26 | NEUROBIOLOGY OF MENTAL ILLNESS PSYCHOSIS PRONENESS
RAQUEL E. GUR
INTRODUCTION
Across medicine, early identification of disorders is recognized as important and at times critical for intervention. A prodrome is an early symptom or set of symptoms, which may be specific (e.g., aura before migraine) or non-specific (e.g., fever, malaise, decreased appetite in infectious illnesses), and is a precursor to a disease. Early detection creates the potential for intervention at a stage where it could disrupt the pathological process or help manage the disorder and ameliorate its course.
The application of the prodrome concept to schizophrenia builds on clinical lore where a prolonged period of two to three years is common before the emergence of psychotic symptoms that meet Diagnostic and Statistical Manual (DSM) criteria for schizophrenia or spectrum psychotic disorders. This period is associated with sub-threshold symptoms and some functional decline. A rapidly growing literature since the mid-1990s has examined the clinical course of individuals with prodromal presentation and gauged conversion rates to schizophrenia. Based on the literature and considering potential implications, the DSM-5 work group has examined how “psychosis risk syndrome” or “attenuated psychotic symptoms syndrome,” terms applied to define the prodromal phase of schizophrenia, should be incorporated into the next diagnostic manual (Carpenter and van Os, 2011). As this debate unfolds, it is critical to evaluate systematically concomitant neurobiological processes that can be examined prospectively to elucidate the pathophysiology of vulnerability to psychosis. Complementally, such work can identify markers of resilience by studying those at risk who do not proceed to a more severe form of the syndrome.
Methodological advances in neuroscience have enabled us to probe brain function in schizophrenia. Because it is a neurodevelopmental disorder, understanding the pathophysiology of schizophrenia is central to establishing the neurobiology pertinent to the continuum of psychosis. The study of at risk individuals with quantitative phenotypic measures of brain and behavior can address fundamental questions regarding the presence of abnormalities at an early phase of presentation and their relation to outcome. Importantly, such measures can be integrated with genomics to elucidate underlying mechanisms of vulnerability and resilience. Therefore, this framework of research can help behavioral neuroscience join the genomic effort underway toward precision medicine.
This chapter will summarize current literature on the prodromal state as examined with neurobehavioral and neuroimaging methods. Findings will be integrated considering the NIMH Research Domain Criteria (RDoC) initiative (http://www.nimh.nih.gov).
NEUROBEHAVIOR
Behavioral performance that can be linked to brain function has been examined in neurocognitive measures. More recently, social cognition measures have been included in evaluating those at clinical risk for psychosis, commonly integrating cognitive and social psychology paradigms. A small literature has focused on olfaction.
NEUROCOGNITION
Impaired cognition is prominent in schizophrenia and is associated with disrupted functioning. Whether cognitive deficits are progressive or stable has been debated. Examination of first-episode patients, before initiation of treatment with antipsychotic medications, and their longitudinal follow-up made it possible to determine that the neurocognitive profile in first-episode patients resembles that evident in individuals with schizophrenia with a more chronic course of illness. The pattern of deficits in the executive and memory domains implicates frontotemporal dysfunction. The extension of neurocognitive assessment with tests applied in schizophrenia research to individuals with attenuated psychotic symptoms has been commonly applied in prodromal investigations. Some inconsistencies are noted as studies vary in ascertainment, assessment instruments, tests applied, and the information provided on potential moderators, such as substance use. Previous reviews of this growing literature have been qualitative. Two recent quantitative meta-analyses highlight the emerging findings reported in multiple studies in the literature (Fusar-Poli et al., 2012b; Giuliano et al., 2012).
Giuliano and colleagues (2012) conducted a meta-analysis of fourteen studies that met specified inclusion criteria, published until February 2011, which examined the psychosis risk syndrome. A sample of 1,215 individuals at risk for psychosis was compared to 851 healthy controls on neurocognitive domains derived from multiple tests that are frequently examined in schizophrenia. Tests included measures of general cognitive abilities, language functions, episodic memory, attention, visuospatial abilities, and working memory. Overall, small to medium effect sizes of neurocognitive impairment were noted in the psychosis risk group, with significant deficits in 9 of the 10 domains examined. The motor skills domain was the only one that did not differ between the groups. Longitudinal follow-up in seven of the studies showed that those in the psychosis risk group, who transitioned to psychosis at follow-up, had medium to large neurocognitive deficits at baseline compared to healthy controls, except for motor speed (Addington et al., 2008; Brewer et al., 2003, 2005; Chung et al., 2008; Dickinson and Gold, 2008; Gschwandtner et al., 2006; Hawkins et al., 2004; Miyake et al., 2000; Pflueger et al., 2007; Pinkham et al., 2007; Reichenberg et al., 2010; Seidman et al., 2010; Smith et al., 2006; Szoke et al., 2008). Given the small samples, the limited period of follow-up, subjects’ attrition, and potential treatment effects, the longitudinal results are preliminary and limited.
Fusar-Poli et al. (2012b) have also focused their meta- analysis on cognitive functioning in individuals who were at clinical high risk for psychosis. They examined 19 studies published before January 2011. Specified and carefully delineated inclusion criteria were applied, and only 19 studies of the 78 studies screened were included (Addington et al., 2008; An et al., 2010; Brewer et al., 2005; Broome et al., 2007; Chung et al., 2008; Frommann et al., 2011; Green et al., 2012; Ilonen et al., 2010; Korver et al., 2010; Koutsouleris et al., 2012; Lindgren et al., 2010; Magaud et al., 2010; Pflueger et al., 2007; Seidman et al., 2010; Silverstein et al., 2006; Simon et al., 2007; Szily and Keri, 2009; van Rijn et al., 2011; Woodberry et al., 2010). The sample consisted of 1,188 clinical risk participants and 1,029 controls. Diffuse deficits across several neurocognitive domains were evident in the clinical risk group. Impairments in general intelligence, executive functions, attention, working memory, verbal fluency, verbal and spatial memory, and social cognition characterized the clinical risk group. Processing speed did not distinguish between the groups. Transition to psychosis was examined in 7 studies, with mean longitudinal follow-up of 19 months (Becker et al., 2010; Brewer et al., 2005; Koutsouleris et al., 2012; Pukrop et al., 2007; Riecher-Rossler et al., 2009; Seidman et al., 2010; Woodberry et al, 2010). The group of converters had lower general intelligence at baseline, and more impaired performance in verbal fluency, verbal and visual memory, and working memory, compared to those who did not develop psychosis at follow-up.
To examine the regional distribution of neurocognitive deficits implicated by the test results, we have entered the z-scores of the prodromal group into the “behavioral imaging” algorithm (Gur et al., 1990). This algorithm uses z-scores from neuropsychological tests to create a three-dimensional representation of brain regions that are likely impaired based on the pattern of deficits. We entered the results from the meta-analyses and averaged both when the same measures were included in both analyses (see Table in Fig. 26.1). The algorithm generates three views (Fig. 26.1) of brain cortex, identifying regions of impaired functioning in individuals at clinical risk. As can be seen, the abnormalities seem more pronounced in the left than the right hemisphere, especially in the juncture of frontal, temporal, and parietal lobes.
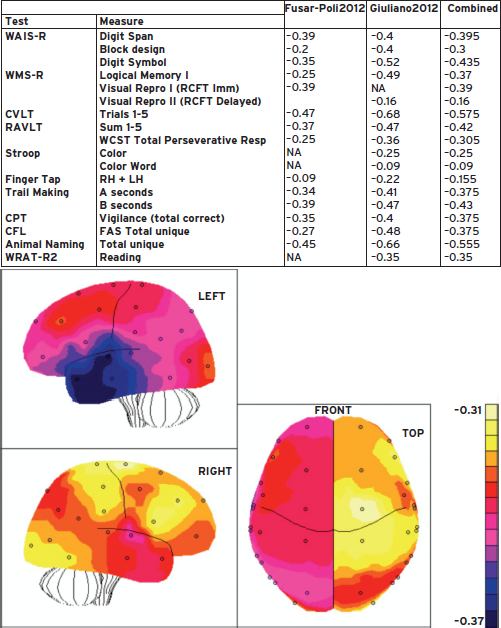
Figure 26.1 Behavioral Image displays of left hemisphere, right hemisphere, and top view. Values are based on the meta-analyses of tests examined and summarized in the table. The color scale indicates effect sizes of psychosis prone individuals relative to healthy controls.
SOCIAL COGNITION
Relative to the large number of studies that investigated neurocognition in clinical risk groups, as highlighted in the meta-analyses previously summarized, a handful of studies examined processes that relate to the perception, interpretation, and response to other people’s behavior. As social cognition is a later addition to studies in schizophrenia and the clinical risk studies are an extension of these efforts, more work is necessary.
Giuliano and colleagues’ (2012) qualitative part of the review of the psychosis risk syndrome presents data on social cognition that is insufficiently represented in the literature for quantitative meta-analysis. Three studies are included, and while deficits in emotion processing and theory of mind tasks are noted, the results are too preliminary to be conclusive (Addington et al., 2008; Chung et al., 2008; Pinkham et al., 2007). Fusar-Poli et al. (2012b) meta-analysis noted a significant impairment in 255 clinical risk participants compared to 235 controls in six studies that included measures of the social cognition domain (Addington et al., 2008; An et al., 2010; Chung et al., 2008; Green et al., 2012; Szily and Keri, 2009; van Rijn et al., 2011). While these preliminary studies are encouraging, and ultimately may yield insights into negative symptoms, this effort is in its initial stages.
OLFACTION
The Giuliano et al. review (2012) mentions only two studies on olfactory identification in the psychosis risk syndrome, with medium mean effect size. Similarly, only two studies (Brewer et al., 2003) examined the transition to schizophrenia spectrum disorders at follow-up. These are preliminary data not yet suitable for meta-analysis, and further research is required.
Thus, the pattern of neurobehavioral performance in individuals at clinical risk for psychosis shows diffuse deficits in multiple domains, similar to those evident in first-episode and more chronic patients with schizophrenia. Specifically, deficits in verbal memory and working memory implicating left frontotemporal dysfunction have been extensively documented in schizophrenia. While longitudinal studies are limited, the literature suggests that those individuals who transition to schizophrenia spectrum disorders manifest neurocognitive deficits already at baseline to a greater extent than those who do not transition. This buttresses the importance of examining schizophrenia related disorders neurodevelopmentally and in integration with neuroimaging.
NEUROIMAGING
The application of neuroimaging methodology to the study of schizophrenia, including first-episode presentation, has enabled the examination of brain structure and function and demonstrated key aberrations in the syndrome (Fusar-Poli et al., 2012c). The extension of this line of research to the investigation of vulnerability to psychosis can provide important information on brain changes associated with the emerging psychotic process. Magnetic resonance imaging (MRI) enables evaluation of brain structure, gray matter and white matter volumes, connectivity, and activity in response to neurobehavioral probes. As the techniques for image acquisition and processing advance rapidly, more refined assessment of brain integrity is feasible.
STRUCTURAL MRI
Detailed morphometric measures can be obtained in individual and group data that provide gray matter, white matter, and CSF whole brain and regional volumes. Parameters commonly scrutinized are highlighted in Figure 26.2.

Figure 26.2 MRI template-warping for parcellating regions of interest. A template in which regions are labeled and demarcated is warped into an individual’s MRI recording the extent of changes required. As a result, axial slices such as seen on the left are labeled as seen on the right column. Regional volumes can then be calculated for quantitative analysis allowing, for example, comparison between psychosis prone and healthy participants.
Structural MRI measures have been tested as endophenotypes in several studies of first-degree family members who are at genetic risk for schizophrenia. Family members show a similar pattern but diminished extent compared to that observed in probands, with increased ventricular volume and decreased gray matter volume. The literature on clinical risk for psychosis, recently reviewed (Fusar-Poli et al., 2012a), is relatively limited in size of samples and extent of follow-up.
A meta-analysis of voxel-based morphometry studies compared psychosis risk and first-episode schizophrenia patients to healthy controls (Fusar-Poli et al., 2012c). Fourteen studies met specified selection criteria and included participants who were never treated with antipsychotic medications. There were 198 individuals at risk for psychosis compared to 254 healthy controls, and 206 first-episode patients compared to 202 controls. Image acquisition in most studies was on a 1.5 Tesla scanner. Gray matter volume reduction was evident in several regions including the right temporal, limbic prefrontal cortex in the clinical risk group, and in the temporal insular cortex and cerebellum in the first-episode group. Notably, the onset of psychosis was associated with decreased gray matter volume in temporal, anterior cingulate, cerebellar, and insular regions. In one study, greater severity of psychotic symptoms was related to right superior temporal lobe gyrus volume decrease. These regions have been implicated in multiple studies of schizophrenia and subserve functions that are aberrant in schizophrenia and relate to cognition and emotion processing.
It is notable that while the neuroanatomic studies, when reporting lateralized effects, suggest greater structural abnormalities in the right hemisphere, the neurocognitive studies suggest greater left hemispheric dysfunction. This seeming discrepancy may reflect the lack of emphasis on laterality in neuroimaging studies, which typically examine data on a voxelwise basis within a hemisphere. It could also reflect greater sensitivity of neuropsychological tests to left hemispheric dysfunction. On the other hand, cognitive deficits could reflect compensatory activation, and if these effects are validated in future studies they could thus suggest avenues for remediation.
There are multiple methodological limitations entailed in MRI meta-analytic approaches, and the studies are mostly cross-sectional and include participants who did not transition to psychosis. However, the results suggest that brain regions that show volume reduction in schizophrenia also show abnormalities in those at risk for psychosis (Fusar-Poli et al., 2012c). Larger samples in a longitudinal design will be important to advance the understanding of underlying neuroanatomical differences between groups. White matter changes have also been reported in schizophrenia, early in the course of illness, as well as in individuals at risk for psychosis (Carletti et al., 2012; Fusar-Poli et al., 2011a).
DIFFUSION TENSOR IMAGING
Diffusion tensor imaging (DTI) quantifies restricted water diffusivity in white matter, enabling non-invasive detection of subtle white matter abnormalities and facilitating the understanding of complex large-scale brain networks. Figure 26.3 illustrates measures obtained in DTI.

Figure 26.3 Diffusion tensor imaging (DTI) illustrates fractional anisotropy (left) and tractography (right). Fractional anisotropy reveals white matter architecture while tractography depicts the integrity of fiber tracts.
Abnormalities in DTI have been reported in schizophrenia, both in chronic patients and in first-episode presentation (Peters et al., 2009), with reduced white matter integrity in frontotemporal tracts. The literature on psychosis risk is limited to several cross-sectional studies, with differing findings including reduced fractional anisotropy in frontal lobe (Bloemen et al., 2010) or in the superior longitudinal fasciculus (Borgwardt et al., 2011). A longitudinal study (Carletti et al., 2012) compared three groups: individuals at risk for psychosis (n = 32) healthy controls (n = 32), and first-episode schizophrenia (n = 15). The psychosis risk and control participants were rescanned on a 1.5 Tesla after 28 months. At baseline, the first-episode group had decreased fractional anisotropy and increased diffusivity relative to controls, and the psychosis risk group was intermediate between the other two groups. At follow-up, further reduction in fractional anisotropy was evident in left frontal region only in those psychosis risk individuals (n = 8) who transitioned to psychosis. This suggests that progressive changes occur at disease onset, which has been reported before for gray matter (Borgwardt et al., 2007; Smeiskova et al., 2010). Again, however, the available data are meager and must be considered very preliminary.
fMRI
Functional MRI has been applied to individuals at risk for psychosis, commonly in small samples with neurobehavioral probes that have shown differences between schizophrenia patients and controls. Neurobehavioral domains examined include working memory, using the n-back paradigm. Overall, psychosis risk groups show decreased activation in the blood oxygenation level-dependent (BOLD) response in dorsolateral and medial prefrontal regions (Fusar-Poli et al., 2012c). The pattern of activity is similar to that seen early in the course of schizophrenia, but less pronounced abnormalities are observed. To evaluate activation changes with disease progression, longitudinal designs are necessary. Such designs have been applied in several fMRI studies, reviewed by Smieskova and colleagues (2010). This small literature suggests that individuals who transition to psychosis differ from those who do not, with the latter group showing normalization. Thus, the application of fMRI holds promise as a tool that may facilitate elucidation of the underlying pathophysiology of the psychotic process (Fig. 26.4).
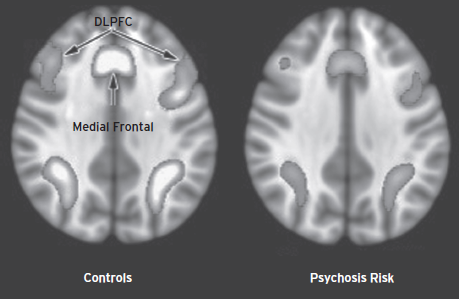
Figure 26.4 Functional MRI (fMRI) BOLD activation in response to a working memory task. A normal pattern of activation (left) and reduced activity in clinical risk for psychosis (right) illustrates the pattern observed in studies applying the n-back paradigm.
CONNECTIVITY
The resting BOLD signal in fMRI paradigms can provide a measure of connectivity, reflecting “cross-talk” integration among brain regions. It examines the time-series correlations among brain regions, indicating which regions show synchronized activation. Aberrations in schizophrenia in frontotemporal connectivity have been reported and have also been seen in those at clinical risk (Crossley et al., 2009). This literature too is at its infancy.
NEUROTRANSMITTERS
Several neurotransmitters that have been related to the pathophysiology of schizophrenia have been examined in those at psychosis risk. Dopamine dysregulation has been linked to psychosis, and positron emission tomography (PET) studies have shown increased dopamine striatal activity in schizophrenia (Fusar-Poli and Meyer-Lindenberg, 2013; Howes et al., 2009). Striatal 6-fluoro-L-dopa F18-dopa was also elevated in psychosis risk individuals and related to symptom severity (Tibbo et al., 2004).
Glutamate has also been implicated in the pathophysiology of schizophrenia and studies have examined individuals at genetic risk for psychosis using magnetic resonance spectroscopy (MRS). Increased glutamine/glutamate ratio in medial frontal cortex was reported in adolescents at genetic risk (Tibbo et al., 2004). In a study integrating fMRI and MRS, 24 psychosis risk individuals were compared to 17 healthy controls (Fusar-Poli et al., 2011c). BOLD response to a verbal fluency task showed that the psychosis risk group had greater bilateral activation than controls in midfrontal gyrus. Glutamate levels in the thalamus were lower in psychosis prone individuals. Furthermore, the pattern of correlations with activation suggests that prefrontal, hippocampal, and temporal functioning are related to thalamic glutamate levels and differentiate those at risk from controls.
ELECTROPHYSIOLOGY
As in schizophrenia research, the investigation of brain function has included several studies that have examined brain activity with electrophysiological measures. For example, P300 amplitude was reported to be smaller in a group of 100 clinical risk individuals, early or late in the prodromal state, compared to 40 healthy controls (Frommann et al., 2008). The findings were interpreted as suggesting that selective left temporoparietal amplitude deficits could be a trait abnormality, while deficits at sagittal midline electrodes may relate to changes that underlie the emergence of psychotic symptoms.
A study integrating P300 and volumetric MRI measures (Fusar-Poli et al., 2011b) examined 39 psychosis risk individuals and 41 healthy controls. P300 at intake was lower, and gray matter was reduced in several regions including the right superior frontal gyrus, left medial frontal gyrus, left inferior frontal gyrus, right orbital gyrus, and right supramarginal gyrus in the psychosis risk group. The longitudinal design made it possible to follow a subgroup, with some subjects transitioning to psychosis. Progressive gray matter alterations in prefrontal and subcortical areas were noted in the psychosis risk group, but no significant changes in P300 amplitude over time were observed.
CHALLENGES AND PROMISES
Advancing the neurobiology of schizophrenia is extremely challenging and requires converging multidisciplinary team science approaches. The bar is even higher when attempting to do what we need to in order to elucidate mechanisms that underlie the pathophysiology early in the course of emerging psychosis. Before turning to the scientific challenges and promises, the context of the debate is important to consider.
Medicine is undergoing a paradigm shift in which “Big Science” is applied to multiple biomarkers in order to permit early identification and targeted interventions that aim at biological processes rather than symptoms. Unfortunately, psychiatry is at risk to stay at the sidelines of the move toward such precision medicine not just because of the complexity of behavior and its organ, the brain, but also because of societal attitudes. Unlike other medical disorders, the burden of stigma and the consequences of false early identification of individuals who may not transition to psychosis weigh heavily when discussing the inclusion of clinical risk in DSM-5. Yet, a shift in research paradigms as envisioned by RDoC provides an opportunity to bypass the stigma by obtaining dimensional data on behavior without emphasis on categorical diagnostic classification. Such a framework can be well integrated into large-scale genomic studies and afford the data essential for addressing fundamental basic and clinical questions that can fill existing gaps in understanding. Such systematic efforts will help answer questions currently enigmatic or with limited data reflecting an early phase of investigation.
The heterogeneity in clinical features and variability in brain behavior measures necessitate large samples and longitudinal follow-up. Large longitudinal samples are also essential for integration of quantitative phenotypic neurobiological data with genomics. Such efforts are required for progress in advancing the understanding of neurodevelopmental trajectories that underlie the continuum of psychosis.
Several methodological challenges merit consideration and discussion. Some uniformity and standardization in the approach to these issues could ultimately lead to consensus in the next stages of this undertaking.
Ascertainment. The population captured in clinical risk studies is based on help-seeking youths. Some sites develop a referral network that may include community mental health providers, schools, and advertisement in the community at large. Is the pattern of presentation related to the method of ascertainment? Demographics? How does the presentation of help-seekers differ from population-based studies? To enable generalizability and sensitivity of measures, consistent information should be provided, even in the form of supplemental data in journal articles.
Comorbidity. There is inconsistency in the literature in reporting comorbid conditions, such as substance use, despite an extensive literature of psychosis in the context of substance use, such as cannabis. Similarly, the role of environmental stressors that can facilitate or curtail the emergence of psychosis needs attention.
Diagnostic instruments. Several instruments have been applied to assess sub-threshold psychotic symptoms. Adopting a common instrument will be helpful in standardization, and research groups can add other instruments to address further questions. Such standardization will help establish common training across research sites and facilitate clinical training of professionals as the field moves forward. There is also a need to focus on negative symptoms early in the course of psychosis, as the clinical presentation is at times mistakenly diagnosed as depression.
Neurobehavioral measures. Core domains should be included in future studies to enable comparison across samples and pulling data together systematically. Easy access to behavioral measurement procedures is necessary to enable data gathering across diverse settings beyond academic sites.
Neuroimaging measures. For more complex brain indices, such as those obtained in neuroimaging and electrophysiology, further considerations on data acquisition and processing are required. In such studies it is crucial to standardize data analytic approaches, and increasingly multimodal neuroimaging is feasible and could help propel the field. Structural, functional, and connectivity data can now be obtained in single sessions, and longitudinal follow-up is feasible. Importantly, the neuroimaging data need to be integrated with clinical and neurocognitive data.
Sample size. The large volume of data generated in neuroimaging studies underscores the need for large samples. Even larger samples are needed if the aim is to integrate neuroimaging data with genomics. The typical concluding paragraph in neuroimaging papers, which states that “larger samples are required” should become limited.
When genomics is running forward and bioinformatics is keeping up, we have unprecedented opportunities with available and developing technologies to attempt to answer questions that are crucial and critical for early intervention. Such knowledge may help pave the way for novel approaches to ameliorate the impact of illness and enable vulnerable individuals to get back on track as much as possible. Concerns over medicating the young and perhaps those not bound to schizophrenia spectrum disorders is understandable. However, this valid concern should not become a paralyzing fear. As the field is adopting behavioral interventions that show promise, as well as discovering natural substances that can help modulate the disease process, it is up to us to be vigilant as health professionals and do no harm. By educating the public, and free of conflict of interest, we can be in a position to impart knowledge gained, admit our ignorance, and strive to obtain the best data possible in the service of those who need it.
As we gain better understanding of the neurodevelopmental trajectories associated with “deep phenotyping” of brain behavior quantitative measures, and have data on adequately powered samples, we will be in a position to evaluate the degree and extent of departure from the normative growth curve. Implications of symptoms, their duration, persistence, intensification, and impact on functioning can be monitored. Such efforts will lead to better understanding of variability in presentation, vulnerability, resilience, and early intervention.
DISCLOSURE
Dr. Gur has no conflicts of interest to disclose.
REFERENCES
Addington, J., Penn, D., et al. (2008). Facial affect recognition in individuals at clinical high risk for psychosis. Br. J. Psychiatry 192:67–68.
An, S.K., Kang, J.I., et al. (2010). Attribution bias in ultra-high risk for psychosis and first-episode schizophrenia. Schizophr. Res. 118:54–61.
Becker, H.E., Nieman, D.H., et al. (2010). Neurocognitive functioning before and after the first psychotic episode: does psychosis result in cognitive deterioration? Psychol. Med. 40:1599–1606.
Bloemen, O.J., de Koning, M.B., et al. (2010). White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol. Med. 40:1297–1304.
Borgwardt, S., McGuire, P.K., et al. (2011). Gray matters! Mapping the transition to psychosis. Schizophr. Res. 133:63–67.
Borgwardt, S.J., Riecher-Rossler, A., et al. (2007). Regional gray matter volume abnormalities in the at-risk mental state. Biol. Psychiatry 61:1148–1156.
Brewer, W.J., Francey, S.M., et al. (2005). Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am. J. Psychiatry 162:71–78.
Brewer, W.J., Wood, S.J., et al. (2003). Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am. J. Psychiatry 160:1790–1794.
Broome, M.R., Johns, L.C., et al. (2007). Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br. J. Psychiatry Suppl. 51:38–42.
Carletti, F., Woolley, J.B., et al. (2012). Alterations in white matter evident before the onset of psychosis. Schizophr. Bull. 38:1170–1179.
Carpenter, W.T., and van Os J. (2011). Should attenuated psychosis syndrome be a DSM-5 diagnosis? Am. J. Psychiatry 168:460–463.
Chung, Y.S., Kang, D.H., et al. (2008). Deficit of theory of mind in individuals at ultra-high-risk for schizophrenia. Schizophr. Res. 99:111–118.
Crossley, N.A., Mechelli, A., et al. (2009). Superior temporal lobe dysfunction and fronto-temporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum. Brain. Mapp. 30:4129–4137.
Dickinson, D., Gold, J.M. (2008). Less unique variance than meets the eye: overlap among traditional neuropsychological dimensions in schizophrenia. Schizophr. Bull. 34:423–434.
Frommann I., Brinkmeyer, J., et al. (2008). Auditory P300 in individuals clinically at risk for psychosis. Int. J. Psychophysiol. 70:192–205.
Frommann, I., Pukrop, R., et al. (2011). Neuropsychological profiles in different at-risk states of psychosis: executive control impairment in the early—and additional memory dysfunction in the late—prodromal state. Schizophr. Bull. 37:861–873.
Fusar-Poli, P., Bonoldi, I., et al. (2012a). Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry 69:220–229.
Fusar-Poli, P., Borgwardt, S., et al. (2011a). Neuroanatomical correlates of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 35:1175–1185.
Fusar-Poli, P., Crossley, N., et al. (2011b). Gray matter alterations relted to P300 abnormalities in subjects at high risk for psychosis: longitudinal MRI-EEG study. NeuroImage. 55:320–328.
Fusar-Poli, P., Deste, G., et al. (2012b). Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry 69:562–571.
Fusar-Poli, P., McGuire, P., et al. (2012c). Mapping prodromal psychosis: a critical review of neuroimaging studies. Eur. Psychiatry 27:181–191.
Fusar-Poli, P., Meyer-Lindenberg A. (2013). Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [18F]/[11C] DOPA PET studies. Schizophr. Bull. 39:33–42.
Fusar-Poli, P., Stone, J.M., et al. (2011c). Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Arch. Gen. Psychiatry 68:881–890.
Giuliano, A.J., Li, H., et al. (2012). Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr. Pharm. Des. 18:399–415.
Green, M.F., Bearden, C.E., et al. (2012). Social cognition in schizophrenia: Part 1. Performance across phase of illness. Schizophr. Bull. 38:854–864.
Gschwandtner, U., Pfluger, M., et al. (2006). Fine motor function and neuropsychological deficits in individuals at risk for schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 256:201–206.
Gur, R.C., Saykin, A.J., et al. (1990). “Behavioral imaging”: III. Inter-rater agreement and reliability of weightings. Neuropsychiatr. Neuropsychol. Behav. Neurol. 3:113–124.
Hawkins, K.A., Addington, J., et al. (2004). Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr. Res. 67:115–122.
Howes, O., Montgomery, A., et al. (2009). Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry 66:13–20.
Ilonen, T., Heinimaa, M., et al. (2010). Differentiating adolescents at clinical high risk for psychosis from psychotic and non-psychotic patients with the Rorschach. Psychiatry Res. 179:151–156.
Korver, N., Nieman, D.H., et al. (2010). Symptomatology and neuropsychological functioning in cannabis using subjects at ultra-high risk for developing psychosis and healthy controls. Aust. N.Z. J. Psychiatry 44:230–236.
Koutsouleris, N., Davatzikos, C., et al. (2012). Early recognition and disease prediction in the at-risk mental states for psychosis using neurocognitive pattern classification. Schizophr. Bull. 38:1200–1215.
Lindgren, M., Manninen, M., et al. (2010). The relationship between psychoticlike symptoms and neurocognitive performance in a general adolescent psychiatric sample. Schizophr. Res. 123:77–85.
Magaud, E., Kebir, O., et al. (2010). Altered semantic but not phonological verbal fluency in young help-seeking individuals with ultra high risk of psychosis. Schizophr. Res. 123:53–58.
Miyake, A., Friedman, N.P., et al. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn. Psychol. 40:49–100.
Peters, B.D., de Haan, L., et al. (2009). Recent-onset schizophrenia and adolescent cannabis use: MRI evidence for structural hyperconnectivity? Psychopharmacol. Bull. 42:75–88.
Pflueger, M.O., Gschwandtner, U., et al. (2007). Neuropsychological deficits in individuals with an at risk mental state for psychosis—working memory as a potential trait marker. Schizophr. Res. 97:14–24.
Pinkham, A.E., Penn, D.L., et al. (2007). Emotion perception and social skill over the course of psychosis: a comparison of individuals “at-risk” for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn. Neuropsychiatry 12:198–212.
Pukrop, R., Ruhrmann, S., et al. (2007). Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr. Res. 92:116–125.
Reichenberg, A., Caspi, A., et al. (2010). Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am. J. Psychiatry 167:160–169.
Riecher-Rossler, A., Pflueger, M.O., et al. (2009). Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol. Psychiatry 66:1023–1030.
Seidman, L.J., Giuliano, A.J., et al. (2010). Neuropsychology of the prodrome to psychosis in the NAPLS Consortium: relationship to family history and conversion to psychosis. Arch. Gen. Psychiatry 67:578–588.
Silverstein, S., Uhlhaas, P.J., et al. (2006). Perceptual organization in first episode schizophrenia and ultra-high-risk states. Schizophr. Res. 83:41–52.
Simon, A.E., Cattapan-Ludewig, K., et al. (2007). Cognitive functioning in the schizophrenia prodrome. Schizophr. Bull. 33:761–771.
Smieskova, R., Fusar-Poli, P., et al. (2010). Neuroimaging predictors of transition to psychosis: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 38:1207–1222.
Smith, C.W., Park, S., et al. (2006). Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 81:211–215.
Szily, E., and Keri, S. (2009). Anomalous subjective experience and psychosis risk in young depressed patients. Psychopathology 42:229–235.
Szoke, A., Trandafir, A., et al. (2008). Longitudinal studies of cognition in schizophrenia: metaanalysis. Br. J. Psychiatry 192:248–257.
Tibbo, P., Hanstock, C., et al. (2004). 3T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am. J. Psychiatry 161:1116–1118.
van Rijn, S., Aleman, A., et al. (2011). Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol. Med. 41:499–508.
Woodberry, K.A., Seidman, L.J., et al. (2010). Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr. Res. 123:188–198.