31 | ANIMAL MODELS OF MOOD DISORDERS
GEORGIA E. HODES AND SCOTT J. RUSSO
WHY USE ANIMAL MODELS?
One of the first questions that arises when planning a research project is what approach to use. How do we accurately model a disease in a rodent to understand the human pathophysiology and gain insight into new treatments? This is increasingly challenging for psychiatric disorders where the human diagnosis is based largely on self-report. Nevertheless, ongoing efforts in neuroscience to develop valid rodent models focus on key behavioral domains that are conserved across species that accurately model symptoms of depressive disorders. While other animal models, including non-human primates, dogs, and even fish, are used to model depression-like behavior, the majority of research is currently being done in the rodent model, which will therefore be the primary focus of this chapter.
Given advances in technology one might question why we still need to model behavior in an animal. Aren’t there computer programs available to model drug or genetic manipulations? The reality is that animal models are currently the key to understanding a multitude of diseases. While there are computer-generated programs such as “Sniffy the virtual rat” (http://www.wadsworth.com/psychology_d/special_features/sniffy.html) that model some aspects of operant conditioning, there is currently no substitute for in vivo research. At this point there is still too much that is unknown about how the brain and body work to accurately model drug action in silico, and thus, rodent studies are critical to determine drug safety before human trials. Adding additional complexity, even when animal models have been used for drug screening purposes, sometimes the findings do not translate to humans. For example a cortisol releasing factor-1 antagonist Pexacerfont recently underwent clinical trials for generalized anxiety disorder. Although the drug was effective in animal models of anxiety, in human trials, the drug was completely ineffective, underscoring the difficulty in treating human behavioral disorders (Coric et al., 2010). Until we have a better understanding of the underlying biology driving mood disorders, we need to depend on animal models to develop safe and effective treatments.
This chapter will focus on animal models of depression, although there is significant overlap between rodent models of mood and anxiety disorder making our discussion relevant to both disorders. We will discuss the behavioral domains tested to explore depression and discuss the benefits and drawbacks of each approach. We will examine models stemming from two basic approaches. The first uses behavioral manipulations (i.e., chronic stress) to induce depression-associated behavior in normal mice. The second uses breeding strategies to try and understand how naturalistic genetic variation can lead to a depression-like phenotype. These approaches and the symptoms of depression in humans that they model are presented in Table 31.1 and discussed throughout the chapter. Both of these approaches are valid and can inform us about the mechanisms of mood disorders and identify new compounds to treat them.
WHAT CONSTITUTES AN ANIMAL MODEL?
A relevant animal model is capable of reproducing one or more core symptoms of the disorder and should have face, construct, and predictive validity (Dalla et al., 2010). Face validity is the ability of the model to mimic the symptoms of the disorder. An example would be a model, like chronic mild stress (CMS) that results in decreased preference for a natural reward (i.e., sucrose water) (Willner, 2005). In this model, animals are exposed to alternating minor stressors for approximately two to four weeks. These stressors can include forced circadian shifts, tilted cages, physical restraint, or wet bedding (Willner, 2005), the main point being that the animal’s environment is constantly changing in uncomfortable ways with no predictable pattern. This model has face validity because it induces anhedonia or the inability to experience pleasure, which is a core symptom of depression. Exposure to CMS reduces sucrose preference as well as decreasing the rewarding properties of intracranial self-stimulation (Dalla et al., 2010). Any model that measurably decreases the animal’s engagement in these types of reward-related behaviors would have good face validity and be considered to model a core symptom of the disorder.
Construct validity evaluates the plausibility or rationale of the model to explain the human disorder. Construct validity is basically addressing the question, does the basis of the model make sense in light of what we know about the human disorder? An example of a model with good construct validity is the early life stress model (Plotsky et al., 2005), though it should be noted that all stress models have a degree of construct validity, since stress is a major precipitating factor in human depression. In this model, animals are separated from their mothers for three hours at a time from postnatal day (PND) 2 to PND 14. During that time they are kept warm but not held or stimulated. In adulthood, they display a number of measures of depression including altered hypothalamic-pituitary-adrenal axis (HPA) activity, increased addiction liability (Moffett et al., 2007), and decreased coping behavior when faced with a stressor (Gardner et al., 2005). Animals that are only separated from their mothers for 15-minute intervals during the same time period show resiliency to stress on all of these domains (Plotsky et al., 2005). From the human literature, studies of orphans in Romania in the 1980s, showed that children who are not held or stimulated during early postnatal life can develop biological and emotional changes including depression, and cognitive deficits (Chugani et al., 2001). These studies support that the early life model of stress has good construct validity. From what we know about human depression, it makes sense that early life adversity can contribute to susceptibility to depression.
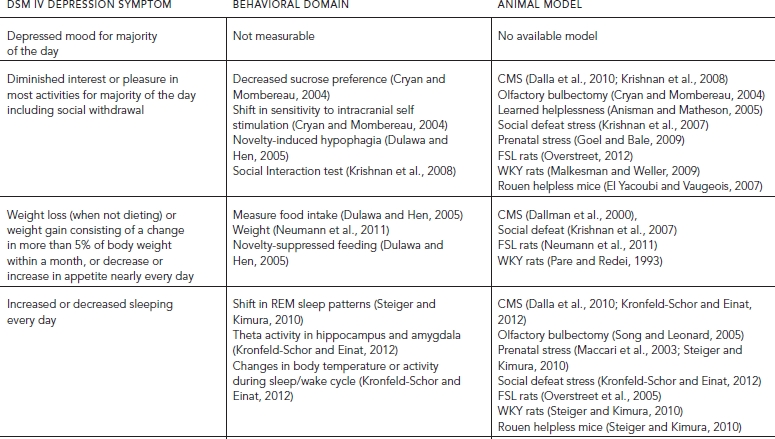
TABLE 31.1. Diagnostic symptoms of depression and the behavioral measures used to model them in animals


Predictive validity is the ability of known treatments to reverse the symptoms induced by the model. An example of a model of depression with good predictive validity is the repeated social defeat model (Krishnan et al., 2007). In this model the experimental mice are exposed each day to a novel aggressor and allowed to physically interact for 10 minutes. Animals are then separated with a perforated Plexiglas barrier that allows for sensory contact but no physical interaction. On the following day, animals are given a social interaction test that measures the time spent in the vicinity of a novel object and a novel animal. Animals that show susceptibility to the stressor will avoid the novel animal, a phenotype that is essentially permanent and only reversed by chronic, but not acute, treatment with antidepressants, including imipramine and fluoxetine (Berton et al., 2006). Importantly, these antidepressant treatments in humans also require chronic administration, thus providing excellent predictive validity. It should be noted that some models fulfill one or more of these criteria, however, ideally a model of depression will fulfill all three criteria. All of the models discussed in this section, chronic unpredictable stress, early life stress, and social defeat stress, have face, construct, and predictive validity.
Another set of criteria that have been used for determining the validity of a model are those proposed by McKinney and Bunny in the late 1960s (McKinney and Bunny, 1969). They proposed that a model should show similarity of symptoms or traits to the disorder being modeled, much like face validity. The model should also have an observable and objectively measurable behavioral change. Like predictive validity, the behavioral change should be sensitive to or reversible with known antidepressant agents, although as these known antidepressants have limited efficacy at best, they may not be the best determinants of a valid animal model for novel drug development. Finally, the model should be reproducible between investigators and laboratories. This is a key issue with some animal models of depression. For example some researchers have had difficulties in replicating the effects of chronic mild stress across laboratories (Cryan and Mombereau, 2004). A number of factors can contribute to difficulties in replicating behavioral effects, including strain of animal, age, sex, temperature differences, health of the animal colony, and so forth.
Lastly, a particularly important form of validity, not often used, involves the degree to which an animal model recapitulates the biological causes of depression in humans. This criterion has been termed pathological validity (Krishnan and Nestler, 2011) and is critical to future drug development efforts. To date, a number of peripheral biomarkers such as changes in release and feedback of cortisol, alterations in cytokine profiles, postmortem brain molecular changes, and structural and functional brain imaging changes have been identified in humans, some of which have also been found in validated animal models. These are summarized in Table 31.2 and referred to throughout the chapter in the context of each model. At this time there is still too much unknown about the etiology of depression to develop bona fide models using clinically accepted diagnostic criteria to establish the pathological validity. However, it a good criterion to keep in mind as the field moves forward.
WHAT IS DEPRESSION AND HOW DO WE MODEL SYMPTOMS?
Depression is a debilitating disorder that currently affects 16.5% of people in United States within their lifetime and can affect up to 6.7% of the population in any given year (Kessler et al., 1993). Women are twice as likely as men to experience an episode of depression within their lifetime (Kessler et al., 1993). Depression is diagnosed based on a heterogeneous cluster of symptoms listed from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). To meet criteria for depression a patient must report depressed mood or lack of interest plus four additional symptoms for two or more weeks. As shown in Table 31.1, the other symptoms include decreased interest and pleasure, weight or appetite change, changes in sleep patterns including either sleeping too much or too little, psychomotor agitation or retardation, fatigue, feelings of worthlessness or guilt, cognitive difficulties including decreases in ability to concentrate or feelings of indecisiveness, and recurrent thoughts of suicide or death (American Psychiatric Association, 2000). Because diagnosis of depression is dependent on expression of a group of symptoms rather than a biomarker-based test it leads to issues in modeling. Two individuals can exhibit very different clusters of symptoms and yet both are diagnosed with the same disorder. For example, patient A reports feelings of sadness accompanied by loss of appetite, insomnia, psychomotor agitation, and suicidal ideation. Patient B reports a lack of interest along with increased appetite and weight gain, hypersomnia, psychomotor retardation, and feelings of indecisiveness. While both patients would receive a diagnosis of major depression, their symptomology is completely different. Given this, animal models tend to focus on endophenotypes or behavioral domains that model these core symptoms rather than trying to fulfill the full criteria for depression diagnosis. In this way, animal models allow us to understand the brain circuits that control the endophenotypes, which may allow us to develop more selective therapeutics that treat the domain rather than the diverse cluster of symptoms that define depression. The next section will include a discussion of common measures used to examine the validity of these models for each depression-like phenotype.
ENDOPHENOTYPES OF DEPRESSION
Behavioral screens used to discern a depressive phenotype can be sorted into three core domains: appetitive tasks, measures of behavioral despair, and ethologically relevant behaviors, such as grooming and social interaction. In addition, researchers use physiological measures that are consistently altered in human subjects with depression, or have otherwise been validated in animal models of depression. These include, but are not limited to, peripheral endocrine, metabolic, and immune markers, along with cellular and structural changes identified through brain imaging. Each of these pathological markers will be discussed throughout the following sections in the context of animal models that exhibit pathological validity (summarized in Table 31.2).
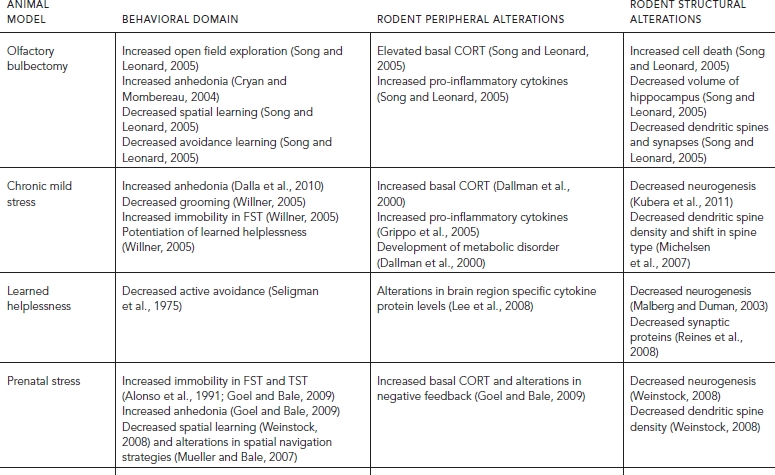
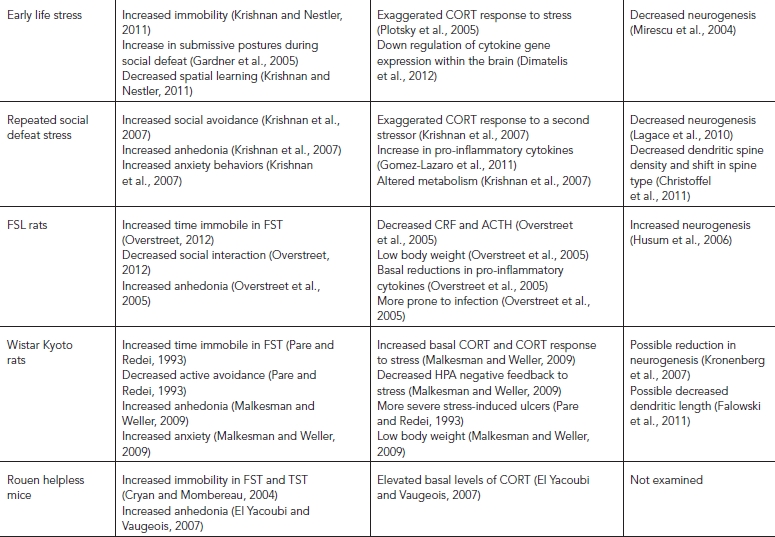
TABLE 31.2. Behavioral and pathological validity of animal models of depression
APPETITIVE TASKS
Appetitive tasks generally measure the core symptom of depression, anhedonia, although some are also negatively affected by anxiety or cognitive deficits. A first-pass screen for anhedonia is done using sucrose preference tests, which consists of giving an animal a choice between a 1% sucrose solution and water. Although many derivations of the procedures have been published, access to a two-bottle choice for sucrose or water is given for an acute period of time (1–2 hours) (Dalla et al., 2010) or overnight (Krishnan et al., 2007). The researcher then measures how much sucrose is consumed versus the total amount of fluid consumed (both sucrose and water) and the percent sucrose preference is reported. While most animals greatly prefer the sucrose over water, a variety of stressors and genetic manipulations result in decreased sucrose preference (Cryan and Mombereau, 2004) while chronic treatment with classic antidepressants can reverse the effects of some chronic stressors on sucrose preference (Willner, 2005). One issue that arises when using this test to compare effects between the sexes is that females show a greater sucrose preference as baseline than males (Dalla et al., 2010). While sucrose preference tests are the most widely published measures of anhedonia, there are many other appetitive tasks that operate on similar principles. For example, sexual activity and the preference a male exhibits toward a sexually receptive female is considered to be a measure of natural reward preference (Willner, 2005). This measure also has validity given that depressed humans exhibit similar sexual dysfunction. The “gold standard” to measure reward function is intracranial self-stimulation (Carlezon and Chartoff, 2007). In this test, an electrode is implanted directly into the medial forebrain bundle, containing dense dopaminergic fibers emanating in the VTA, and the animals are allowed to press a lever to stimulate dopamine release. The more current necessary to invoke an operant response is thought to reflect reward thresholds and are significantly increased in stress disorders, meaning that they need greater activation of the circuitry to find things reinforcing.
Other appetitive tasks make use of food restriction or food rewards to measure a depression-like behavior. Novelty-suppressed feeding (NSF) is a task that measures the latency of an animal to eat in a novel environment. In this task, animals are food-restricted over night and then placed in a new environment the following day. One pellet of food is placed in the center of the novel space and the latency to approach and feed is measured (Dulawa and Hen, 2005). This was originally considered an anxiety test, however, evidence that latency to eat could be reversed or shortened by giving the animals chronic antidepressants led people to use the test as a measure of depression (Santarelli et al., 2003). In addition, a biological process in the hippocampus called neurogenesis, thought to control antidepressant efficacy, was shown to be necessary for the antidepressant effects on latency to feed in this task (Santarelli et al., 2003). A variation of this test is called novelty-induced hypophagia (NIH). The NIH test does not require food restriction. Instead animals are trained to eat a novel palatable substance in the home cage (Dulawa and Hen, 2005). Once the animal is reliably eating the substance, the latency to feed is measured in a novel environment (Dulawa and Hen, 2005). While this again taps into aspects of anxiety/exploratory-based behavior, chronic, but not acute, treatment with antidepressants shortens the latency to eat in the novel environment.
BEHAVIORAL DESPAIR
A number of depression tests are considered measures of behavioral despair. These include the forced swim test (FST) (Fig. 31.1 A, B), tail suspension test (TST) (Fig. 31.1 C, D), and active avoidance tests. Behavioral despair tests can be thought to model feelings of sadness, psychomotor retardation, and fatigue. However, they are all confounded if the animal has a motor deficit and the active avoidance tasks are additionally confounded by cognitive deficits, since the animal has to learn how to escape or avoid a shock.
The FST was developed by Porsolt in the late 1970s. In this test, rats are placed in a cylinder containing 15 cm of room temperature water for 15 minutes. Twenty-four hours later they are again placed in the same cylinder and the amount of time the animal spends floating (immobile) is measured (Porsolt et al., 1978). The concept behind the test is that animals with higher levels of emotional despair would give up struggling sooner and remain immobile, thereby forgoing an attempt to escape the aversive environment. Importantly, the FST was the first animal model of depression that could be used as a rapid screening device for antidepressants, since tricyclics, MAOIs, and atypical antidepressants all decrease immobility on this test (Detke and Lucki, 1996). Variations of this test were later used to differentiate between different types of antidepressants. Drugs that engaged the norepinephrine (NE) system, such as tricyclic antidepressants, resulted in decreased immobility and increased climbing behavior. Drugs such as selective serotonin reuptake inhibitors (SSRIs) decreased immobility but also increased their swimming behavior (Detke and Lucki, 1996). An additional modification shortening the test to one day was then validated for use in mice and since has been used in a number of genetic mutant mice to screen for potential depression phenotypes (Krishnan and Nestler, 2011). The TST is essentially a variation of FST developed so that despair could be measured without the use of water. Removing the water element was thought to prevent hypothermia and increase repeatability (Steru et al., 1985) because water level and temperature can alter behavioral outcome. In this test mice are suspended by their tails for six minutes and the amount of time spent immobile is recorded as a behavioral measure of despair (Steru et al., 1985). The benefit of these types of tests is that they are rapid and allow for high throughput screening of new compounds. In addition, they are fairly accurate at detecting the efficacy of current antidepressants. However, they lack predictive validity in that acute antidepressants effectively decrease the time spent immobile in both the FST and TST even though chronic treatment is necessary to alleviate depression symptoms in humans. In general, these types of tests work well for determining antidepressant efficacy and screening potential new drugs based on the monoamine hypothesis, but their validity as a model for depression or use in testing novel mechanisms of action is highly questionable.
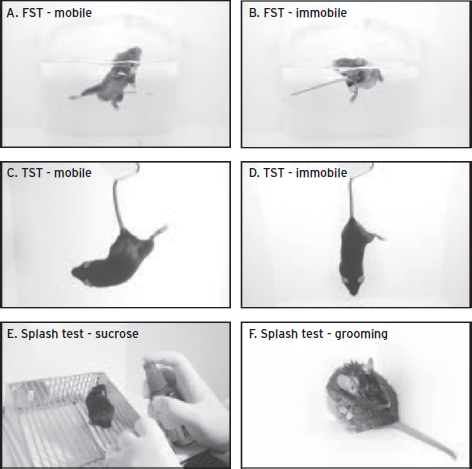
Figure 31.1 Behavioral assays of depression-like behavior in mice. Common behavioral tests used to assay depression-associated behavior. (A) Time spent swimming in the forced swim test (FST) is considered an antidepressant measure, as SSRIs will increase engagement in this type of behavior. (B) The time spent floating or in an immobile posture is considered prodepressant, as stress manipulations increase this behavior. (C) Struggling behavior in the tail suspension test (TST) is considered an antidepressant measure, as antidepressants increase engagement in this activity. (D) The amount of time the animal spends immobile is considered a depression-like response, as stress based manipulations increase the amount of time spent immobile. (E) In the splash test, an animal is sprayed with a 10% sucrose solution to make their coats sticky. (F) The amount of time spent grooming is measured as an indicator of a depression-like state; animals that are stressed spend less time grooming and this can be reversed by chronic antidepressant treatment. (Photography credit: Christopher Wood.)
Active escape avoidance is another commonly used measure of behavioral despair. In an active avoidance task animals are required to perform an operant behavior allowing them to escape an aversive environment. The operant response requirement can vary from simple (i.e., running through a doorway from one side of a cage to another) to complex (i.e., pushing a lever, turning a wheel, or poking their nose into a hole). Usually the stimulus that makes the environment aversive is a mild foot shock. A subset of animals that are stressed will fail to engage in active avoidance behavior (Krishnan and Nestler, 2011). This task has face validity and it is often used to test learned helplessness behavior, since animals experiencing repeated stress learn that they have no control over their environment and therefore don’t attempt escape (Overmier and Seligman, 1967). Chronic treatment with antidepressants can reverse active avoidance deficits induced by the learned helplessness paradigm (Malberg and Duman, 2003), therefore the model has good predictive validity.
ETHOLOGICALLY RELEVANT BEHAVIORS
A number of behavioral tests for depression measure the degree to which ethologically relevant behaviors are altered. These are behaviors that the animal would normally engage in within their natural environment. Two examples of this type of test include social avoidance behavior (Fig. 31.3) and the splash test (Fig. 31.1 E, F). Social avoidance behavior is defined as the amount of time an animal spends in the presence of a novel animal versus a novel object. This test is used to model two core symptoms of depression: lack of interest or pleasure, since rodents are social organisms and find these interactions to be naturally rewarding, and psychomotor retardation, as we can measure the total distance traveled within the environment as well as the time spent near the target or in the corners. While a version of this test, often referred to as sociability test, is also used extensively to test features of autism and memory, in depression, it is commonly used as a behavioral endpoint following repeated social defeat stress (Fig. 31.2). During testing the experimental animal is placed in a novel environment with a novel object, consisting of a small wire mesh cage. The amount of time the animal spends within the interaction zone with a novel animal placed inside the mesh cage is measured. A ratio of the time spent in the interaction zone with the novel animal divided by the time spent in the same area without the novel mouse can be used to determine if an animal exhibits social avoidance. Under control conditions, most animals prefer the social target and increase their interaction time when the novel mouse is placed in the mesh cage. Conversely, susceptible mice avoid the novel animal and typically hide within the corners of the arena. This model has predictive, construct, and face validity, since chronic, but not acute, antidepressant treatment can reverse avoidance behavior in mice (Berton et al., 2006) and there is an extensive literature showing the contribution of repeated social subordination in the development of depressive disorders in humans (Gilbert, 2006). The model also has pathological validity; a number of biological targets have been identified in this model that are similarly changed in postmortem brain from humans with depression (Krishnan et al., 2007; Wilkinson et al., 2011).

Figure 31.2 Repeated Social Defeat Stress and Social Interaction Testing. Repeated social defeat stress is an animal model of depression with strong face, construct, and predictive validity. The experimental mouse is housed with a new aggressor every day for 10 days. (A) The smaller C57BL/6J mouse is given a 10-minute physical interaction with a larger CD-1 mouse that is screened to show aggression within his home cage. (B) CD-1 aggressor mouse is pinning the C57BL/6J mouse, one type of aggressive behavior. (C) The C57BL/6J mouse is displaying a submissive posture to the CD-1 mouse. (D) After the physical interaction, the C57BL/6J mouse is separated by a divider that allows for sensory, but not physical, stimulation for the next 24 hours. (Photography credit: Christopher Wood.)
Another ethologically relevant measure of a depressed state is a decrease in grooming behavior. All organisms including humans and rodents tend to decrease grooming when they are sick or stressed (Isingrini et al., 2010). These types of tests can be used to model lack of interest in normal activities of daily life, another core symptom of depression. Rodent studies such as the splash test score grooming intensity as a behavioral measure of an animal’s psychological state. In this test animals are sprayed with a 10% sucrose solution that makes their coat sticky. The amount of time the animal spends grooming is then measured as an indicator of a depression-like state. This test has predictive and face validity since animals experiencing chronic stress will spend less time grooming, like depressed humans, and chronic, but not acute, antidepressants have been shown to reverse this effect (Isingrini et al., 2010). Together these endophenotypes give us multiple functional behavioral outputs that are relevant to depressive-like states in humans. It should be noted however, that each test focuses on aspects of core symptoms of depression and are most effective when used as part of a larger test battery providing a powerful set of tools to explore the underlying biology of depression.
ANIMAL MODELS OF DEPRESSION
While there are no clinically accepted genetic or biological causes of depression in humans, it is clear that stress exposure increases depression onset and severity. Therefore, most models of depression utilize various environmental stressors to induce a depression-like behavioral phenotype and then study the underlying biological consequences of such stress on the key behavioral domains described earlier. Environmental models use “normal” animals and manipulate their environment through physical or psychological stress, surgical manipulation, or other methods to produce a depression-like phenotype. Examples of this type of stressor include early life stress, CMS, and olfactory bulbectomy. Genetic models make use of naturally occurring or trait-based selective breeding or gene knockout/transgenic technology to produce a depressive state. These studies can vary from examining natural strain differences in depression-associated behavior to conditional removal or overexpression of genes of interest, although the latter is beyond the scope of the current chapter and is discussed in detail in subsequent chapters. Recently, there has been increasing interest in epigenetic models of depression. These models examine the impact of environmental stressors on parent-offspring heritability of traits that are outside of genetic sequence differences. Alternatively they consider differences in the stress response not driven by genetic sequence differences. While epigenetics is clearly an important area of research, it is discussed in detail in subsequent chapters and is beyond the scope of the current discussion. The following section will discuss examples of a variety of these animal models of depression and discuss their validity within the context of the behavioral domains previously described.
The majority of these studies have been performed in rats, with only a small subset in mice (Cryan and Mombereau, 2004). In this model the olfactory bulbs are removed resulting in a variety of behavioral, cellular, neuroendocrine, and immune changes consistent with depression (Cryan and Mombereau, 2004). Animals that undergo olfactory bulbectomy are highly aggressive and hyperactive, including increased investigation of novel environments and increased nocturnal activity (Song and Leonard, 2005). They also show cognitive impairment similar to patients with depression. These cognitive impairments include decreased abilities to perform spatial learning tasks that engage the hippocampus, such as the radial arm maze and Morris water maze, along with deficits in active avoidance tasks (Song and Leonard, 2005). Olfactory bulbectomy results in anhedonia measured by decreased sucrose preference (Cryan and Mombereau, 2004) and reduced sexual activity (Song and Leonard, 2005). These deficits are due to the removal of the olfactory bulbs, as inducing anosmia by flushing zinc through the nasal cavities does not cause any significant depression phenotype (Song and Leonard, 2005). The removal of the olfactory bulbs also leads to cell death and degeneration in a number of areas implicated in depression in humans, including the hippocampus, amygdala, dorsal raphe, cortex, and locus coeruleus. Within the hippocampus there is a reduction in volume along with a decrease in the number of dendritic spines. These changes have also been reported in the piriform cortex (Song and Leonard, 2005) and the amygdala (Cryan and Mombereau, 2004). Chronic antidepressant treatment can reverse behavioral and structural changes (Song and Leonard, 2005). In addition, removal of the olfactory bulbs also induces changes similar to those seen in depressed humans within the immune system. These include increases in serum concentrations of the pro-inflammatory cytokine interleukin (IL)-1β and prostaglandin E2 along with a decrease in circulating levels of the anti-inflammatory cytokine IL-10 (Song and Leonard, 2005). Olfactory bulbectomized animals also have elevated basal corticosterone (CORT) levels, the rodent homolog for the stress hormone cortisol in humans. While this model does not have good construct validity and only moderate face validity, it does have excellent predictive validity, as chronic antidepressant treatments consistently reverse the behavioral effects of olfactory bulbectomy (Cryan and Mombereau, 2004). The paradigm also has excellent reproducibility across labs. Interestingly, olfactory bulbectomy is one of the few models of depression that does not show sex differences in the behavioral measures. Both males and females show behavioral effects of olfactory bulbectomy in tests of locomotor activity, exploration of a novel environment, anxiety, and sucrose preference (Song and Leonard, 2005) making it a test of interest for those studying depression in females.
CHRONIC MILD STRESS
In the chronic mild stress (CMS) paradigm, sometimes referred to as chronic variable or unpredictable stress (CVS or CUS), animals are exposed to variable stressors for a length of time extending between 6 (LaPlant et al., 2009) and 28 days (Willner, 2005). The stressors are typically minimally invasive but are given in a varied and unpredictable order. Animals can be exposed to more than one stressor in a day and the stressors are administered during all phases of their light:dark cycle. Stressors include restraint, tail suspension, disruption of the light/dark cycle, cage tilt, food and/or water restriction, changing of cage mates, temperature reductions, exposure to soiled or wet bedding, and random foot-shocks (Willner, 2005). Animals exposed to CMS generally show anhedonic behaviors, such as decreased sucrose consumption, impairments in natural reward associations, and changes in responsiveness to intracranial self-stimulation (Dalla et al., 2010). Animals exposed to CMS display increased immobility in the FST (Willner, 2005) and are more likely to show decreased active avoidance if they are subsequently exposed to the learned helplessness paradigm described in the following section in this chapter (Willner, 2005). They also show deficits in grooming, sexual behavior, and immune function (Willner, 2005). Biological profiling shows that CMS can induce increases in circulating levels of the pro-inflammatory cytokines tumor necrosis factor (TNF) α and IL1β (Grippo et al., 2005) both in the brain and the body. Four weeks of CMS exposure also results in increased basal levels of CORT (Grippo et al., 2005) along with disruptions in circadian release of CORT leading to reduced peak levels and an elongation of the trough of the cycle (Dallman et al., 2000). This dysregulation of normal HPA feedback results in changes in the storage of calories and fat leading to the development of a metabolic syndrome (Dallman et al., 2000). CMS can reduce neurogenesis in rodents although this may be due to effects on cell survival rather than cell proliferation (Kubera et al., 2011). CMS can also alter spine density in the prefrontal cortex and hippocampus of rodents consistent with work in humans showing reduced volume of these structures by magnetic resonance imaging (MRI) (Michelsen et al., 2007). Importantly, many of these effects can be reversed with chronic, but not acute, antidepressant exposure, giving this model good predictive validity. Also, an abbreviated six-day variation of this model can induce depression-like behaviors in females, but not males, making it an ideal model for understanding biological differences in stress responses between males and females (LaPlant et al., 2009). The utility of CMS to understand the neurobiology of depression in both males and females makes it a very useful tool to test new compounds for the treatment of depression and possibly identify targets for individualized approaches to treatment in men and woman.
LEARNED HELPLESSNESS
The learned helplessness paradigm was developed in the late 1960s. In the original experiments, dogs were given a single session of multiple inescapable shocks and then presented with an active avoidance task (Overmier and Seligman, 1967). They were allowed to escape the shock by jumping over a barrier in a shuttle box. In control animals that were not exposed to a previous bout of inescapable shock, the animals quickly learned to escape the aversive environment. Dogs that had experienced a prior uncontrollable shock failed to learn to escape the shuttle box and would exhibit helpless behavior (i.e., dogs would lie down and whimper) (Seligman, 1972). This test was later adapted for use in rodents and is now considered to be a primary depression test. Adaptations of the protocol have included yoked controls, allowing the researcher to directly compare behavioral and biological effects of having control over shock termination. Here, both groups of animals are exposed to the shock before avoidance training but only one group can control the termination of the shock (Seligman et al., 1975). Animals that can terminate the stressor, usually by performing an operant task, learn to escape in a subsequent active avoidance task, whereas yoked animals receiving the same shock without control over termination show a much greater degree of helplessness behavior. Interestingly, there are individual differences in susceptibility to inescapable stress; a third of the animals exposed to inescapable shock display susceptibility and engage in helplessness behavior, whereas a majority of animals are resilient to the stress and still learn to escape (Russo et al., 2012). This suggests that there may be an epigenetic component, since many of these studies are performed in genetically identical inbred animals, though further studies are needed. The difference in susceptibility also highlights a degree of face validity to the model, since most humans are resilient in the face of stress and don’t develop significant depression symptoms. The model also has good construct validity, as loss of control over one’s environment is highly stressful and can contribute to onset of a depressive episode (Gilbert, 2006) and all classes of antidepressant reverse learned helplessness in adult male rats (Zazpe et al., 2007). The model has some pathological validity, since helpless rodents have decreased hippocampal synaptic proteins such as PSD 95 and synaptophysin, and decreased neurogenesis (Malberg and Duman, 2003), which are consistent with some of the structural changes observed by MRI in humans. Both behavioral and structural deficits can be reversed by chronic, but not acute, treatment with antidepressants (Malberg and Duman, 2003), indicating that the model has good predictive validity (Malberg and Duman, 2003; Reines et al., 2008). Helpless animals also show reductions in IL-2 levels in brain regions implicated in depression including the hippocampus and prefrontal cortex (Lee et al., 2008). In the periphery IL-2 is involved with T-cell development and the recognition of “self” within the immune system (Lee et al., 2008) and within the brain it has been demonstrated to control emotion associated behaviors (Lee et al., 2008). However, females do not have the same stress-related behavioral deficits in LH (Shors et al., 2007), nor do they exhibit any chronic stress-related decrease in neural plasticity (Shors et al., 2007). Therefore the utility of the test is limited, especially when studying females. Regardless, LH models important aspects of mood disorders, and therefore, warrants further mechanistic studies to determine pathological validity across a wider array of biomarker targets.
DEVELOPMENTAL STRESSORS
Stress exposure during critical periods of development has been shown to produce long-lasting changes in depression and anxiety-like behavior (Russo et al., 2012). Prenatal stress paradigms involve exposing pregnant females to stress and examining the effects of this stress exposure on the adult offspring. The effects of prenatal stress depends in part on the critical prenatal period when the mother is exposed to the stressor (Goel and Bale, 2009; Weinstock, 2008). Any number of prenatal stressors, including CMS (Goel and Bale, 2009), restraint stress, foot shock, and swim stress have been used (Weinstock, 2008). Stress during the early prenatal period (PND 1–7) results in a depression-like phenotype in male but not female mice (Goel and Bale, 2009), as measured by increased immobility in the FST and TST. Male mice exposed to gestational stress at this time point also displayed decreased sucrose preference (Goel and Bale, 2009). Interestingly, during this period, stress leads to dysmasculinization, causing males to engage in female-like behavioral strategies in their exploration of the Barnes maze (Mueller and Bale, 2007). Physiological changes in HPA-axis sensitivity to stress have been shown. Male mice exhibit larger stress-induced increases in CORT along with alterations of gene expression involved with negative feedback of the HPA (Goel and Bale, 2009). Later gestational stress can lead to a more pronounced depression-like phenotype in females than males, although this depends heavily on the behavioral domain being studies (Weinstock, 2008). Restraint stress (PND 15- parturition) leads to greater immobility in the FST in females than males (Alonso et al., 1991). However, other studies have reported that late gestational restraint stress (PND 14–20) impacts cognitive abilities in males but not females (Weinstock, 2008). Both restraint stress and foot shock starting at PND 8 have been reported to decrease spatial learning in males (Weinstock, 2008). These mice also had reductions in dendritic spine densities within the hippocampus that were thought to be associated with the cognitive deficit (Weinstock, 2008). Other studies performed only in males have found late gestational restraint stress also leads to adult reductions in cell proliferation within the hippocampus (Weinstock, 2008). Like early gestational stress, late gestational stress leads to hypercortisolemia in adults along with dysregulation of HPA negative feedback (Weinstock, 2008). Late gestational restraint stress also leads to alterations in circadian rhythm and changes in REM sleep (Maccari et al., 2003). These alterations include increases in paradoxical and fragmented sleep along with a decrease in deep slow wave sleep (Maccari et al., 2003). This model has good predictive validity, since treatment of offspring exposed to late gestational stress with chronic antidepressants in adulthood can reverse behavioral effects in the FST and alter glucocorticoid receptor expression (Maccari et al., 2003).
Early life stress can also produce significant depression-like phenotypes (Russo et al., 2012). Most studies have used maternal separation during the early postnatal period followed by behavioral, hormonal, and molecular analysis of the separated offspring during adulthood (Plotsky and Meaney, 1993). Studies typically separate pups for either 15 or 180 minutes a day during the postnatal period. Offspring separated for 15 minutes a day did not differ from normal facility reared controls or else showed resilience to stress exposure later in life (Plotsky et al., 2005). This phenomenon is referred to as stress tolerance and may promote active coping strategies (Russo et al., 2012). Conversely, animals exposed to early life stress have displayed greater immobility, and decreased spatial learning (Krishnan and Nestler, 2011). Rats exposed to maternal separation and then put through a repeated social defeat paradigm in adulthood used a passive coping mechanism to the stressor and engaged in more submissive postures and behavior (Gardner et al., 2005). Other studies have demonstrated that early life stress can make animals more prone to self-administer alcohol and cocaine (Moffett et al., 2007). They also exhibit greatly exaggerated HPA responses to stress (Plotsky et al., 2005) and have decreased cell proliferation in hippocampus leading to a smaller overall volume (Mirescu et al., 2004). Interestingly, studies in rodents have demonstrated that most early life stress affects male, but not female, rodents in adulthood (Diehl et al., 2007). In general it is currently thought that male animals are more susceptible to most forms of early life stress than females (Goel and Bale, 2009), whereas females are more susceptible to stress after puberty (Dalla et al., 2010). More research is clearly needed to understand how stress susceptibility changes across the life span in both sexes. In sum, early life stress models are excellent for understanding a host of biological and behavioral changes important in depression. They exhibit excellent face, construct, and predictive validity as well as a growing body of evidence that suggests the model has pathological validity.
REPEATED SOCIAL DEFEAT STRESS
Repeated social defeat stress (Fig. 31.2) can unmask behavioral differences in vulnerability to stress across a wide spectrum of behavioral domains. In this model, experimental mice are placed into the home cage of a novel larger aggressive mouse each day for 10 days (Krishnan et al., 2007). The larger mouse quickly establishes dominance through physical interaction for a brief five to ten minute period, and then a perforated Plexiglas divider is placed in the cage to physically separate the two animals, allowing them to experience continued sensory cues. At the end of 10 days, experimental mice are separated into resilient and susceptible populations based on their social interaction score (Fig. 31.3), described earlier in the behavioral domains section of this chapter. Susceptibility and resilience scores are calculated as a ratio of the time spent near the novel animal divided by the time spent near the novel object. Animals that prefer the novel animal are considered resilient and behave like control animals that have not been exposed to stress. Animals that avoid the novel animal and spend more time near the novel object are considered susceptible. Approximately two-thirds of the mice exhibit social avoidance behavior and are considered susceptible, while the remaining one third do not show avoidance behavior and are considered resilient (Krishnan et al., 2007). Interestingly, both susceptible and resilient mice exhibit significant anxiety phenotypes, yet only susceptible mice show depression-like phenotypes measured by social avoidance, anhedonia, disruptions of the circadian system, and metabolic changes, including weight gain (Krishnan et al., 2007). The fact that only a subset of animals shows vulnerability to the effects of stress is again consistent with human responses to stress and provides construct validity. Some of the behavioral effects, including social avoidance and metabolic syndrome, can be reversed with chronic, but not acute, antidepressant treatment (Berton et al., 2006). Mechanistically, social defeat stress leads to a transient decrease in cell proliferation in both susceptible and resilient mice (Lagace et al., 2010), though reduced neurogenesis has been functionally implicated in resiliency, which may be somewhat counter to the reduced hippocampal volume identified in depression in humans (Lagace et al., 2010). Susceptibility to social defeat stress increases dendritic spine density on medium spiny neurons in the nucleus accumbens, a region critical for emotion and reward (Christoffel et al., 2011). These changes in spine profiles correlated with social avoidance behavior. Social defeat stress also increases circulating levels of pro-inflammatory cytokines in susceptible mice (Gomez-Lazaro et al., 2011). While social defeat is clearly a powerful model of depression exhibiting face, construct, and predictive validity, one of the major limitations of this type of stress is that it is difficult to test in female mice. Female mice do not normally attack each other, making it difficult to establish antagonistic interactions based on these dominance hierarchies. A few recent studies using a female rodent that is monogamous and territorial (California mouse; Peromyscus californicus) (Trainor et al., 2011) or nursing dams have effectively established social dominance in females (Shimamoto et al., 2011). Importantly, these studies have shown similar effects as males in measures such as anhedonia, drug addiction, and metabolic effects. However, more research is clearly necessary to determine whether the same molecular cascades and neural circuitry are affected in both sexes.
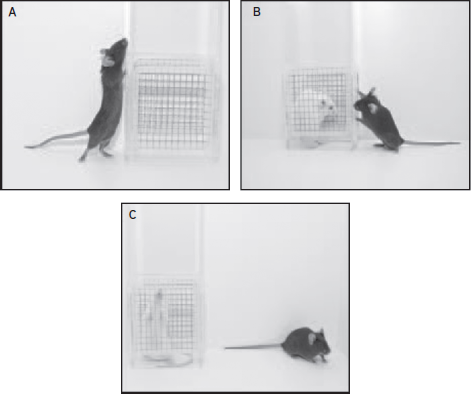
Figure 31.3 Social interaction testing. The social interaction test is used as a behavioral endpoint for stress-based manipulations such as repeated social defeat stress. In this test an animal is placed in a novel environment and allowed to interact with a novel object or a novel animal. (A) The time the mouse spends near a novel object is measured. (B) The amount of time spent interacting with the novel animal is measured. Control and resilient mice spend more time with the novel animal than the novel object. (C) The amount of time spent avoiding the novel animal is measured. Stress-susceptible mice will spend more time with the novel object than the novel animal. (Photography credit: Christopher Wood.)
SELECTIVE BREEDING MODELS
While there are no known genetic causes of depression, there is clearly a strong familial linkage (Gilbert, 2006), suggesting that certain heritable complex genetic traits may predispose one to depression. For years, researchers have utilized selective breeding strategies to uncover the genetic basis of disease and, not surprisingly, there are a few lines, including the Flinder’s sensitive line (FSL) and the Wistar-Kyoto (WKY) line, that show increased depression-associated phenotypes. Although the FSL was not originally investigated as a model of depression, it became clear that some of the neurochemical changes recapitulated alterations in humans with depression. The FSL rats were derived from Sprague–Dawley rats by selecting animals that were sensitive to cholinergic and anticholinergic compounds (Overstreet, 2012). It was later determined that FSL rats show increased time spent immobile in the FST and decreased social interaction behavior (Overstreet, 2012). In addition, FSL rats are less motivated to work for a natural reward and have lower body weight and alteration in REM sleep (Overstreet et al., 2005). Interestingly, male FSL rats show greater behavioral deficits than females; females only show reductions in latency to immobility but not in time spent immobile (Dalla et al., 2011). The male response in the FST has been used to screen novel antidepressant compounds because they can be tested in concert with the Flinder’s resistant line (FRL), which do not show the same behavioral deficits and are not responsive to acute antidepressant treatment. Interestingly, FSL rats are only responsive to chronic, antidepressant treatment, making this line particularly useful for its predictive validity. Pathological changes are also shown in the FSL rats. They have reductions in CRF and ACTH, similar to the subset of depressed subjects with hypocortisolemia and psychomotor retardation (Overstreet et al., 2005). The FSL rats also show abnormalities in immune function. There is a reduction in the number of natural killer cells in FSL rats, and they are more vulnerable to infection. However, FSL rats show basal reductions in pro-inflammatory cytokines such as IL-6 (Overstreet et al., 2005) that are normally elevated in the blood of subjects with depression (Dowlati et al., 2010). While FSL rats have increased cell proliferation in the dorsal hippocampus compared to the FRL line, during aging, they have a sharp decline in cell proliferation concurrent with a decrease in serotonergic enervation of the hippocampus thought to increase depression associated behavior (Husum et al., 2006). These findings warrant future investigation in that they may provide insight into the mechanisms of increased depression prevalence during aging. In general, the model shows good predictive validity in male animals. The face and pathological validity is mixed, as some symptoms do overlap with those of depression, whereas a number of biological measures do not. There is also a degree of construct validity, as humans with depression have abnormal sensitivity to cholinergic agents (Overstreet, 2012). Importantly, the model shows good reproducibility, at least in male rats, as multiple labs have found similar effects of antidepressants on the FST response (Dalla et al., 2011; Overstreet, 2012). However the fact that females show less depression behavior than males offers an important caveat, since females of most other rodent strains and women exhibit greater stress-induced depression-like behavior and higher depression prevalence, respectively (Dalla et al., 2010).
As mentioned previously, the WKY rats are also predisposed to greater depression behavior as a result of selective breeding. WKY rats were originally bred as a control for a hypertensive line (Malkesman and Weller, 2009). WKY rats compared to other strains show greater stress susceptibility, including increased immobility in the FST, decreased active avoidance when exposed to the learned helplessness paradigm, and increased vulnerability to stress-induced ulcers (Pare and Redei, 1993). Females show greater immobility than males in their response in the FST and also develop more severe ulcers, although this varies across the estrous cycle (Pare and Redei, 1993). In addition, WKY rats show greater anhedonia, anxiety-like behavior, and stress-induced weight loss compared with other strains (Malkesman and Weller, 2009). This cluster of behavioral symptoms have led researchers to classify this as a model of comorbid depression and anxiety disorder (Malkesman and Weller, 2009). Mechanistically, WKY rats display dysregulation of the HPA axis including increased levels of CORT and ACTH along with an elongation of the elevation in CORT and a decrease in ACTH levels following an acute swim stress (Malkesman and Weller, 2009). Little is known about differences in brain plasticity, as most studies have compared the WKY rats to the spontaneously hypertensive strain, but these studies have shown that WKY rats have lower rates of cell proliferation and survival (Kronenberg et al., 2007). This strain has recently been used to explore the antidepressant and anxiolytic effects of novel antidepressant drugs acting on the kappa-opioid system (Carr et al., 2010). The model has good face and predictive validity, but lacks construct validity, as the basis for the susceptibility of WKY rats to stress is not yet understood. Reproducibility between laboratories is mixed because differences in breeding strategies by commercial breeders can lead to significant genetic drift (Overstreet, 2012).
In addition to selective breeding models in rat, Rouen helpless or “depressed” mice have been heavily used to study the mechanisms of depression and for novel antidepressant discovery. These mice were originally generated in 1995 by breeding CD-1 mice with high and low immobility scores in the TST (Cryan and Mombereau, 2004). Fourteen generations later these mice were shown to display increased immobility in the FST and TST, alterations in REM sleep, anhedonia, and elevated basal levels of CORT (El Yacoubi and Vaugeois, 2007). The helpless phenotype appears more often in females than males, making it a valid line for studying sex differences (Yacoubi et al., 2011). The model has good predictive validity as a number of classic antidepressants are able to reverse the behavioral effects in both sexes (El Yacoubi and Vaugeois, 2007). Reproducibility is also good, as multiple labs have identified similar behavioral phenotypes (Svenningsson et al., 2006). This model was used to first identify a functional role for P11 (Svenningsson et al., 2006), a protein that interacts with serotonin 1B receptors. P11 is decreased in helpless male and female mice, as well as in postmortem tissue from subjects with depression (El Yacoubi and Vaugeois, 2007; Svenningsson et al., 2006~) and is proposed to be a potential novel target for the development of new antidepressants. The selective breeding strategy is one method of developing animal models of depression with pathological validity for discovering complex genetic variations that may underlie depression. However, there are some major drawbacks to selective breeding models. The generation of animals is time consuming and expensive, multiple overlapping genetic factors make it impossible to understand contributions of specific genes, and genetic drift occurring over time can affect the reproducibility of phenotypes (Overstreet, 2012; Yacoubi et al., 2011). Despite these factors, selective breeding may help to understand how these complex psychiatric phenotypes are maintained in a population and eventually, through the use of high-throughput genetic sequencing, we may even determine the complex genetic contributions driving depression susceptibility.
SUMMARY AND CONCLUSIONS
In this chapter we have discussed the validity of each major animal model of mood disorders currently being used to uncover novel mechanisms of psychiatric illness and to develop new therapeutics. These models range from those based on stress exposure to induce a depression-like phenotype in genetically identical mice to models that use selective breeding to examine the complex genetic contributions to depression between strains. Some of the models make use of acute or short-term stress exposure such as learned helplessness, whereas others depend on exposure to chronic stress, such as CMS and social defeat stress. All of the models discussed at least partially fulfill the criterion for face, construct, and predictive validity, which is critical to establishing animal models that accurately mimic the disease. However, as we have discussed throughout this chapter, we need to judge the validity of these animal models with greater stringency and incorporate new criteria, such as reproducibility across labs and pathological validity. Pathological validity is especially important and will lead to a greater understanding of the underlying biology of depression. Over time, this may help clinicians to develop diagnostic criteria based on these biological changes and move drug discovery efforts beyond monoamine-based antidepressants, which only lead to successful remission in approximately 40% of the population (Krishnan and Nestler, 2008). New, rapid onset treatments such as ketamine (Berman et al., 2000) are undergoing clinical trials and show promise, however, all currently approved antidepressants are based on serendipitous discovery and do not directly target the underlying pathology of mood disorders. Additionally, diagnosis is based largely on non-overlapping clusters of behavioral symptoms that are often shared across disorders, making it difficult to achieve specificity in diagnosis. Thus, we need to develop biomarkers and diagnostic tests for the core symptoms of depression, and diagnose and treat based on these factors. For this, the use of valid animal models is critical and we need to continually develop and expand on our models to cover an even greater range of behavioral deficits. Moreover, few basic drug discovery studies use female animals as experimental subjects (Beery and Zucker, 2010), despite the fact that in humans, there are twice as many women that suffer from depression as men (Kessler et al., 1993). While there are a few models that accurately reflect this sex difference in depression prevalence, many do not. Thus, we need to be cognizant of this limitation in validity and understand that there may be individual- or sex-based differences in our models that will impact our results. In summary, the field has made tremendous progress in developing animal models of mood disorders that, in some cases, have a high degree of validity in modeling human depression. By using a battery of tests to understand distinct behavioral domains we can gain a greater insight into the biology of depression behavior. Over time, we can use these animal models that mimic the pathophysiology of depression to develop biomarker diagnostic tests and eventually more effective treatments with fewer negative side effects.
Preparation of this review was supported by grants from the National Institute of Mental Health: R01 MH090264 (SJR), and National Institute on Drug Abuse training grant 5TDA07135-28 (GEH).
DISCLOSURES
Dr. Russo has no conflicts of interest to disclose. His work is funded by a NIMH R01 MH090264 and J&J IMHRO Rising Star Award.
Dr. Hodes has no conflicts of interest to disclose. She is funded by NIDA (5TDA07135-28) and the Brain and Behavior Research foundations NARSAD Young Investigator award.
REFERENCES
Alonso, S.J., Arevalo, R., et al. (1991). Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol. Behav. 50(3):511–517.
American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, 4th Edition. Washington DC: Author.
Anisman, H., and Matheson, K. (2005). Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 29(4–5):525–546.
Beery, A.K., and Zucker, I. (2010). Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35(3):565–572.
Berman, R.M., Cappiello, A., et al. (2000). Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47(4):351–354.
Berton, O., McClung, C.A., et al. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762):864–868.
Carlezon, W.A., Jr., and Chartoff, E.H. (2007). Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat. Protoc. 2(11):2987–2995.
Carr, G.V., Bangasser, D.A., et al. (2010). Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 35(3):752–763.
Christoffel, D.J., Golden, S.A., et al. (2011). IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 31(1):314–321.
Chugani, H.T., Behen, M.E., et al. (2001). Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. NeuroImage. 14(6):1290–1301.
Coric, V., Feldman, H.H., et al. (2010). Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress. Anxiety 27(5):417–425.
Cryan, J.F., and Mombereau, C. (2004). In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatry 9(4):326–357.
Dalla, C., Pitychoutis, P.M., et al. (2010). Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 106(3):226–233.
Dalla, C., Pitychoutis, P.M., et al. (2011). Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr. Top. Behav. Neurosci. 8:97–118.
Dallman, M.F., Akana, S.F., et al. (2000). Bottomed out: metabolic significance of the circadian trough in glucocorticoid concentrations. Int. J. Obes. Relat. Metab. Disord. 24(Suppl 2):S40–S46.
Detke, M.J., and Lucki, I. (1996). Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav. Brain. Res. 73(1–2):43–46.
Diehl, L.A., Silveira, P.P., et al. (2007). Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain. Res. 1144:107–116.
Dimatelis, J.J., Pillay, N.S., et al. (2012). Early maternal separation leads to down-regulation of cytokine gene expression. Metab. Brain. Dis. 27(3):393–397.
Dowlati, Y., Herrmann, N., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67(5):446–457.
Dulawa, S.C., and Hen, R. (2005). Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 29(4–5):771–783.
El Yacoubi, M., and Vaugeois, J.M. (2007). Genetic rodent models of depression. Curr. Opin Pharmacol. 7(1):3–7.
Falowski, S.M., Sharan, A., et al. (2011). An evaluation of neuroplasticity and behavior following deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery 69(6):1281–1290.
Gardner, K.L., Thrivikraman, K.V., et al. (2005). Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136(1):181–191.
Gilbert, P. (2006). Evolution and depression: issues and implications. Psychol. Med. 36(3):287–297.
Goel, N., and Bale, T.L. (2009). Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J. Neuroendocrinol. 21(4):415–420.
Gomez-Lazaro, E., Arregi, A., et al. (2011). Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress 14(5):537–548.
Grippo, A.J., Francis, J., et al. (2005). Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 84(5):697–706.
Husum, H., Aznar, S., et al. (2006). Exacerbated loss of cell survival, neuropeptide Y-immunoreactive (IR) cells, and serotonin-IR fiber lengths in the dorsal hippocampus of the aged Flinders sensitive line “depressed” rat: implications for the pathophysiology of depression? J. Neurosci. Res. 84(6):1292–1302.
Isingrini, E., Camus, V., et al. (2010). Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One 5(4):e10404.
Kessler, R.C., McGonagle, K.A., et al. (1993). Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 29(2–3):85–96.
Krishnan, V., Berton, O., et al. (2008). The use of animal models in psychiatric research and treatment. Am. J. Psychiatry 165(9):1109.
Krishnan, V., Han, M.H., et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2):391–404.
Krishnan, V., and Nestler, E.J. (2008). The molecular neurobiology of depression. Nature 455(7215):894–902.
Krishnan, V., and Nestler, E.J. (2011). Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 7:121–147.
Kronenberg, G., Lippoldt, A., et al. (2007). Two genetic rat models of arterial hypertension show different mechanisms by which adult hippocampal neurogenesis is increased. Dev. Neurosci. 29(1–2):124–133.
Kronfeld-Schor, N., and Einat, H. (2012). Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology 62(1):101–114.
Kubera, M., Obuchowicz, E., et al. (2011). In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35(3):744–759.
Lagace, D.C., Donovan, M.H., et al. (2010). Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. USA 107(9):4436–4441.
LaPlant, Q., Chakravarty, S., et al. (2009). Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol. Psychiatry 65(10):874–880.
Lee, Y.T., Wang, W.F., et al. (2008). Effects of escapable and inescapable stressors on behavior and interleukin-2 in the brain. Neuroreport 19(12):1243–1247.
Maccari, S., Darnaudery, M., et al. (2003). Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci. Biobehav. Rev. 27(1–2):119–127.
Malberg, J.E., and Duman, R.S. (2003). Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28(9):1562–1571.
Malkesman, O., and Weller, A. (2009). Two different putative genetic animal models of childhood depression—a review. Prog. Neurobiol. 88(3):153–169.
Mckinney, W.T., Jr., and Bunny, W.E., Jr. (1969). Animal model of depression. I. Review of evidence: implications for research. Arch. Gen. Psychiatry 21(2):240–248.
Michelsen, K.A., van den Hove, D.L., et al. (2007). Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC. Neurosci. 8:107.
Mirescu, C., Peters, J.D., et al. (2004). Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci. 7(8):841–846.
Moffett, M.C., Vicentic, A., et al. (2007). Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol. 73(3):321–330.
Mueller, B.R., and Bale, T.L. (2007). Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 91(1):55–65.
Neumann, I.D., Wegener, G., et al. (2011). Animal models of depression and anxiety: what do they tell us about human condition? Prog. Neuropsychopharmacol. Biol. Psychiatry 35(6):1357–1375.
Overmier, J.B., and Seligman, M.E. (1967). Effects of inescapable shock upon subsequent escape and avoidance responding. J. Comp. Physiol. Psychol. 63(1):28–33.
Overstreet, D.H. (2012). Modeling depression in animal models. Methods Mol. Biol. 829:125–144.
Overstreet, D.H., Friedman, E., et al. (2005). The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci. Biobehav. Rev. 29(4–5):739–759.
Pare, W.P., and Redei, E. (1993). Sex differences and stress response of WKY rats. Physiol. Behav. 54(6):1179–1185.
Plotsky, P.M., and Meaney, M.J. (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain. Res. Mol. Brain. Res. 18(3):195–200.
Plotsky, P.M., Thrivikraman, K.V., et al. (2005). Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30(12):2192–2204.
Porsolt, R.D., Anton, G., et al. (1978). Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 47(4):379–391.
Reines, A., Cereseto, M., et al. (2008). Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: correlation with behavioral response. Neuropsychopharmacology 33(8):1896–1908.
Russo, S.J., Murrough, J.A., et al. (2012). Neurobiology of resilience. Nat. Neurosci. 15(11):1475–1484.
Santarelli, L., Saxe, M., et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301(5634):805–809.
Seligman, M.E. (1972). Learned helplessness. Annu. Rev. Med. 23 407–412.
Seligman, M.E., Rosellini, R.A., et al. (1975). Learned helplessness in the rat: time course, immunization, and reversibility. J. Comp. Physiol. Psychol. 88(2):542–547.
Shimamoto, A., Debold, J.F., et al. (2011). Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology. (Berl.) 218(1):271–279.
Shors, T.J., Mathew, J., et al. (2007). Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol. Psychiatry 62(5):487–495.
Song, C., and Leonard, B.E. (2005). The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 29(4–5):627–647.
Steiger, A., and Kimura, M. (2010). Wake and sleep EEG provide biomarkers in depression. J. Psychiatr. Res. 44(4):242–252.
Steru, L., Chermat, R., et al. (1985). The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl.) 85(3):367–370.
Svenningsson, P., Chergui, K., et al. (2006). Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311(5757):77–80.
Trainor, B.C., Pride, M.C., et al. (2011). Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One 6(2):e17405.
Weinstock, M. (2008). The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32(6):1073–1086.
Wilkinson, M.B., Dias, C., et al. (2011). A novel role of the WNT-Dishevelled-GSK3{beta} signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J. Neurosci. 31(25):9084–9092.
Willner, P. (2005). Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52(2):90–110.
Yacoubi, M.E., Popa, D., et al. (2011). Genetic association between helpless trait and depression-related phenotypes: evidence from crossbreeding studies with H/Rouen and NH/Rouen mice. Int. J. Neuropsychopharmacol. 1–12.
Zazpe, A., Artaiz, I., et al. (2007). Reversal of learned helplessness by selective serotonin reuptake inhibitors in rats is not dependent on 5-HT availability. Neuropharmacology 52(3):975–984.