11 | BLOOD–BRAIN BARRIER OPENING AND DRUG DELIVERY USING FOCUSED ULTRASOUND AND MICROBUBBLES
ELISA E. KONOFAGOU
INTRODUCTION
Current treatments of neurological and neurodegenerative diseases are limited due to the lack of a truly noninvasive, transient, and regionally selective brain drug delivery method (Pardridge, 2005). The brain is particularly difficult to deliver drugs to because of the blood–brain barrier (BBB). The impermeability of the BBB is due to tight junctions connecting adjacent endothelial cells and highly regulatory transport systems of the endothelial cell membranes (Abbott et al., 2006). The main function of the BBB is ion and volume regulation in order to ensure conditions necessary for proper synaptic and axonal signaling (Stewart and Tuor, 1994). However, the same impermeability properties that keep the brain healthy are the reason for the difficulties in its efficient pharmacological treatment. The BBB prevents most neurologically active drugs from entering the brain, and, as a result, has been determined as the rate-limiting factor in brain drug delivery (Pardridge, 2005). Until a solution to the trans-BBB delivery problem is found, treatments of neurological diseases will remain impeded.
BBB PHYSIOLOGY: STRUCTURE AND FUNCTION
The BBB is a specialized substructure of the vascular system, consisting of endothelial cells connected together by tight junctions. The luminal and abluminal membranes line the inner wall of the vessel and act as the permeability barrier. The combination of tight junctions and these two membranes characterizes the BBB as having low permeability to large and ionic substances. However, certain molecules such as glucose and amino acids are exceptions because they are actively transported. It has also been shown that lymphocytes can traverse the BBB by going through temporarily opened tight junctions of the endothelial walls. The astrocytes have been proven to offer a protective mechanism of the neurons to any mechanical effect (Abbott et al., 2006).
BBB AND NEUROTHERAPEUTICS
Several neurological disorders remain intractable to treatment by therapeutic agents because of the BBB, the brain’s natural defense. By acting as a permeability barrier, the BBB impedes entry from blood to the brain of virtually all molecules with higher than 400 Da of molecular weight, thus rendering many potent neurologically active substances and drugs ineffective simply because they cannot be delivered to where they are needed. As a result, traversing the BBB remains the rate-limiting factor in brain drug delivery development (Pardridge, 2005, 2006).
FOCUSED ULTRASOUND
Focused ultrasound (FUS) utilizes the same concept of acoustic wave propagation as the more widely known diagnostic ultrasound applications. However, instead of acquiring and displaying echoes generated at several tissue interfaces for imaging, FUS employs concave transducers that usually have a single geometric focus, at which most of the power is delivered during sonication in order to induce mechanical effects, thermal effects, or both. Note that the more widely used “high-intensity focused ultrasound (HIFU)” name of the method is not used here for BBB opening since the intensities used are low, that is, on the level of what is used in diagnostic ultrasound.
BBB OPENING USING FUS AND MICROBUBBLES
Blood–brain barrier opening induced by ultrasound at or near ablation intensities was first observed while accompanied by neuronal damage (Bakay et al., 1956; Ballantine et al., 1960; Patrick et al., 1990; Vykhodtseva et al., 1995). After reducing the acoustic intensity and duty cycle (the time the power is on relative to the time the power is off), BBB opening was still observed, but without the macroscopic damage detected as lesions (Mesiwala et al., 2002). With the addition of intravenously (IV) injected microbubbles prior to sonication, BBB opening was determined to be transient (Hynynen et al., 2001) in the presence of Optison™ (Optison™; Mallinckrodt Inc., St. Louis, MO), which are albumin-coated, octafluoropropane-filled microbubbles of 3–4.5 μm in diameter and are usually used to enhance blood vessels on clinical ultrasound images through opacification. The BBB opening procedure could also be monitored with MRI and MR contrast agents (Hynynen et al., 2001). This showed the potential of opening the BBB without damaging parenchymal cells, such as neurons. Further investigation entailed study of this phenomenon with Optison™ to search for a difference in threshold of BBB opening and neuronal damage and understand the mechanism of the opening in rabbits, with (Hynynen et al., 2003; McDannold et al., 2004; Sheikov et al., 2004) or without (Hynynen et al., 2005) a craniotomy. The advantage of having microbubbles present in the blood supply is that it allows for the reduction of the ultrasound intensity, the containment of most of the disruption within the vasculature, and the reduction of the likelihood of irreversible neuronal damage (Choi et al., 2005, 2006, 2007a, 2007b; Hynynen et al., 2003, 2005, 2006; McDannold et al., 2004, 2005, 2006; Sheikov et al., 2004, 2006). Although there are many indications that damage can be contained to minimal hemorrhage (Hynynen et al., 2006), the complete safety profile remains to be assessed. In addition, indications to various mechanisms such as the dilation of vessels, temporary ischemia, mechanically induced opening of the tight junctions, and the activation of various transport mechanisms have been reported (Mesiwala et al., 2002; Sheikov et al., 2004, 2006).
Our group has demonstrated feasibility of BBB opening through intact skull and skin and successful imaging of the BBB opening in the area of the hippocampus at submillimeter imaging resolution using a 9.4T MR scanner in both wild-type (Choi et al., 2005, 2007a, 2007b; Konofagou et al., 2009) and Alzheimer’s mice (Choi et al., 2008). Our group also concentrates on a specific brain region (e.g., the hippocampus), which is key in neurodegenerative disease, such as Alzheimer’s, and can be successfully and reproducibly targeted (Konofagou and Choi, 2008). Delivery of molecules of up to 2,000 kDa in molecular weight was also demonstrated (Choi et al., 2010). Preliminary histology indicated no structural damage in the area of the hippocampus (Baseri et al., 2010). Finally, it is important to note that the microbubbles used for BBB opening have been approved by the Food and Drug Administration (FDA) for human use in contrast echocardiography, for example, for the detection of myocardial infarction (Kaufmann et al., 2007). It is equally important to specify that the pressure amplitudes used for BBB opening are of similar range to ultrasound diagnostic levels (<1.5–2 MPa) and, therefore, assumed safe for human use (Christensen, 1988) while the pulse duration is by orders of magnitude longer.
MICROBUBBLES IN CONTRAST ULTRASOUND AND ASSOCIATED BIOEFFECTS
Currently, in the United States, microbubbles are only FDA approved for echocardiography in patients with suboptimal images of the cardiac chambers. However, microbubbles have shown promise for imaging myocardial perfusion using intermittent contrast destruction pulses. Therefore, most in vivo bioeffects studies have focused on the heart (Miller, 2007). For a given frequency, separate pressure thresholds exist for microbubble destruction and the onset of bioeffects (Chen et al., 2002; Li et al., 2003, 2004). Safe cardiac perfusion imaging would then be done with the microbubble clearance pulse being between these thresholds. Extravascular drug delivery to the brain would then be performed near the threshold for transient opening, but well below the conditions for permanent damage. Human doses for commercially available microbubbles used in contrast echocardiography could provide a useful benchmark for therapy trials. However, the human dose for imaging purposes varies widely (Table 11.1). A typical dose ranges between 6 and 12 × 107 microbubbles per kg (60–120 microbubbles per mg). Mean diameters are given, but detailed information of the polydispersed size distributions is lacking. Thus, the effects of microbubble size and concentration on safety are difficult to decouple from previous studies using these commercial agents. Our ability to generate and isolate microbubbles of distinct and narrow size distributions with well-defined concentrations will allow us to probe these effects in the proposed study.
TABLE 11.1. Clinically used contrast agents and their specifications

Several studies have shown an increase in bioeffects with increasing microbubble dose. For Definity and Optison, increases in rat cardiomyocyte cell death and premature heartbeats and microvessel leakage were found after exposure to ultrasound, also called sonication (Li et al., 2003; Miller et al., 2005). Similar dose–response relationships have been observed for BBB opening (Kaps et al., 2001; Yang et al., 2007). Miller et al. (2005) compared insonation of Optison, Definity, and Imagent in the rat heart and found that microvascular effects were similar when expressed as the number of microbubbles injected. They concluded that shell type and encapsulated gas have little effect on bioeffects. Given the polydispersed size distribution of the different formulations, however, the effects of size are difficult to glean from that study. However, little is known about the effects of microbubble size on bioeffects. Christiansen et al. (2003) found that intra-arterial injection was more effective than intravenous injection for gene transfection through sonoporation. This result was attributed to the difference in microbubble sizes delivered to the insonified region. Several biophysical studies have shown remarkable size dependence for microbubble oscillation and destruction (Borden et al., 2005; Chomas et al., 2001).
CLINICAL RELEVANCE OF BBB DISRUPTION
NEURODEGENERATIVE DISEASE
Over 4 million U.S. men and women suffer from Alzheimer’s disease: 1 million from Parkinson’s disease, 350,000 from multiple sclerosis, and 20,000 from ALS (amyotrophic lateral sclerosis). Worldwide, these four diseases account for more than 20 million patients. Although great progress has been made in recent years toward the understanding of neurodegenerative diseases like Alzheimer’s, Parkinson’s, multiple sclerosis, ALS, and others, few effective treatments and no cures are currently available. Aging greatly increases the risk of neurodegenerative disease, and the average age of Americans is steadily increasing. Today, over 35 million Americans are over the age of 65. Within the next 30 years, this number is likely to double, putting more and more people at increased risk of neurodegenerative disease. Alzheimer’s disease, which has emerged as one of the most common brain disorders, begins in the hippocampal formation and gradually spreads to the remaining brain at its most advanced stages, and it is characterized partly by deposition of amyloid plaques in the brain tissue but also in the blood vessels themselves (Iadecola, 2004). For the purpose of this study, we will focus on the treatment of Alzheimer’s disease through the FUS-induced BBB opening, and therefore, the targeted region in the brain will be the hippocampus.
DRUG DELIVERY IN NEURODEGENERATIVE DISEASE
Over the past decade, numerous small- and large-molecule products have been developed for treatment of neurodegenerative diseases with mixed success. When administered systemically in vivo, the BBB inhibits their delivery to the regions affected by those diseases. A review of the Comprehensive Medicinal Chemistry database indicates that only 5% of the more than 7,000 small-molecule drugs treat the central nervous system (CNS) (Pardridge, 2006). With these, only four CNS disorders can be treated: depression, schizophrenia, epilepsy, and chronic pain (Ghose et al., 1999; Lipinski, 2000). Despite the availability of pharmacological agents, potentially devastating CNS disorders and age-related neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and amythrophic lateral sclerosis, remain undertreated mainly because of the impermeability of the BBB (Pardridge, 2005, 2006). The goal of this proposal is thus to optimize the FUS method and elucidate its mechanism in order to ultimately deliver therapeutics to the brain and significantly facilitate treatment of currently intractable and devastating neurodegenerative diseases.
A successful drug delivery system requires transient, localized, and noninvasive targeting of a specific tissue region. None of the current techniques clinically used, or currently under research, address these issues within the scope of the treatment of neurodegenerative diseases. As a result, the present situation in neurotherapeutics enjoys few successful treatments for most CNS disorders. Some of those routes of administration are listed in Table 11.2. Several pharmaceutical companies use the technique known as “lipidization,” which is the addition of lipid groups to the polar ends of molecules to increase the permeability of the agent (Fischer et al., 1998). However, the effect is not localized as the permeability of the drug increases not only in the targeted region, but also over the entire brain and body. There can thus be a limit to the amount absorbed before the side effects become deleterious (Fischer et al., 1998).
TABLE 11.2. Techniques shown to induce trans-BBB transport or BBB disruption

A second set of techniques under study are neurosurgically based drug delivery methods, which involve the invasive implantation of drugs into a region by a needle (Blasberg et al., 1975; Fung et al., 1996). The drug spreads through diffusion and is localized to the targeted region, but diffusion does not allow for molecules to travel far from their point of release. In addition to this, invasive procedures traverse untargeted brain tissue, causing unnecessary damage. As a result, effective drugs have recently been shelved after reports of adverse effects. Other techniques utilize solvents mixed with drugs or adjuvants (pharmacological agents) attached to drugs to disrupt the BBB through dilation and contraction of the blood vessels [Pardridge, 2005, 2006, 2007]). However, this disruption is not localized within the brain, and the solvents and adjuvants used are potentially toxic. This technique may constitute a delivery method specific to the brain, but it requires special attention to each type of drug molecule and a specific transport system resulting in a time-consuming and costly process while still not being completely localized to the targeted region. FUS in combination with microbubbles constitutes thus the only truly transient, localized, and noninvasive technique for opening the BBB. Due to these unique advantages over other existent techniques (Table 11.2), FUS may facilitate the delivery of already developed pharmacological agents and could significantly impact how devastating CNS diseases are treated.
However, despite the fact that FUS is currently the only technique that can open the BBB locally and noninvasively, several key aspects of this phenomenon remain unexplored. A clear correlation of BBB opening with microbubbles has been shown (Choi et al., 2005; Hynynen et al., 2001; McDannold et al., 2004). Although the presence of microbubbles allows for a reduction in the necessary acoustic pressure for BBB opening, it also allows for the possibility of disrupting the microbubble through inertial cavitation (Leighton, 1994; Neppiras, 1980; Pardridge, 2007). The resulting effects not only can open the tight junctions but also could induce irreversible damage to the blood vessels and their surrounding cells (Baseri et al., 2010). Recent studies have indicated that BBB opening may occur without necessarily incurring inertial cavitation, without (Hynynen et al., 2003) or with (McDannold et al., 2006) craniotomy. However, it is not clear how the different types of mechanical effects lead to BBB opening and how the role of the microbubble can be optimized. Given the strong coupling of microbubble size and concentration to the response to sonication, a mechanistic study to BBB opening by contrast-assisted focused ultrasound must include these parameters. Control over both ultrasound and microbubble parameters is essential for the proper optimization and understanding of the FUS technique. However, to our knowledge no study to date has included a thorough investigation of both of these components.
FUS-FACILITATED BBB OPENING IN DRUG DELIVERY FOR TREATMENT OF NEURODEGENERATIVE DISEASE
Realizing the strong premise of this technique for facilitation of drug delivery to specific brain regions, we showed that the BBB can be opened reliably and reproducibly in the hippocampal region in mice (Choi et al., 2005, 2006, 2007a, 2007b, 2007c, 2008, 2009, 2010; Konofagou et al., 2008, 2009). By developing a better understanding of the underlying physical parameters that are responsible for the opening of the BBB, namely, the ultrasound and microbubble parameters, we will be in a position to fully exploit this methodology and to do so safely. The feasibility of the technique at optimized ultrasound and microbubble parameters for reversible BBB opening, as determined in vivo, has been tested on wild-type mice as a first step to identify the potential of this technique in the treatment of neurodegenerative diseases (Choi et al., 2008). The MR imaging methods developed allow for high sensitivity, high spatial resolution, and high temporal resolution. The latter is achieved through the slow diffusion of intraperitoneally injected gadolinium. The added potential of combining this ultrasound technique with any therapeutic agent may renew possibilities in potentially employing available pharmacological agents, whose development has currently been abandoned because of poor BBB penetration. This may thus result in the novel and effective treatment of several potentially devastating neurological and neurodegenerative diseases. As previously indicated, we will concentrate on the feasibility of noninvasive and localized treatment of Alzheimer’s disease by specifically targeting the hippocampus. However, the FUS technique can, in principle, be combined and applied in the case of any neurological disease. Therefore, findings of this study may impact not only treatment of a specific disease but also the entire field of brain diseases. In summary, FUS stands to make a very important impact in the brain drug delivery, and the proposed study aims at its optimization through understanding of the type of interaction between the microbubble, the tissue, and the FUS beam.
DRUG DELIVERY THROUGH THE OPENED BBB
The delivery of many large agents using focused ultrasound and microbubbles has been demonstrated in previous studies by our group and others: MRI contrast agents such as Omniscan (573 Da) (Choi et al., 2007b) and Magnevist® (938 Da) (Choi et al., 2007), Evans Blue (Kinoshita et al., 2006), Trypan Blue (Raymond et al., 2008), Herceptin (148 kDa) (Kinoshita et al., 2006), horseradish peroxidase (40 kDa) (Sheikov et al., 2008), doxorubicin (544 Da) (Treat et al., 2007), multisized Dextran (Choi et al., 2010), and rabbit anti-Aβ antibodies (Raymond et al., 2008). Despite the promise shown by the delivery of such a variety of compounds, several questions about the effectiveness of the delivery remain. In particular, it is still not known whether therapeutic molecules can cross through the BBB opening into the intracellular neuronal space so that they can trigger the required downstream effects for neuronal regeneration.
METHODS FOR INDUCING AND ASSESSING BBB OPENING
FUS AND MICROBUBBLES
The experimental setup is shown in Figure 11.1. The FUS transducer (center frequency: 1.5 MHz; focal depth: 60 mm; outer radius: 30 mm; inner radius: 11.2 mm, model cdc7411–3, Imasonic, Besançon, France) is used to perform sonication immediately following bubble administration. The transducer is driven by a function generator (Agilent Technologies, Palo Alto, CA) through a 50-dB power amplifier (ENI Inc., Rochester, NY). A cone filled with degassed and distilled water is attached to the transducer system. The transducer is attached to a computer-controlled positioner (Velmex Inc., Bloomfield, NY). The PCD, a 5-cm cylindrically focused broadband hydrophone (Sonic Concepts, Bothell, WA), with a cylindrical focal region (height 19 mm, diameter 3.64 mm) is placed at 60o from the longitudinal axis of the FUS beam. The PCD and the FUS transducer are confocally aligned. The acoustic emissions from the microbubbles are captured with the PCD and collected using a digitizer (model 14200, Gage Applied Technologies, Inc., Lachine, QC, Canada) through a 20 dB amplifier (model 5800, Olympus NDT, Waltham, MA). Microbubbles (Definity®: mean diameter range: 1.1–3.3 μm, Lantheus Medical Imaging, MA, or lipid-shelled microbubbles manufactured in-house and size isolated using differential centrifugation [57]) are activated and used within 24 hr after activation. Following activation, a 1:20 dilution solution is prepared using 1× phosphate-buffered saline (PBS) and slowly injected into the tail vein (1 µl per gram of mouse body weight). Both transducers use pulsed-wave FUS (burst rate: 10 Hz; burst duration: 20 ms; duty cycle: 20%) in two 30-s sonication intervals with a 30-s intermittent delay. Peak-rarefactional acoustic pressures of 0.15, 0.30, 0.45, and 0.60 MPa are typically used as they have been shown to provide the best tradeoff between safety and BBB opening (Baseri et al., 2010). One side of the hippocampus in the horizontal orientation is sonicated in each mouse. Acoustic parameters other than the pressure have also been studied with respect to their role in BBB disruption. One of those is the pulse length (Choi et al., 2011). In that study, mouse brains were pulse sonicated (center frequency: 1.5 MHz, peak-negative pressure: 0.3 MPa, pulse length [PL]: 2.3 µs, pulse repetition frequency [PRF]: 6.25, 25, 100 kHz) continuously or with a burst length of 1,000 pulses (burst repetition frequency [BRF]: 0.1, 1, 2, or 5 Hz) through the intact scalp and skull for 11 min. One minute after the start of sonication, fluorescence-tagged dextran (60 µg/g, molecular weight: 3 kDa) and Definity® microbubbles (0.05 µl/g) were intravenously injected. After 20 min of circulation, the mice were transcardially perfused, and the brains were sectioned and imaged using fluorescence microscopy. In order to determine the microbubble size dependence, mice have been injected intravenously with lipid-shelled bubbles of either 1–2, 4–5, or 6–8 µm in diameter while the concentration was 107 numbers/mL (Choi et al., 2009).
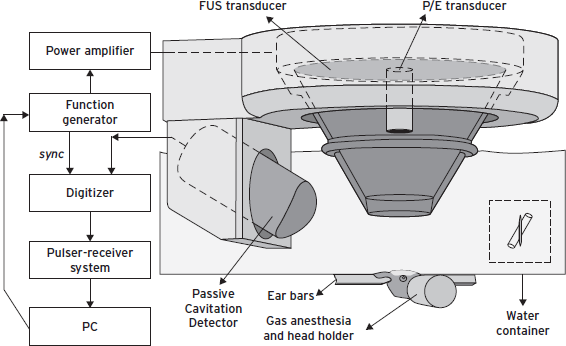
Figure 11.1 Block diagram and illustration of the experimental setup. The PCD was positioned at 60° relative to the longitudinal axis of the FUS beam. The overlap between the focal regions of PCD and FUS occurring inside the murine brain is illustrated in the inset.
CONFIRMATION OF BBB OPENING BY MAGNETIC RESONANCE IMAGING
A vertical-bore 9.4 T MR system (Buker Biospin, Billerica, MA) was used to confirm the blood–brain barrier opening in the murine hippocampus. Each mouse was anesthetized using 1%–2% of isoflurane gas and was positioned inside a single resonator. The respiration rate was monitored throughout the procedure using a monitoring or gating system (SA Instruments Inc., Stony Brook, NY). Prior to introducing the mouse into the scanner, intraperitoneal (IP) catheterization was performed. Two different protocols were used for MR imaging. The first protocol was a three-dimensional (3D), T1-weighted SNAP gradient echo pulse sequence, which acquired horizontal images using TR/TE = 20/4 ms, a flip angle of 25°, NEX of 5, a total acquisition time of 6 min and 49 s, a matrix size of 256 × 256 × 16 pixels and a field of view (FOV) of 1.92 × 1.92 × 0.5 cm3, resulting in a resolution of 75 × 75 × 312.5 µm3. The second protocol was a 3D T2*-weighted GEFC gradient echo pulse sequence, which acquired horizontal images using TR/TE = 20/5.2 ms, a flip angle of 10°, NEX of 8, a total acquisition time of 8 min and 12 s, a matrix size of 256 × 192 × 16 pixels, and a FOV of 2.25 × 1.69 × 0.7 cm3, resulting in a resolution of 88 × 88 × 437.5 µm3. Both protocols were applied approximately 30 min after IP injection of 0.30 ml of gadodiamide (590 Da, Omniscan®, GE Healthcare, Princeton, NJ), which allowed sufficient time for the gadodiamide to diffuse into the sonicated region.
ACOUSTIC EMISSION SIGNAL ACQUISITION AND ANALYSIS
The acoustic emission signals acquired by the PCD are sampled at 25 MHz to accommodate the highest memory limit of the digitizer involved in each case. A customized spectrogram function (30 cycles, i.e., 20 µs, Chebyshev window; 95% overlap; 4096-point FFT) in MATLAB® (2007b, Mathworks, Natick, MA) is used to generate a time–frequency map, which provided the spectral amplitude in time. The spectrogram can then clearly indicate how the frequency content of a signal changes over time. Therefore, the onset of the broadband response and its duration could be clearly demonstrated on the spectrogram.
The acoustic emissions are quantified in vivo. A high-pass, Chebyshev type 1, filter with a cutoff of 4 MHz was first applied to the acquired PCD signal. The acoustic emission collected by the focused hydrophone was used in the quantification of the ICD; the harmonic (nf, n = 1, 2, . . . , 6), subharmonic (f/2) and ultraharmonics (nf/2, n = 3, 5, 7, 9) frequencies produced by stable cavitation (Farny et al., 2009) were filtered out by excluding 300-kHz bandwidths around each harmonic and 100-kHz bandwidths around each sub- and ultraharmonic frequency. These bandwidths were designed to filter for the broadband response and to ensure that the stable cavitation response was not included in the ICD calculation. The root mean square (RMS) of the spectral amplitude (VRMS) could then be obtained from the spectrogram after filtering. To maximize the broadband response compared to the sonication without microbubbles, only the first 50 µs of sonication were considered in the ICD calculation, which was performed by integrating the VRMS variation within an interval of 0.75 µs (i.e., calculating the area below the VRMS curve between 0.095 ms and 0.145 ms). In order to remove the effect of the skull in the ICD calculation, the VRMS in the case without microbubbles was also calculated and was subtracted from the results with the microbubbles to obtain the net bubble response. A Student’s t-test was used to determine whether the ICD was statistically different between different pressure amplitudes. A p-value of p < 0.05 was considered to denote a significant difference in all comparisons.
ACOUSTIC PARAMETER DEPENDENCE AND MECHANISM OF BBB OPENING
The BBB opening pressure threshold is identified to fall between 0.30 and 0.45 MPa in the case of the 1-to-2-µm bubbles and between 0.15 and 0.30 MPa in the 4- to-5- and 6- to-8-µm cases (Choi et al., 2009; Tung et al., 2010). At every acoustic pressure, both the region of contrast enhancement in the MRI imaging and the amplitude of broadband emissions increased with the bubble diameter. The IC threshold is found to be bubble independent and to lie between 0.30 and 0.45 MPa for all bubble sizes (Fig. 11.2). In fluorescence imaging, the PL of 2.3 µs was found to be sufficient for BBB opening and Dextran delivery (Choi et al., 2011).

Figure 11.2 Spectrogram during the first 0.2 ms sonication. Broadband acoustic emissions were detected at (B) 0.45 MPa and (C) 0.60 MPa but not at (A) 0.30 MPa. Corresponding MRI images confirm that the blood–brain barrier could be opened at 0.30 MPa, that is, without inertial cavitation [60,64]. The arrows indicate the location of BBB opening.
MOLECULAR DELIVERY THROUGH THE BBB OPENING
A molecular delivery study (Choi et al., 2007c, 2010) indicated that the range of molecular size for trans-BBB delivery spreads to well beyond the 574 Da (Gadolinium; Fig. 11.2) to 67 kDa (Albumin; Fig. 11.3[v]) and 2,000 kDa (Dextrans; Fig. 11.3[iii]). As expected, at 2,000 kDa (or, ~20 nm), the fluorescent region is the smallest (since the molecule is the largest and thus diffusion the slowest) and mostly outside of the hippocampus. Therefore, FUS-induced BBB opening was shown feasible for noninvasive, local, and transient opening of the BBB for drug delivery of agents of several tens of kDa, providing thus the opportunity of delivering available pharmacological agents to specific brain regions for treatment of neurological disease.
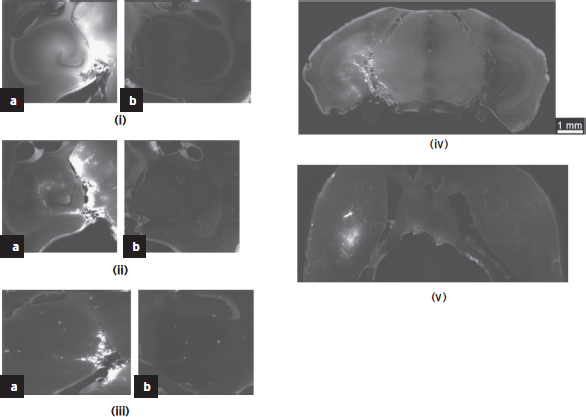
Figure 11.3 Study of the molecular size through the bbb opening using dextrans and fluorescence imaging. Horizontal slice of Dextran of molecular weight equal to (i) 3, (ii) 70, and (iii) 2000 kDa on the (a) left (targeted) and (b) right (not targeted) hippocampus; (iv) coronal slice of the entire brain at 70 kDa Dextran showing the fluorescent left hippocampus (crescent shaped); (v) fluorescent albumin (67 kDa) permeated in the putamen through the opened BBB.
SAFETY AND REVERSIBILITY OF BBB OPENING
In order to determine the safety window of the FUS technique, through histological and immunohistological techniques (Baseri et al., 2010), we have identified the safe operating parameters of ultrasound exposure for neurons, astrocytes, and endothelial cells (Fig. 11.4). In summary, BBB opening starts occurring at around 0.2–0.3 MPa rarefactional pressure amplitude and beyond. At pressures under 0.6 MPa (Fig. 11.4[i]), no extravasation of red blood cells (RBC) or neuronal damage was observed in the regions of the hippocampus exhibiting the most pronounced BBB opening. Beyond 0.6 MPa (Fig. 11.4[ii]), RBC extravasation was detected, and beyond 0.9 MPa neuronal damage was observed. These preliminary findings suggest that there is overlap between the feasibility and safety windows within the pressure range of 0.3–0.6 MPa; that is, the BBB can be opened throughout the entire hippocampus without endothelial or neuronal damage at those pressures (Fig. 11.4; Baseri et al., 2010; Konofagou and Choi, 2008). FUS-induced BBB opening was reported to close within 72 hours in rabbits (Hynynen et al., 2001). The blood-brain barrier closure occurs within the first 24 hours after BBB opening at low pressures and within 2–3 days at higher pressures and monodispersed bubbles (Samiotaki et al., 2012).

Figure 11.4 Comparison between MRI (left) and histology (center [1×] and right [200×] near the region of most enhanced BBB opening according to the MRI) after FUS-induced BBB opening on the left hippocampus at (i) 0.45 and (ii) 0.75 MPa peak rarefactional pressure. It shows that at lower pressures (i) the endothelial and neurons are intact while at higher pressures (ii) there is extravasation of red blood cells (indicated by arrowhead) and neuronal death (indicated by arrow). This indicates the safety window of the FUS technique in BBB opening.
Dynamic contrast-enhanced (DCE) MRI has been performed before and after the intraperitoneal injection of gadodiamide over 60 min (Vlachos et al., 2010). The general kinetic model (GKM) and reference region model (RRM) were used to estimate the permeability in the entire brain (Vlachos et al., 2010). At 0.3 MPa and 4- to-5-µm bubbles (Feshitan et al., 2009), the permeability were found to equal 0.02 ± 0.0123 min-1 and increase by at least 100 times in the region of BBB opening compared with the control side (Fig. 11.5). Cavitation (Fig. 11.2) and permeability (Fig. 11.5) findings demonstrated that the inertial cavitation threshold is independent of the bubble size while both the ICD and MR amplitude increased at larger bubble sizes, also indicating a correlation between the cavitation and permeability increase (Tung et al., 2010). The fact that the permeability increased with the pressure and microbubble size indicated that the BBB opening occurs at multiple sites within the capillary tree and that the BBB opening is larger with larger microbubbles, most likely due to the larger area of contact between the bubble and the capillary wall.
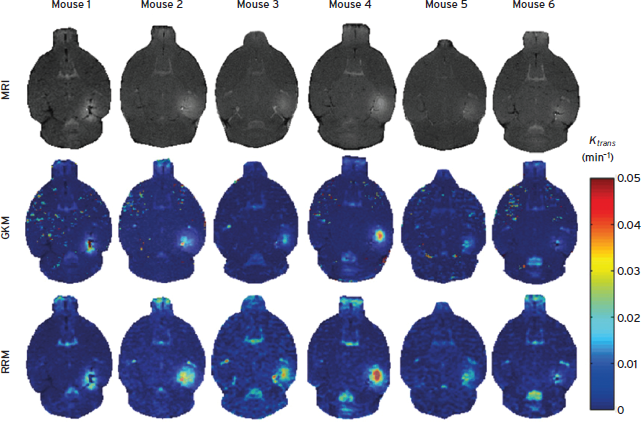
Figure 11.5 T1 images (first row) and their corresponding permeability maps generated from GKM (second row) and RRM (third row) for all mice. The transverse slice with maximum T1 signal enhancement is selected. The Ktrans values are indicated in the color bar. The maps have been superimposed over the corresponding DCE-MR images. In the case of Mouse 1, the last acquired DCE-MR image is presented instead of a regular T1[61].
BBB OPENING IN LARGE ANIMALS
A 500-kHz FUS transducer was used transcranially in Macaca Mulatta monkeys (Marquet et al., 2011). This transducer was mounted on a stereotaxic frame, enabling treatment planning using the brain atlas. In vivo experiments were conducted in six monkeys (30 sonications) using Definity or 4–5 μm, custom-made, lipid-shelled microbubbles. The BBB opening was confirmed using T1-weighted, spoiled gradient pulse-echo MR sequence at 3 T and gadodiamide IV injection. Damage was assessed using a T2 sequence with the same system. To obtain the BBB closing time line, the T1-weighted MR sequence was repeated along with gadodiamide IV injection over 4 days. MR images were registered to a monkey brain atlas allowing targeting quality assessment. Postprocessing was performed on combined pre- and postcontrast agent T1 images to quantify BBB disruption at the targeted regions, that is, caudate, hippocampus (Fig. 11.6), and visual cortex.
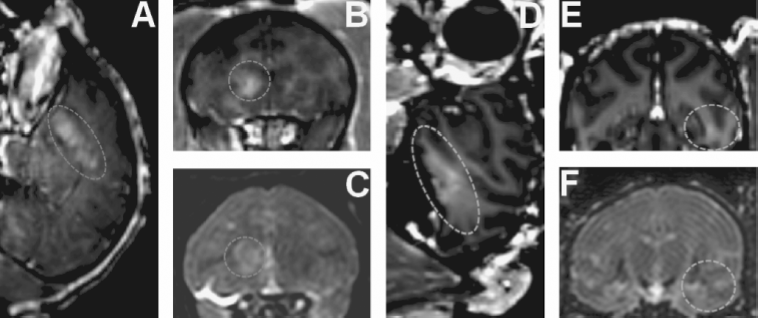
Figure 11.6 In vivo bbb opening in monkeys. ( A, C) BBB opening experiment targeting the caudate using custom-made microbubbles and applying 0.6 MPa (dashed line shows region of interest). (D, F) BBB opening experiment targeting hippocampus using Definity® microbubbles and applying 0.6 MPa (dashed line shows region of interest). (A, B, D, E) 3D Spoiled Gradient-Echo (SPGR) T1-weighted sequence was applied after intravenous (IV) injection of gadodiamide 1 hr after sonication. (A, D) Sagittal slices at the region of interest. (B, E) Corresponding coronal slices. (C, F) 3D T2-weighted sequence. An edema was visible using custom-made microbubbles while no damage was detected using Definity® microbubbles.
The blood-brain barrier opening was achieved in six different animals using peak negative pressures ranging from 0.2 to 0.6 MPa. No damage was detected at pressures below 0.45 MPa. The actual BBB-opened location was found to be in very good agreement with the targeted one under close to normal incidence angles with the absolute targeting error being less than 1 mm laterally and less than 4 mm axially. The maximum opening-volume-to-target overlap was 85% with an average of 52% depending on the brain region targeted (Deffieux and Konofagou, 2010; Marquet et al., 2011). Initial findings on the closing time line showed that the BBB was fully restored within 2 days after treatment.
THERAPEUTIC DELIVERY THROUGH THE BBB OPENING
Neurotrophic delivery to the brain has been proven essential in reversing the neuronal degeneration process but so far has been hindered by the blood–brain barrier. In a recent study by our group, not only was it shown that the brain-derived neurotrophic factor (BDNF) can cross the ultrasound-induced BBB opening but also that it can trigger signaling pathways in the pyramidal neurons of mice in vivo from the membrane to the nucleus (Fig. 11.7) (Baseri et al., 2012). This opens entirely new avenues in the brain drug delivery where focused ultrasound in conjunction with microbubbles can generate downstream effects at the cellular and molecular level and thus increase the drug’s efficacy and potency in controlling or reversing the disease.
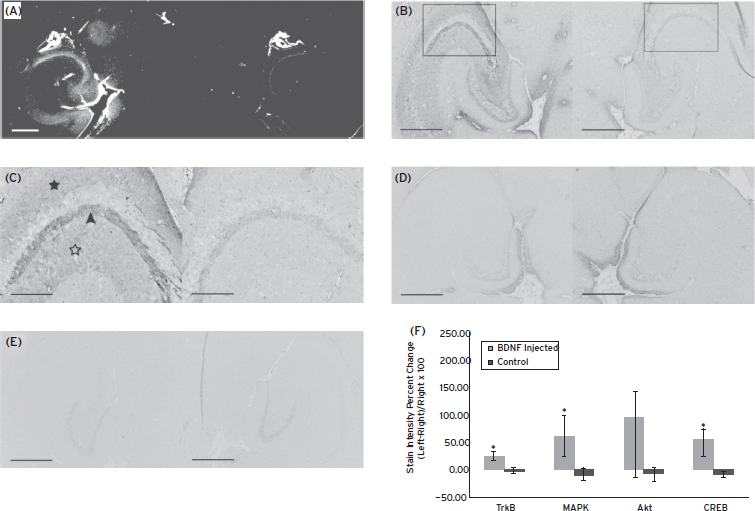
Figure 11.7 ( A) Fluorescent image of a 100-micron frozen brain section from a mouse that was sacrificed 20 min after sonication. The sonicated hippocampus (left) shows much higher fluorescent intensity than the unsonicated hippocampus (right), depicting blood–brain barrier opening and the extravasation of fluorescent-tagged (Alexa Fluor 594) BDNF in the sonicated region; (B) a 5-micron frozen section from the same mouse was immunohistochemically stained using a primary antibody against phosphorylated MAPK (pMAPK). Consistent with the fluorescent image in (A), the intensity of DAB staining is much greater in the left sonicated hippocampus compared with the right control; the black box shows the enlarged area in (C), where immunoreactivity to pMAPK is shown in mossy fiber terminals (arrowhead), suprapyramidal CA3 dendrites (black star), and the axons of the Schaffer collateral system (hollow star). (D) Immunohistochemical staining of a 5-micron frozen section from a mouse that was sacrificed 3 min after sonication; the same primary antibody against pMAPK was used. No difference in DAB intensity is observed between the sonicated and the control hippocampus. (E) Negative control for the same mouse in (A); no primary antibody (against pMAPK) was added to this 5-micron frozen section during the staining procedure. All magnifications are 40× and scale bars are 500 μm except for (C), which is 100× and 200 μm, respectively. In (F), immunohistology stain intensity analysis shows percentage change between the left (FUS) and the right (no FUS) sides of the mice brains. A significant difference (p < 0.05, N = 3; depicted by asterisks) was found between the BDNF-administered animal group and the control (no BDNF) animal group for the TrkB, MAPK, and CREB antibodies. Bars represent mean ± standard deviation.
SUMMARY
The studies described this chapter demonstrate that FUS in conjunction with microbubbles was hereby shown to effectively and reproducibly open the blood–brain barrier transcranially in vivo with its recovery occurring within the first 24 hours. The permeability of the FUS-opened BBB was shown to increase by at least two orders of magnitude, indicating facilitation of drug delivery through FUS. Molecules of a wide range of sizes were capable of traversing the opened BBB without any associated structural damage. A dependence of the BBB permeability on the pressure and the microbubble size indicated that multiple sites of BBB opening within the ultrasound beam occur simultaneously while each BBB opening site increases with the microbubble size. Finally, a new pulse sequence was designed that showed feasibility at very short pulse lengths, and transcranial BBB opening in larger animals, such as nonhuman primates and humans, was shown feasible in simulations and ex vivo experiments as well as in vivo primate monkeys (Fig. 11.6) (Marquet et al., 2011; Tung et al., 2011).
The preclinical studies described point to the considerable progress that has been made in understanding the characteristics of the BBB as well as the safety and efficacy of delivering small molecules into the brain parenchyma using focused ultrasound with microbubbles. Studies are ongoing in order to ensure successful clinical translation in the near future.
DISCLOSURE
Dr. Konofagou has no conflicts of interest to disclose. The research in the chapter was supported by NIH R01 EB009041, NIH R21 EY018505, NSF CAREER 064471, the Kinetics Foundation, and the Kavli Institute.
REFERENCES
Abbott, N.J., Ronnback, L., et al. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7:41–53.
Bakay, L.,H. Ballantine, T., Jr., et al. (1956). Ultrasonically produced changes in the blood-brain barrier. AMA Arch. Neurol. Psychiatry 76:457–467.
Ballantine, H.T., Jr., Bell, E., et al. (1960). Progress and problems in the neurological applications of focused ultrasound. J. Neurosurg. 17:858–876.
Baseri, B., Choi, J.J., et al. (2012). Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood–brain barrier using focused ultrasound and microbubbles. Phys. Med. Biol. 57:N65–N81.
Baseri, B., Choi, J.J., et al. (2010). Safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultras. Med. Biol. 36(9):1445–1459.
Blasberg, R., Patlak, C., et al. (1975). Intrathecal chemotherapy brain tissue profiles after ventriculocisternal perfusion. J. Pharmacol. Exp. Ther. 195:73–83.
Borden, M., Kruse, D., et al. (2005). Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE. Trans. Ultrason. Ferroelect. Freq. Contr. 52:1992–2002.
Chen, S., Kroll, M.H., et al. (2002). Bioeffects of myocardial contrast microbubble destruction by echocardiography. Echocardiography 19:495–500.
Choi, J.J., Feshitan, J.A., et al. (2009). The dependence of the ultrasound-induced blood-brain barrier opening characteristics on microbubble size in vivo. In: Ebbini, E., ed. 8th International Symposium on Therapeutic Ultrasound, AIP Conference Proceedings. Minneapolis, MN, pp. 58–62.
Choi, J.J., Pernot, M., et al. (2007a). Spatio-temporal analysis of molecular delivery through the blood-brain barrier using focused ultrasound. Phys. Med. Biol. 52:5509–5530.
Choi, J.J., Pernot, M., et al. (2005). Feasibility of transcranial, localized drug-delivery in the brain of Alzheimer’s-model mice using focused ultrasound. In: IEEE Ultrasonics Symposium, 2005, pp. 988–991.
Choi, J.J., Pernot, M., et al. (2006). Noninvasive blood-brain barrier opening in live mice. AIP Conf. Proc. 829:271–275.
Choi, J.J., Pernot, M., et al. (2007b). Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 33:95–104.
Choi, J., Selert, K., et al. (2011). Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc. Natl. Acad. Sci. USA 108(40):16539–16544.
Choi, J.J., Small, S.A., et al. (2006). Optimization of blood-brain barrier opening in mice using focused ultrasound. In: IEEE Ultrasonics Symposium, 2006, pp. 540–543.
Choi, J.J., Wang, S., et al. (2008). Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound. Ultrason. Imaging 30:189–200.
Choi, J.J., Wang, S., et al. (2007c). Molecular delivery and microbubble dependence study of the FUS-induced blood-brain barrier opening in vivo. In: Ultrasonics Symposium, 2007 IEEE International Ultrasound Symposium Proceedings, pp. 1192–1195.
Choi, J.J., Wang, S., et al. (2010). Molecules of various pharmacologically- relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med. Biol. 36(1):58–67.
Christensen, D.A. (1988). Ultrasonic Bioinstrumentation. New York: John Wiley.
Christiansen, J., French, B.A., et al. (2003). Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound. Med. Biol. 29:1759–1767.
Chomas, J.E., Dayton, P., et al. (2001). Threshold of fragmentation for ultrasonic contrast agents. J. Biomed. Opt. 6:141–150.
Deffieux, T.A., and Konofagou, E.E. (2010).Numerical study and experimental validation of a simple transcranial focused ultrasound system applied to blood-brain barrier opening. IEEE-UFFC Trans. 57(12):2637–265,.
Farny, C.H., Holt, R.G., et al. (2009). Temporal and spatial detection of HIFU-induced inertial and hot-vapor cavitation with a diagnostic ultrasound system. Ultrasound Med. Biol. 35:603–615.
Feshitan, J.A., Chen, C.C., et al. (2009). Microbubble size isolation by differential centrifugation. J. Colloid. Interface Sci. 329:316–324.
Fischer, H., Gottschlich, R., et al. (1998). Blood-brain barrier permeation: molecular parameters governing passive diffusion. J. Membr. Biol. 165:201–211.
Fung, L., Shin, M., et al. (1996). Chemotherapeutic drugs released from polymers distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm. Res. 13:671–682.
Ghose, A., Viswanadhan, V.N., et al. (1999). A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1:55–68.
Hynynen, K., McDannold, N., et al. (2001). Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220:640–646.
Hynynen, K., McDannold, N., et al. (2003). Non-invasive opening of BBB by focused ultrasound. Acta Neurochir. Suppl. 86:555–558.
Hynynen, K., McDannold, N., et al. (2006). Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J. Neurosurg. 105:445–454.
Hynynen, K., McDannold, N., et al. (2005). Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 24:12–20.
Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5:347–360.
Kaps, M., Seidel, G., et al. (2001). Pharmacokinetics of echocontrast agent infusion in a dog model. J. Neuroimaging 11:298–302.
Kaufmann, B.A., Wei, K., et al. (2007). Contrast echocardiography. Curr. Probl. Cardiol. 32:51–96.
Kinoshita, M., McDannold, N., et al. (2006). Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA 103:11719–11723, Aug 1.
Konofagou, E. and Choi, J.J. (2008). Ultrasound-induced treatment of neurodegenerative diseases across the blood-brain barrier. In Al-Jumaily, A., and Alizad, A. eds. Biomedical Applications of Vibration and Acoustics in Therapy, Bioeffects and Modelling. New York: ASME Press, pp. 63–80.
Konofagou, E.E., Choi, J., et al. (2009). Characterization and optimization of trans-blood-brain barrier diffusion in vivo. In: Ebbini, E. ed. 8th International Symposium on Therapeutic Ultrasound, AIP Conference Proceedings. Minneapolis, MN, pp. 418–422.
Leighton, T. (1994). The Acoustic Bubble, 1st Edition. London: Academic.
Li, P., Armstrong, W.F., et al. (2004). Impact of myocardial contrast echocardiography on vascular permeability: comparison of three different contrast agents. Ultrasound Med. Biol. 30:83–91.
Li, P., Cao, L.Q., et al. (2003). Impact of myocardial contrast echocardiography on vascular permeability: an in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med. Biol. 29:1341–1349.
Lipinski, C. (2000). Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 44:235–249.
Marquet, F., Tung, Y.-S., et al. (2011). Noninvasive, transient and selective blood- brain barrier opening in non-human primates in vivo. PLoS ONE, 6(7):e22598.
McDannold, N., King, R.L., et al. (2004). MRI monitoring of heating produced by ultrasound absorption in the skull: in vivo study in pigs. Magn. Reson. Med. 51:1061–1065.
McDannold, N., Vykhodtseva, N., et al. (2006). Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys. Med. Biol. 51:793–807.
McDannold, N., Vykhodtseva, N., et al. (2005). MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med. Biol. 31:1527–1537.
Mesiwala, A.H., Farrell, L., et al. (2002). High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med. Biol. 28:389–400.
Miller, D.L. (2007). Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation. Prog. Biophys. Mol. Bio. 93:314–330.
Miller, D., Li, P., et al. (2005). Influence of contrast agent dose and ultrasound exposure on cardiomyocyte injury induced by myocardial contrast echocardiography in rats. Radiology 237:137–143.
Neppiras, E.A. (1980). Acoustic cavitation. Phys. Rep. 61:159–251.
Pardridge, W.M. (2005). The blood-brain barrier: bottleneck in brain drug development. in NeuroRx 2:3–14.
Pardridge, W.M. (2006). Molecular Trojan horses for blood-brain barrier drug delivery. Disc. Med. 6:4.
Pardridge, W.M. (2007). Drug targeting to the brain. Pharm. Res. 24:1733–1744.
Patrick, J.T., Nolting, M.N., et al. (1990). Ultrasound and the blood-brain barrier. Adv. Exp. Med. Biol. 267:369–381.
Raymond, S.B., Treat, L.H., et al. (2008). Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS ONE 3:e2175.
Samiotaki, M., Vlachos, F., et al. (2012). A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn. Reson. Med. vol. 67(3):769–777.
Sheikov, N., McDannold, N., et al. (2006). Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med. Biol. 32:1399–1409.
Sheikov, N., McDannold, N., et al. (2008). Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 34(7):1093–1104.
Sheikov, N., McDannold, N., et al. (2004). Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 30:979–989.
Stewart, P.A., and Tuor, U.I. (1994). Blood-eye barriers in the rat: correlation of ultrastructure with function. J. Comp. Neurol. 340:566–576.
Treat, L.H., McDannold, N., et al. (2007). Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 121:901–907.
Tung, Y.-S., Vlachos, F., et al. (2011). The mechanism of the interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J. Acous. Soc. Amer. 108(40), 130(5):3059–3067.
Tung, Y., Vlachos, F., et al. (2010). In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening. Phys. Med. Biol., 55(20):6141–6155.
Vlachos, F., Tung, Y.S., et al. (2010). Permeability assessment of the focused ultrasound-induced blood-brain barrier opening using dynamic contrast-enhanced MRI. Phys. Med. Biol. 55:5451–5466.
Vykhodtseva, N.I., Hynynen, K., et al. (1995). Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med. Biol. 21:969–979.
Yang, F.Y., Fu, W.M., et al. (2007). Quantitative evaluation of focused ultrasound with a contrast agent on blood-brain barrier disruption. Ultrasound Med. Biol. 33:1421–1427.