
50 | ANIMAL MODELS OF ADDICTION
RAFAEL MALDONADO, J. DAVID JENTSCH, BRIGITTE L. KIEFFER, AND CHRISTOPHER J. EVANS
Drug addiction, or substance use disorder (SUD), is a chronic brain disease characterized by the compulsive use of drugs, loss of control over drug-taking in spite of their adverse consequences, and relapse even after long periods of drug abstinence (Koob and Volkow, 2010; O’Brien et al., 1998). Substance use disorder is considered the result of a series of transitions from voluntary use in search of a hedonic effect, to loss of control over this behavior, and ultimately to compulsive behavior (Everitt et al., 2008). Important in the context of mental illness is the high comorbidity of depressive illness and anxiety with SUD and that drugs of abuse taken acutely often alleviate symptoms of these afflictions, yet during abstinence the symptoms of depression and anxiety are exacerbated. Indeed, SUD is comorbid with many psychiatric diseases (clinically referred to as dual diagnosis), including schizophrenia, where incidence of both cigarette and cannabinoid smoking is exceptionally high (Santucci, 2012). In current research of SUD, animal models recapitulate the phenotypes contributing to abuse susceptibility through initial drug taking, habitual drug taking, abstinence, and finally relapse (Fig. 50.1). These models have begun to unravel the molecular, cellular, and behavioral adaptations regulating addictive behaviors in research, which has greatly enriched our understanding of the neurocircuitry mediating learning, motivation, mood, and stress (Koob and Volkow, 2010; Russo et al., 2010). This chapter systematically explores animal models that contribute insights to the addiction cycle as outlined in Figure 50.1. We first cover models assessing reward-related and reinforcement behaviors. We follow this with descriptions of models for abstinence and relapse, and new genetic models that increasingly are facilitating addiction research. Finally, we discuss animal model contributions to susceptibility for initiating additive behaviors.
Maladaptive patterns of reward-seeking and -taking involving natural rewards have been reported to share similarities with those observed in drug addicts. Animal models have revealed that natural rewards activate the mesocorticolimbic system, which mediates the hedonic and motivational aspects of different rewarding stimuli in a manner very similar to drugs of abuse. Repeated activation of this reward pathway leads to neuroadaptive changes that are involved in the progressive behavioral changes caused by natural rewards and drugs. Similar structural plasticity changes in the mesocorticolimbic system have recently been reported after repeated exposure to natural rewards, such as highly caloric food (Johnson and Kenny, 2010), palatable food (Guegan et al., 2012), or sex (Pitchers et al., 2010), all of which can drive the development of compulsive behavior. The similarities and differences among the behavioral disorders promoted by drugs and natural rewards represent a complex and fascinating topic that has been investigated in animal models. Although this area is out of the scope of this chapter, it has been reviewed elsewhere (Olsen, 2011).
MODELS OF REWARD AND REINFORCEMENT
Animal models of reward and reinforcement are the fundamental tools for substance abuse research. Drug consumption is promoted and maintained by the rewarding and reinforcing properties of the drug. These rewarding effects play a crucial role in the initiation of the addictive processes (Koob and Le Moal, 2008). However, it is important to note that although drug consumption is a requirement for the development of addiction, drug intake does not necessarily induce an addicted state. In fact, addiction is defined as a chronic relapsing mental disease and is difficult to comprehensively mimic with the animal models currently available. Most of the animal models described in this chapter have been designed for evaluating particular properties of drugs leading to the development of this disease (reward/reinforcing properties) or some specific manifestations of the disease (tolerance, withdrawal, or relapse).
Animal models for reward and reinforcement use either investigator administration of drug (non-contingent), or self-administration of drug (contingent) in which drug taking is under the control of the animal (Fig. 50.2). Contingent or non-contingent administration paradigms can elicit different behavioral responses that can be reflected at the level of neuronal adaptations. The analysis of drug-induced changes in dendritic spine density and dendritic branching that controls inter-neuronal communication is one example in which differential adaptations in multiple brain regions depend on whether the drug is given contingently or non-contingently (Robinson and Kolb, 2004). Human addictions are most effectively recapitulated in contingent models. However, the value of non-contingent models should not be discounted because they can provide information on adaptations that occur only because of the presence of the drug as well as insight into a subset of reward-related behaviors using uncomplicated drug administration procedures.

Figure 50.1 Addiction cycle. In addicted individuals, recreational drug use switches to compulsive drug intake. The addiction cycle typically includes intoxication/withdrawal/craving episodes (Koob and Volkow, 2010). Exiting the cycle requires maintenance of abstinence. Drug abstinence is characterized by a negative affect that strongly contributes to relapse and is often triggered by drug cues, the drug itself, or stress. All aspects of the cycle are subject to modification by genetic–environment interactions.
All animal models measuring reinforcement and reward require learning. Some acute models of drug administration engage only goal-directed learning, whereas others engage habit learning after extensive repetition of drug administration paradigms. Animal studies in recent years have identified different brain circuitry mediating goal-directed and habit learning with circuitry between the medial prefrontal cortex and dorsomedial striatum mediating goal-directed learning and the sensory-motor cortex to the dorsolateral striatum mediating habit learning (Balleine and O’Doherty, 2010). Reinforcement assays require instrumental actions to obtain the reward, and a learning curve is necessary to develop the instrumental association between an action (e.g., lever push, nose poke, or eye movement) and its contingent effect. Reinforcement can be demonstrated by an increase in responding to a rewarding stimulus (positive reinforcement) or responding to avoid or delay an aversive stimulus (negative reinforcement), and animals will clearly work for both. Models of drug self-administration may not readily distinguish between these two motivational states given that the cycle of addiction includes a dysphoric phase as a consequence of absence of the drug (see Fig. 50.1). Furthermore, certain intrinsic aspects of the models may be aversive (e.g., the anxiety of a novel environment or surgery for catheterization) and the drug may relieve these aversive components as a result of anxiolytic, analgesic, or dissociative properties. Modeling the relief of an aversive stimulus has important implications for certain aspects of human behavior in which amelioration of pain, depression, or anxiety favor drug use. However, the circuitry recruited for euphoric reward will undoubtedly differ from the circuitry recruited to obfuscate an aversive stimulus (Koob and Volkow, 2010).
SELF-ADMINISTRATION
Self-administration methods are considered the most reliable and predictive animal models to directly evaluate the reinforcing properties of a drug. These procedures mimic drug-taking behavior in humans and are widely used by many researchers. The neurobiological mechanisms involved in drug self-administration in animals are assumed to be similar to those underlying drug intake in humans (Maldonado et al., 2011). Operant and non-operant procedures can be used in these self-administration models. Non-operant paradigms involve the consumption of a freely available drug, are centered on the amount of drug consumed, and are restricted in rodents to oral self-administration. These non-operant methods are widely used to study alcohol-rewarding effects, and they have also been used less frequently with other drugs of abuse, such as nicotine, cocaine, amphetamine, and morphine (Sanchis-Segura and Spanagel, 2006). Oral ethanol non-operant self-administration procedures present high reliability and predictive value as models of human alcohol consumption. Indeed, rodents drink alcohol when using the oral route, whereas they hardly consume this drug through other routes of self-administration (Sanchis-Segura and Spanagel, 2006; Spanagel and Zieglgansberger, 1997). In contrast, rodents show only modest motivation to self-administer other drugs by the oral route, including opioids or psychostimulants (Meisch, 2001), and very specific and sometimes biased procedures are required to induce their oral consumption. Therefore, the behavioral responses evaluated in those particular situations do not necessarily reflect the rewarding properties of these drugs. In the most currently used oral alcohol self-administration procedures, rodents have simultaneous access to two bottles, one containing an aqueous alcohol solution and another containing water (Spanagel and Zieglgansberger, 1997). The consumption of alcohol in these paradigms is greatly influenced by the genetic background and animal species used. The alcohol concentration represents another critical factor that can even bias the outcome because low concentrations may have a mild-sweet taste, whereas overly high concentrations have an aversive flavor (Sanchis-Segura and Spanagel, 2006). Other critical factors in these paradigms are the temporal accessibility to alcohol (Vengeliene et al., 2012), and the kind and number of available bottles and/or other reinforcers (Tordoff and Bachmanov, 2003). In general, alcohol consumption increases when rodents are given restricted access to the drug (e.g., every other day) or a higher number of alternative alcohol solutions are presented.
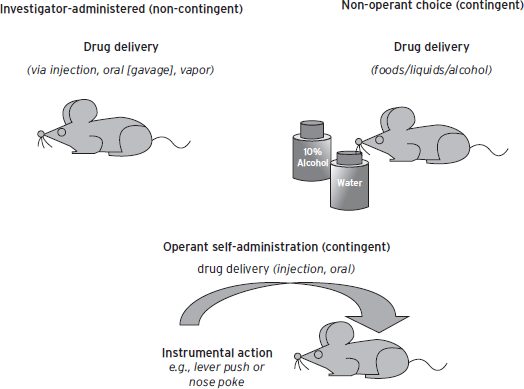
Figure 50.2 Drug administration.
Operant paradigms require that the animal performs an instrumental response to self-administer the drug. In these models, the animal learns to maintain a behavioral response, typically by pressing a lever or nose poking in a hole, to obtain a positive reinforcer (drug delivery) that is delivered contingently after completion of the schedule requirement (Maldonado et al., 2011). The operant chamber levers and nose poking holes also transmit operant response. The response in the active manipulandum is linked to the delivery of the drug, whereas the response in the inactive manipulandum results in the delivery of the drug vehicle or lacks any programmed consequence. These operant procedures are considered by most researchers to be reliable models of drug consumption in humans with a high predictive value. The route of administration most commonly used in these operant procedures is intravenous delivery of all the prototypical drugs with the exception of alcohol, which usually requires the delivery of an oral solution. However, these operant procedures are not specifically associated with any route of administration, and multiple additional routes have been successfully used, such as intracranial, intraventricular, or intragastric delivery.
Different single and complex schedule requirements to obtain the reinforcer can be programmed in these operant paradigms. The schedules most frequently used are the fixed ratio and progressive ratio schedule of reinforcement programs. Under a fixed ratio schedule, the drug is delivered each time a preselected number of responses are completed in the active manipulandum. Under the progressive ratio schedule, the response requirement to deliver the drug escalates according to an arithmetic progression. The common index of performance evaluated in this last schedule is the highest number of responses that the animal accomplishes to obtain a single infusion of drug (the break point), which provides information about its motivation for the drug. The analysis of this instrumental response provides valuable data about different behavioral aspects of drug consumption. After acquisition of the operant task, the behavioral response can be extinguished by exposing the animals to an additional training in which the reward is no longer available. The operant behavior to seek the drug can be then reinstated by using different stimuli.
Rodents typically maintain an operant behavior to self-administer all the prototypical drugs of abuse, including opioids, psychostimulants, synthetic drugs, nicotine, and cannabinoids (Maldonado et al., 2011). In the case of cannabinoids, rodents self-administer synthetic cannabinoids but not D9-tetrahydrocannabinol, the main psychoactive component in Cannabis sativa, which is only self-administered by monkeys (Tanda et al., 2000). In rodents, these operant self-administration models were first validated in rats, and then adapted to mice. Reliable operant models of acquisition and relapse of morphine, cocaine, ecstasy, alcohol, nicotine, and synthetic cannabinoids self-administration are also now available in mice (Martin-Garcia et al., 2009; Mendizabal et al., 2006; Soria et al., 2008). Application of these new models to the different lines of genetically modified mice will yield more insights in the future.
CONDITIONED PLACE PREFERENCE
In these paradigms, the subjective effects of a drug are repeatedly paired to a previously neutral stimulus. Through this repeated conditioning process, the neutral stimulus acquires the ability to act as a conditioned stimulus, and the animal will prefer or avoid this conditioned stimulus depending on the rewarding or aversive effects produced by the drug (Fig. 50.3). The most commonly used paradigms apply a spatial environmental stimulus as the conditioned stimulus and the animal will show a conditioned place preference or aversion for the environment associated with the effects of the drug or its withdrawal. Although a conditioned approach/avoidance toward specific stimuli can also occur in humans as a result of drug consumption (Bardo and Bevins 2000), the place conditioning paradigms are not primarily intended to model any particular feature of human behavior. These paradigms mainly represent an indirect assessment of the rewarding or aversive effects of a drug or its withdrawal, by measuring the response of the animal toward the conditioned stimulus. Drugs of abuse display a differential ability to produce conditioned place preference. Opioids and psychostimulants easily produce robust place preference over a wide range of experimental conditions, whereas other drugs, such as ethanol, cannabinoids, or nicotine, produce more inconsistent results. The caveats of the place preference assay are many, with issues of memory function, circadian rhythm, and that the animals non-contingently receive the drug in a novel environment, which has consequences for eliciting stress. Despite this, conditioned place preference has been an informative and widely utilized behavioral model for understanding parameters that disrupt or enhance the rewarding effects of abused drugs.
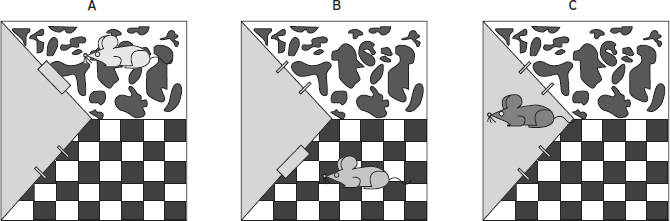
Figure 50.3 (A) Drug administered non-contingently (repeated three to five times on alternate days) and the animal confined to one compartment (“cow pattern” in this example but the side would be a randomized group). (B) Drug vehicle given to the animal confined to the other compartment (“checked pattern”) on alternate days from drug. (C) Animal put in the neutral chamber (gray triangle) with access to both the drug- and vehicle-paired compartments.
INTRACRANIAL SELF-STIMULATION
Intracranial electric self-stimulation (ICSS) procedures were essential in the discovery of the brain reward circuits. These paradigms are now widely used to study the effects of acute and chronic drugs of abuse administration and withdrawal in the activity of the reward circuits (Sanchis-Segura and Spanagel, 2006). In this paradigm, animals are trained to maintain an operant behavior so as to obtain an electric pulse through an electrode that has been previously implanted in a reward-related brain site, most frequently the lateral hypothalamic area. The threshold of the minimal current needed to promote intracranial electric self-stimulation is estimated. A drug that stimulates the reward circuit will decrease this threshold, which is related to its rewarding properties, whereas a drug having aversive effects will enhance the minimal current required to maintain the self-stimulation (Markou and Koob, 1993). The ICSS threshold increases after prolonged drug administration and during withdrawal. This is interpreted as a desensitized reward system and parallels other behavioral and imaging evidence that SUD can, over time, attenuate the basal functioning of the dopaminergic reward circuitry (Kenny, 2007).
MODELS OF WITHDRAWAL AND RELAPSE
Withdrawal from drugs of abuse can be an important aspect of SUD because withdrawal can promote a state at both the behavioral and cellular level that supports negative reinforcement. Drug withdrawal has multiple phenotypes, including physical symptoms, craving, and the modulation of affect (Koob and Volkow, 2010). Symptoms of acute and protracted withdrawal are sensitizers for relapse and can serve as major drivers for the addiction cycle (see Fig. 50.1). Although the acute physical withdrawal behaviors caused by individual drugs appear very different, the prolonged withdrawal phenotype (negative affect, craving behavior, and vulnerability to stress) begin to emerge as common phenotypes. This is reflected by merging transcript profiles in the extended amygdala, one of the brain regions involved in emotional responses following protracted abstinence of several drugs of abuse (Le Merrer et al., 2012).
PHYSICAL WITHDRAWAL
Depressant drugs such as opioids, alcohol, or benzodiazepines have dramatic acute withdrawal symptoms. In the case of alcohol and benzodiazepines these result in seizures, which can be lethal. In acute withdrawal from opioids, there are a classical set of physical symptoms that can be readily measured in rodents and include jumping, wet dog shakes, weight loss, paw tremors, diarrhea, and piloerection. In the most frequently used model of opioid withdrawal, the symptoms are triggered by administration of an opioid receptor antagonist such as naloxone. Similar antagonist-precipitated withdrawal assays have been used for measures of nicotine and cannabinoid physical withdrawal, although the symptoms are considerably less robust than opioid withdrawal (Trigo et al., 2010). Cocaine and other psychostimulants do not elicit physical withdrawal symptoms that can be easily measured in animal models or human subjects. This demonstrates that physical withdrawal is not a predicate for addiction.
WITHDRAWAL AND AFFECTIVE STATE/CRAVING
Although many studies have focused on the physical aspects of withdrawal, the affective state and drug craving (the desire to attain the drug state) are aspects that deserve focus, given their role in triggering relapse and modeling the primary mental affliction of SUD. In the case of opioid withdrawal, antagonist precipitation cannot be used to assess changes in affect because antagonists such as naloxone and naltrexone are highly aversive in rodents that have never been exposed to an opioid drug. This aversion in drug-naïve mice has been shown to be caused by a constitutive hedonic tone as a result of proenkephalin-derived opioid peptides activating mu-opioid receptors (Skoubis et al., 2005). Affective state and craving is best modeled in animals simply withdrawn from the drug. Recent studies have assessed animals during both acute and protracted withdrawal. Significantly, although physical signs of withdrawal diminish rapidly and within a few days after opioid withdrawal, some phenotypes strengthen, including social interaction deficits and depressive phenotypes (Goeldner et al., 2011). The strengthening of such behaviors after protracted abstinence is associated with deficits in serotonin and can be reversed with the serotonin membrane transporter blocker fluoxetine. Incubation after acute withdrawal also enhances drug-seeking induced by drug-related stimuli (Weiss, 2010).
RELAPSE
Relapse susceptibility is an extremely persistent and robust phenotype in both humans and animals (Wikler and Pescor, 1970) and can get progressively stronger in response to some triggers during abstinence (Weiss, 2010). Relapse models in animals first require the animal to learn a drug self-administration task, although a place-preference paradigm could also be used. The animal receives extinction training so as to erode the established associations, followed by test sessions in which behavior spontaneously recovers or is reinstated by a drug-related cue, the drug, or stress (Sinha et al., 2011). The use of extinction training is not an optimum method to reduce drug-seeking behavior because it does not accurately recapitulate the human experience and, in fact, changes the underlying neuroadaptations mediating drug-seeking and -taking. However, without extinction, extinguishing drug-seeking may not be practically feasible given the strength and longevity of reward-related behaviors. In humans, relapse happens when the motivation to abstain is overwhelmed by the motivation to take the drug. This stands in contrast to animal models in which the motivation to take the drug overwhelms extinction-related learning. New research should focus on the development of models that emphasize conflict between the motivational states that excite and inhibit drug-taking and the role for cognitive control circuitry in these circumstances.
MODELS OF DRUG ADDICTION
As discussed, the animal models available for evaluating drug reward and reinforcement, as well as withdrawal, have been very useful in clarifying the neurobiological basis of drug taking and specific features of the addictive process. However, addiction is a chronic relapsing disorder characterized by compulsive drug use maintained despite adverse consequences for the user (O’Brien et al., 1998). Behavioral models that resemble the main diagnostic criteria for addiction have only recently been studied using animal models. Two independent research groups have validated behavioral models of compulsive drug-seeking in rodents, resembling addictive behavior in humans (Belin et al., 2008; Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004). These models evaluate the persistence of drug-seeking and -taking in the face of punishment, resistance to extinction, and exaggerated motivation to obtain the drug. In one animal model, the motivation for the drug determined by the break point (the maximal amount of work that the animal performs before cessation of responding) was abnormally high under these conditions. Indeed, a break point greater than 500 operant actions to obtain a single cocaine injection in “addict rats” was reported in these models (Deroche-Gamonet et al., 2004). Although there is significant promise in this area of work, it remains relatively underutilized and studied.
GENETIC ANIMAL MODELS FOR ADDICTION RESEARCH
Multiple studies in humans (family twin and adoption studies) point to a major genetic component in the etiologies of SUD (Ho et al., 2010). Rodent models have been developed to identify genes and pathways related to this disease. Three major approaches are well exampled in alcohol research (Crabbe, 2008). The first approach has used inbred lines with different SUD propensity. A second approach has used genetic selection for phenotypes related to SUD. A third approach has been the targeted modification of the genome, a strategy that has probably been the most informative in identification of relevant primary targets and reward-dependent mechanisms. What is becoming clear from genetic association analysis in both human and animal subjects is that SUD is an exceptionally complex disorder with multiple genetic contributions that modify susceptibility to different drugs and different aspects of the disorder in selective environments (Ho et al., 2010). This complexity of genetic contribution and interaction with environment makes human analysis an ominous task requiring large cohorts for valid analysis. However, recently the identification of one locus in a cluster of nicotinic receptors, the CHRNA5-A3-B4 cluster on chromosome 15, has been repeatedly associated with both lung cancer and smoking (Bierut, 2010). Ongoing research is elucidating how this locus modulates disease susceptibility in animal models with genetically down- or up-regulated levels of the nicotinic receptor subunits in this area of the genome (Fowler et al., 2011; Gallego et al., 2012).
During the past decade there has been an explosion of genetically modified mouse models to determine the effects of specific genes on SUD. The endogenous opioid system provides an outstanding example of progress that can be achieved with targeted modification of genome (Le Merrer et al., 2009). The genetic ablation of the mu-opioid receptor (mu knockout mice) eliminates the place preference, self-administration, and analgesic effects of drugs such as morphine and fentanyl. Intriguingly the mu knockouts additionally exhibit diminished or abolished reward-associated behaviors for nicotine, alcohol, and cannabinoids, which do not directly activate opioid receptors. These data are suggestive of the recruitment of the endogenous opioid system for the rewarding experience by several non-opioid drugs. Indeed proenkephalin knockout mice, which lack one of the three precursors of endogenous opioid peptides, exhibit the same reduced-reward phenotype as mu-opioid receptor knockout mice.
Genetically modified mice have also contributed to our understanding of the dark side of addiction. Kappa-opioid receptor knockout mice as well as mice null for the gene encoding kappa-opioid receptor ligands (prodynorphin), both exhibit a phenotype of reduced stress (Bruchas et al., 2010). Mice null for the endogenous kappa-opioid system do not show stress-induced relapse for cocaine and relieve the aversive component of tetrahydrocannabinol in place preference assays (Maldonado et al., 2011). Kappa antagonists are now a major focus for drug development for the treatment of relapse and other stress-related disorders. The era of developing new animal models by genetic modification has not ended and the new era of conditional knockout and knockin models are revealing cell types, circuits, and brain regions involved in selective aspects of SUD. Furthermore, as genetic analysis begins to reveal new genes of interest, such as in the case of the alpha 5 nicotinic receptor for smoking, animal models provide the tools to understand the genetic influence mechanistically and perhaps enlighten therapeutic approaches.
ANIMAL MODELS OF ADDICTION SUSCEPTIBILITY
A variety of biological and environmental factors have been hypothesized to prospectively (causally) influence SUD. These hypothesized relationships can often be studied in a controlled, experimental manner in animal models because of the capacity to selectively manipulate all factors but the one under study and prospectively examine the characteristics of the phenotypic relationship. These procedures overcome the confounds associated with covariation of phenotypes (Lynch et al., 2002) in human subjects as well as the limitations of purely retrospective analyses.
SEX
In many ways, the potential for animal models of vulnerability to drug abuse is best demonstrated by studies of sex effects on drug reinforcement. In most Western societies, the use of licit and illicit substances with addiction liability is more common in males than females (Lynch et al., 2002). On the other hand, it has been suggested that among those persons who sample the drug, the development of a compulsive, clinically significant form of drug-seeking and -taking occurs more readily in females (Lynch et al., 2002). Because of the absence of social factors that likely inhibit drug sampling in human females, rodent models are useful in interrogating the influence of purely biological dimensions of sex on the phases of drug use, abuse, and dependence that follow initial sampling. Significantly, female rats have been shown, in multiple laboratories and using multiple preparations, to more readily acquire drug self-administration, self-administer larger amounts of drug, to reinstate drug self-administration, and develop inflexible patterns of drug intake (Lynch et al., 2002; Perry and Carroll, 2008). Subsequent studies have revealed the partial contributions of estrogenic hormones to these differences (Lynch et al., 2002), but it remains unknown how genetic aspects of sex contribute to gender differences. These results demonstrate the potential for animal models to reveal important biological predetermining factors, particularly when those factors are sometimes overridden by environmental or psychosocial factors.
BIOBEHAVIORAL PHENOTYPES
Substance use disorders cosegregate with a number of biobehavioral preclinical or clinical phenotypes. One such relationship is the apparent comorbidity between SUD and several mental disorders, including attention deficit/hyperactivity disorder (ADHD) (Groman et al., 2009). Children diagnosed with ADHD are at greater risk for the development of a SUD during their lifetime, particularly if they are not successfully medicated. There are many theories regarding the causal basis of this comorbidity. On one hand, some argue that substance use in the teenage and adult years represents a form of “self-medication” in which patients consume nicotine and/or stimulants because of their ability to remediate inattentive and/or hyperactive symptoms. A different view, however, is that individuals with ADHD are susceptible for substance misuse because they exhibit novelty seeking, behavioral impulsivity, and/or differential reward sensitivity, which independently predispose them for dyscontrolled reward-seeking behaviors (Groman et al., 2009). These and other phenotypes that segregate with SUD may represent predisposing factors that are worthy of causal and biological dissection in animal models.
NOVELTY-SEEKING
Novelty-seeking traits in humans—defined as the propensity to be attracted to environments associated with novelty, sensation, and/or risk—are associated with SUD, and novelty reactivity in rats has been conceptually linked with novelty-seeking in humans (Dellu et al., 1996). One of the earliest demonstrations that traits related to novelty reactivity predict individual differences in response to illicit substances of abuse was that, in outbred rats, the magnitude of the locomotor response evoked by a novel environment positively predicted subsequent acquisition of an instrumental response reinforced by drugs (Piazza et al., 1989). This relationship is present in both male and female subjects, and is under genetic control in that it can be enriched by breeding for the phenotype. So-called “high responders” (in the novel environment) are therefore more susceptible to the reinforcing effects of stimulants and opiates under some circumstances.
On the other hand, psychomotor reactivity to novelty may only be one dimension of novelty-seeking in humans because the trait is primarily defined as the propensity to seek out and participate in novel and exciting circumstances. With that in mind, it is interesting that individual differences in novelty preference or novelty reinforcement in rats has also been linked to drug self-administration phenotypes in a manner that extends beyond the explanatory value of simple novelty reactivity (Belin et al., 2011), suggesting that the overall relationship is quite complex.
IMPULSIVITY
Novelty-seeking has been conceptually linked to another dimension of temperament often referred to as impulsivity—a tendency to act or react with limited forethought and/or a tendency of one’s behavior to be driven by immediate, rather than delayed, outcomes associated with behavior (Evenden, 1999). High impulsivity is itself a phenotype that segregates with SUD and that likely explains the dysregulated pattern of drug intake in some individuals with drug abuse and dependence (Jentsch and Taylor, 1999). Because of evidence that exposure to drugs of abuse is sufficient to elicit impulsive patterns of responding (Izquierdo and Jentsch, 2012; Jentsch and Taylor, 1999), it was unclear that impulsivity in SUD was a preexisting vulnerability factor.
Recent studies in rodents, however, have dramatically clarified this view. Rapid response impulsivity (acting without forethought) and impulsive choice (diminished sensitivity to delayed, as opposed to immediate, outcomes) both predict high liability for drug self-administration (Dalley et al., 2007; Izquierdo and Jentsch, 2012; Perry and Carroll, 2008), although different aspects of impulsivity have been revealed to dissociably relate to distinct aspects of self-administration (e.g., initial acquisition versus motivation to consume the drug versus escalation versus extinction versus reinstatement). Impulsivity traits in animal models are under genetic control and are linked to reduced dopamine D2-like receptor availability/function in brain (Dalley et al., 2007; Laughlin et al., 2011). Although all the details of this relationship remain to be exposed, it is clear that a multidimensional model that incorporates both the phenotypic complexity of impulsivity and drug reinforcement is required. In this sense, the results of the animal models not only generally clarify the causal nature of the relationship between impulsivity and drug abuse phenotypes, but can also generate new hypotheses about the behavioral and molecular architecture of this relationship that can be subsequently tested in humans.
REWARD SENSITIVITY
It has been suggested that individual differences in the positive subjective response to reward is an independent predictor of liability for SUD and that so-called reward sensitivity can be phenotypically indexed by specific behavioral paradigms, such as the sweet flavor preference (Carroll et al., 2008). In other words, high preference for sweet flavors indicates high reward sensitivity, and the hypothesis is that reward sensitivity in turn translates into individual differences in sensitivity to drug reward and/or reinforcement. In a comprehensive series of studies, Carroll and colleagues have provided empirical support for this hypothesis in rat models. Saccharin preference (a phenotype that indicates sweet, but not calorie, preference) is a genetically influenced trait, in the sense that it can be enriched by breeding for the phenotype. Rats bred for high saccharin preference more readily self-administer cocaine and opioids, compared with rats bred for low saccharin preference, with a greater effect in females than males (Perry and Carroll, 2008).
SUMMARY
The concept that animal models can disambiguate the causal nature of the relationship between putative biological and behavioral susceptibility factors and SUD is not new, but recent years have seen a dramatic increase in the sophisticated use of these systems to do so. For those relationships now exposed (e.g., among novelty-seeking, impulsivity, reward sensitivity, and drug self-administration), it is now possible to begin taking apart the genetic, molecular, and circuit mechanisms that mediate the relationship. Presumably, these biological factors are useful for a variety of reasons, including their ability to serve as biomarkers of risk and as targets for prevention of SUD.
DISCLOSURES
Dr. Maldonado receives financial support and/or research contracts from Laboratorios Esteve SA, Ferrer SA, Pharmaleads, and a grant from Marató TV3. He currently holds a patent under exploitation by Panlab SA.
Dr. Jentsch and Dr. Evans have no conflicts of interest to disclose. They are both funded by the NIDA and NINDS.
Dr. Kieffer has no conflicts of interests to disclose. She is funded by academic institutions (CNRS, INSERM and Université de Strasbourg and the National Institutes of Health (National Institute of Drug Addiction, grant #05010 and National Institute on Alcohol Abuse and Alcoholism, grant #16658).
REFERENCES
Balleine, B.W., and O’Doherty, J.P. (2010). Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35(1):48–69.
Bardo, M.T., and Bevins, R.A. (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153(1):31–43.
Belin, D., Berson, N., et al. (2011). High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36(3):569–579.
Belin, D., Mar, A.C., et al. (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science 320(5881):1352–1355.
Bierut, L.J. (2010). Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol. Sci. 31(1):46–51.
Bruchas, M.R., Land, B.B., et al. (2010). The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 1314:44–55.
Carroll, M.E., Morgan, A.D., et al. (2008). Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav. Pharmacol. 19(5–6):435–460.
Crabbe, J.C. (2008). Review: neurogenetic studies of alcohol addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363(1507):3201–3211.
Dalley, J.W., Fryer, T.D., et al. (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315(5816): 1267–1270.
Dellu, F., Piazza, P.V., et al. (1996). Novelty-seeking in rats: biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology 34(3):136–145.
Deroche-Gamonet, V., Belin, D., et al. (2004). Evidence for addiction-like behavior in the rat. Science 305(5686):1014–1017.
Evenden, J.L. (1999). Varieties of impulsivity. Psychopharmacology 146(4):348–361.
Everitt, B.J., Belin, D., et al. (2008). Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363(1507):3125–3135.
Fowler, C.D., Lu, Q., et al. (2011). Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471(7340):597–601.
Gallego, X., Molas, S., et al. (2012). Overexpression of the CHRNA5/A3/B4 genomic cluster in mice increases the sensitivity to nicotine and modifies its reinforcing effects. Amino Acids 43(2):897–909.
Goeldner, C., Lutz, P.E., et al. (2011). Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol. Psychiatry 69(3):236–244.
Groman, S.M., James, A.S., et al. (2009). Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 33(5):690–698.
Guegan, T., Cutando, L., et al. (2012). Operant behavior to obtain palatable food modifies neuronal plasticity in the brain reward circuit. Eur. Neuropsychopharmacol.
Ho, M.K., Goldman, D., et al. (2010). Breaking barriers in the genomics and pharmacogenetics of drug addiction. Clin. Pharmacol. Ther. 88(6):779–791.
Izquierdo, A., and Jentsch, J.D. (2012). Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology 219(2):607–620.
Jentsch, J.D., and Taylor, J.R. (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146(4):373–390.
Johnson, P.M., and Kenny, P.J. (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 13(5):635–641.
Kenny, P.J. (2007). Brain reward systems and compulsive drug use. Trends Pharmacol. Sci. 28(3):135–141.
Koob, G.F., and Le Moal, M. (2008). Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363(1507):3113–3123.
Koob, G.F., and Volkow, N.D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35(1):217–238.
Laughlin, R.E., Grant, T.L., et al. (2011). Genetic dissection of behavioral flexibility: reversal learning in mice. Biol. Psychiatry 69(11):1109–1116.
Le Merrer, J., Becker, J.A., et al. (2009). Reward processing by the opioid system in the brain. Physiol. Rev. 89(4):1379–1412.
Le Merrer, J., Befort, K., et al. (2012). Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict. Biol. 17(1):1–12.
Lynch, W.J., Roth, M.E., et al. (2002). Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacol. (Berl.). 164(2):121–137.
Markou, A., and Koob, G.F. (1993). Intracranial self-stimulation thresholds as a measure of reward. In: Sahgal, A., ed. Behavioral Neuroscience: A Practical Approach. New York: Oxford University Press, pp. 93–115.
Martin-Garcia, E., Barbano, M.F., et al. (2009). New operant model of nicotine-seeking behaviour in mice. Int. J. Neuropsychopharmacol. 12(3):343–356.
Meisch, R.A. (2001). Oral drug self-administration: an overview of laboratory animal studies. Alcohol 24(2):117–128.
Mendizabal, V., Zimmer, A., et al. (2006). Involvement of kappa/dynorphin system in WIN 55,212–2 self-administration in mice. Neuropsychopharmacology 31(9):1957–1966.
O’Brien, C.P., Childress, A.R., et al. (1998). Conditioning factors in drug abuse: can they explain compulsion? J. Psychopharmacol. 12(1):15–22.
Olsen, C.M. (2011). Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 61(7):1109–1122.
Perry, J.L., and Carroll, M.E. (2008). The role of impulsive behavior in drug abuse. Psychopharmacology 200(1):1–26.
Piazza, P.V., Deminiere, J.M., et al. (1989). Factors that predict individual vulnerability to amphetamine self-administration. Science 245(4925):1511–1513.
Pitchers, K.K., Balfour, M.E., et al. (2010). Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol. Psychiatry 67(9):872–879.
Robinson, T.E., and Kolb, B. (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Suppl 1):33–46.
Russo, S.J., Dietz, D.M., et al. (2010). The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33(6):267–276.
Sanchis-Segura, C., and Spanagel, R. (2006). Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11(1):2–38.
Santucci, K. (2012). Psychiatric disease and drug abuse. Curr. Opin. Pediatr. 24(2):233–237.
Sinha, R., Shaham, Y., et al. (2011). Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology 218(1):69–82.
Skoubis, P.D., Lam, H.A., et al. (2005). Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur. J. Neurosci. 21(5):1379–1384.
Soria, G., Barbano, M.F., et al. (2008). A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology 199(4):593–603.
Spanagel, R., and Zieglgansberger, W. (1997). Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol. Sci. 18(2):54–59.
Tanda, G., Munzar, P., et al. (2000). Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat. Neurosci. 3(11):1073–1074.
Tordoff, M.G., and Bachmanov, A.A. (2003). Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol Clin. Exp. Res. 27(4):600–606.
Trigo, J.M., Martin, E.-Garcia, et al. (2010). The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depen. 108(3):183–194.
Vanderschuren, L.J., and Everitt, B.J. (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305(5686):1017–1019.
Vengeliene, V., Noori, H.R., et al. (2013). The use of a novel drinkometer system for assessing pharmacological treatment effects on ethanol consumption in rats. Alcohol Clin. Exp. Res. 37(Suppl 1):E322–E328.
Weiss, F. (2010). Advances in animal models of relapse for addiction research. In: Kuhn, C.M., and Koob, G.F., eds. Advances in the Neuroscience of Addiction. Boca Raton, FL: CRC Press.
Wikler, A., and Pescor, F.T. (1970). Persistence of “relapse-tendencies” of rats previously made physically dependent on morphine. Psychopharmacologia 16(5):375–384.