral series” – the frequencies of light absorbed and emitted by different atoms. A long-sought-after way to marry electromagnetism and matter had been realized.
"If your experiment needs statistics, you ought to have done a better experiment."
Ernest Rutherford
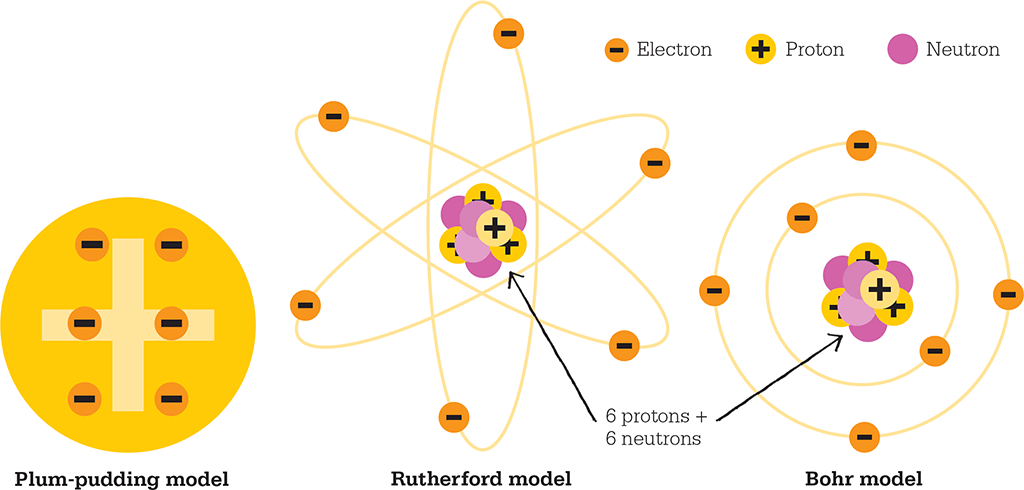
The plum-pudding model of the atom with the electrons spread across a diffuse nucleus was replaced by Rutherford’s model with electrons in orbit around a small, dense nucleus. Bohr refined Rutherford’s model by adding quantized orbits for the electrons. Here, a carbon atom is illustrated.
Going inside the nucleus
Once this picture of the nuclear atom had been accepted, the next stage was to ask what lay inside the nucleus. In experiments reported in 1919, Rutherford found that his beams of alpha particles could generate hydrogen nuclei from many different elements. Hydrogen had long been recognized as the simplest of all the elements and thought of as a building block for all other elements, so Rutherford proposed that the hydrogen nucleus was in fact its own fundamental particle, the proton.
The next development in atomic structure was James Chadwick’s 1932 discovery of the neutron, in which Rutherford once again had a hand. Rutherford had postulated the existence of the neutron in 1920 as a way to compensate for the repulsive effect of many point-sized positive charges crammed into a tiny nucleus. Like charges repel each other, so he theorized that there must be another particle that somehow dissipates the charge or binds the jostling protons tightly together. There was also extra mass in elements heavier than hydrogen, which could be accounted for by a third, neutral but equally massive subatomic particle.
However, the neutron proved difficult to detect and it took nearly a decade of searching to find it. Chadwick was working at the Cavendish Laboratory under the supervision of Rutherford. Guided by his mentor, he was studying a new kind of radiation that had been found by the German physicists Walther Bothe and Herbert Becker when they bombarded beryllium with alpha particles.
Chadwick duplicated the Germans’ results and realized that this penetrating radiation was the neutron Rutherford had been looking for. A neutral particle, such as the neutron, is much more penetrating than a charged particle, such as a proton, because it feels no repulsion as it passes through matter. However, with mass slightly greater than a proton, it can easily knock protons out of the nucleus, something that otherwise only extremely energetic electromagnetic radiation can do.
"The difficulties disappear if it be assumed that the radiation consists of particles of mass 1 and charge 0, or neutrons."
James Chadwick
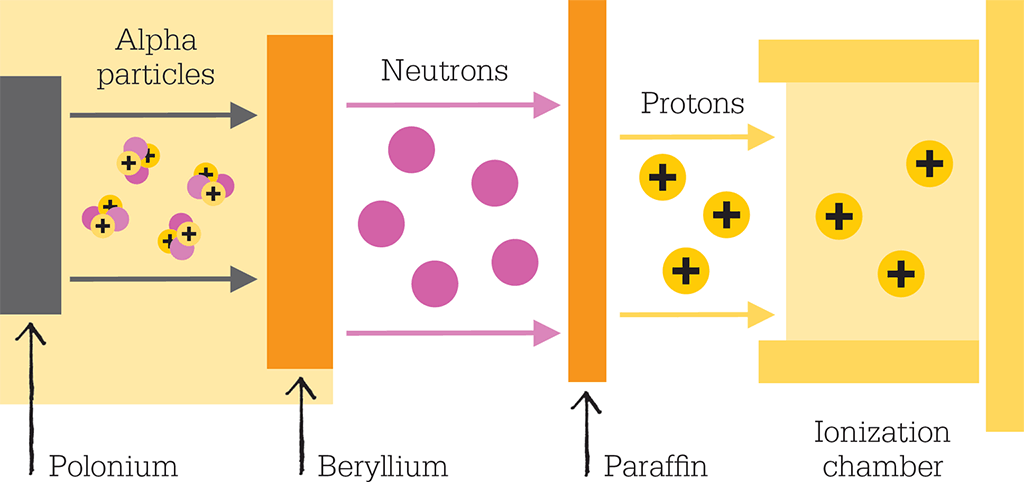
James Chadwick discovered the neutron by bombarding beryllium with alpha particles from radioactive polonium. The alpha particles knocked neutrons out of the beryllium. Then the neutrons dislodged protons from a layer of paraffin, and these protons were detected by an ionization chamber.
Electron clouds
The discovery of the neutron completed the picture of the atom as a massive nucleus with electrons in orbit around it. New discoveries in quantum physics would refine our view of electrons in orbit around a nucleus. Modern models of the atom feature “clouds” of electrons, which represent only those areas in which we are most likely to find an electron, according to its quantum wavefunction.
The picture has been further complicated by the discovery that neutrons and protons are not fundamental particles, but are made of arrangements of smaller particles called quarks. Questions to do with the true structure of the atom are still actively being researched.
ERNEST RUTHERFORD

Brought up in rural New Zealand, Ernest Rutherford was working in the fields when the letter from J J Thomson arrived informing him of a scholarship to Cambridge University. In 1895, he was made a research fellow at the Cavendish Laboratories, where he conducted experiments alongside Thomson that led to the discovery of the electron. In 1898, aged 27, Rutherford took up a professorial post at McGill University in Montreal, Canada. It was there that he carried out the work on radioactivity that won him the 1908 Nobel Prize in Physics.
Rutherford was an accomplished administrator, too, and during his lifetime headed up the three top physics research laboratories. In 1907, he took the chair in physics at the University of Manchester where he discovered the atomic nucleus. In 1919, he returned to the Cavendish as director.
Key works
1902 The Cause and Nature of Radioactivity, I & II
1909 The Nature of the Particle from Radioactive Substances
See also: John Dalton • August Kekulé • Wilhelm Röntgen • Marie Curie • Max Planck • Albert Einstein • Linus Pauling • Murray Gell-Mann