85 | THE NEUROBIOLOGY OF AGGRESSION
R. JAMES R. BLAIR
Aggression, here defined as any form of behavior directed toward the goal of harming or injuring another living being who is motivated to avoid such treatment, is a natural and adaptive phenomenon. However, it can become maladaptive if it is exaggerated, persistent, or expressed out of context (Nelson and Trainor, 2007). As such it is a serious social concern as well as a considerable economic burden on society. Indeed, aggressive and antisocial behaviors are the leading cause of all child and adolescent referrals to mental health clinicians (Berkowitz, 1993). Moreover, each antisocial individual costs society up to ten times more than their healthy counterparts in aggregate health care and social service expenditures (Nelson and Trainor, 2007). An increased risk for aggression can be seen in a variety of psychiatric disorders, including but not limited to mood and personality disorders. Understanding the neurobiology of aggression is thus of considerable importance.
TAXONOMIES OF AGGRESSION
Work with animals has distinguished several forms of aggression (Gregg and Siegel, 2001). We briefly consider the two that, according to the current literature, might be most directly applied to understanding human aggression. These are predatory and reactive aggression.
PREDATORY AGGRESSION
Predatory aggression occurs during food seeking in certain omnivorous and carnivorous species. It involves methodological stalking, well-directed pouncing, and quiet biting attack. If predatory aggression is induced through stimulation of the brain, there will be attacks on live prey but also bites of dead prey. However, attacks on conspecifics will not be initiated. Electrical stimulation of a circuit including dorsolateral hypothalamus and the ventral half of the periaqueductal gray (PAG) has been shown to initiate predatory aggression in both rats and cats (Panksepp, 1998).
REACTIVE AGGRESSION
Animals demonstrate a gradated and instinctual response to threat. Distant threats induce freezing, and then, as the threats draw closer, they induce flight, and finally reactive aggression when they are very close and escape is impossible (Blanchard et al., 1977). As such, reactive aggression involves unplanned, enraged attacks on the object perceived to be the source of the threat or frustration. The animal exhibits piloerection, autonomic arousal, hissing, and growling during their attack. Reactive aggression appears to be mediated via a circuit that runs from the medial amygdala downward, largely via the stria terminalis to the medial hypothalamus and from there to the dorsal half of the PAG (Gregg and Siegel, 2001; Collin et al., 2011; Nelson and Trainor, 2007; Panksepp, 1998; ). There have been suggestions that this is a social behavior network from which aggressive behavior is an emergent property (Nelson and Trainor, 2007). However, more recent data suggest rather that overlapping but distinct neuronal subpopulations are involved in different social behaviors such as fighting and mating (Collin et al., 2011). It has also been argued that orbitofrontal cortex (OFC) has an inhibitory impact on this network (Nelson and Trainor, 2007), a claim that will be considered in greater detail in the following.
HUMAN AGGRESSION
In work on human aggression, a fundamental distinction is drawn between instrumental (proactive/planned) and reactive (affective/defensive/impulsive) aggression. This distinction has been made for some time (Crick and Dodge, 1996) even if consideration of the implications of this distinction for the neurobiology of human aggression is only more recent (Blair, 2001).
Instrumental aggression involves the planned execution of aggression. It can involve both overt and covert actions executed with forethought and a degree of planning. The anticipated outcome is positive as seen from the viewpoint of the aggressor: acquisition of territory or goods, improvement of social status, gratification of a perceived need. Typically, there is a relative absence of intense emotion.
In humans, reactive aggression is unplanned aggression that can be most often characterized as impulsive. These acts are often overt, explosive, and involve the active confrontation of the victim. Accompanying emotions are almost always negative (fear of retaliation, anger, sadness, frustration, and irritation). One notable feature distinguishing human reactive aggression from that studied in animals is that it has been associated with frustration (Berkowitz, 1993). Frustration occurs when an individual continues to do an action in the expectation of a reward but does not actually receive that reward (Berkowitz, 1993).
Instrumental and reactive aggression cluster differentially in confirmatory factor analyses with moderate correlations between the two dimensions (Crick and Dodge, 1996). Studies indicate that approximately 10% of children show elevated levels of instrumental and reactive aggression, 3% show elevated instrumental aggression only, and 6% show elevated reactive aggression only (Dodge et al., 1997). Instrumental and reactive aggression have distinct trajectories. (Those with both forms followed a pattern similar to those with instrumental aggression only.) Thus, Dodge and colleagues (1997) observed that children with reactive aggression had an earlier onset (4.5 years vs. 6.5 years for proactive), were more likely (21%) to have experienced physical abuse or aversive parenting, had poorer peer relations, and inadequate problem-solving patterns. The instrumentally aggressive children were more likely to have had aggressive role models in the family and to view aggression positively (Dodge et al., 1997). Similarly, different psychiatric conditions are associated with risks for different forms of aggression. Thus, patients with mood and anxiety conditions (e.g., bipolar disorder and posttraumatic stress disorder, as well as patients with intermittent explosive disorder and borderline personality disorder [BPD]) are at risk for increased reactive aggression. In contrast, individuals with the personality disorder psychopathy show an increased risk for instrumental aggression coupled with an increased risk for reactive aggression (Frick et al., 2005).
It should be noted that the instrumental–reactive dichotomy of aggression has received significant criticism (Bushman and Anderson, 2001). Specifically, whether individual aggressive acts can be reliably classified as reactive or instrumental has been questioned. This criticism may be overstated. Most would agree that the aggression of an individual punching a person who has startled him or her is different from that of an individual pointing a gun at another person and demanding his or her wallet. Moreover, there are identifiable differences in the neural circuitry mediating reactive and instrumental aggression, as detailed in the following. But the criticism of the dichotomy was not without merit. How should one classify the aggression of the person who shoots someone five days after discovering the victim had been having an affair with the shooter’s spouse? There is a clear reactive component (anger, frustration, and confrontation), and yet the action is planned and, by using a gun, definitively instrumental. In short, it may be necessary to consider forms of aggression beyond the instrumental–reactive dichotomy such as aggression in which the functional contributions of the neural systems engaged in instrumental and those engaged in reactive aggression are both involved.
INSTRUMENTAL AGGRESSION
There have been suggestions that the animal work on the neurobiology of predatory aggression may provide information on human instrumental aggression (Gregg and Siegel, 2001). However, this appears unlikely. Instrumental aggression is a flexible way of achieving an individual’s goals rather than an instinctual response to the presence of a prey animal. It is flexible and highly influenced by the individual’s learning environment; for example, environmental factors play a large role in determining the choice of weapons from fists to pistols. In contrast, whereas predatory aggression in animals may serve the goal of providing the animal with food, it is an instinctual motor program initiated by the presence of prey in the environment that occurs in a relatively fixed fashion; that is, ending with a bite to the neck. Moreover, predatory aggression in animals is not displayed to conspecifics. In contrast, instrumental aggression in humans is almost always displayed to conspecifics. As such, it appears unlikely that most instrumental aggression in humans recruits the subcortical circuits identified in animal work that mediate predatory aggression. Instead, when considering the neurobiology of instrumental aggression in humans, we are considering the neurobiology of instrumental motor responding generally (regions implicated include, e.g., premotor cortex, striatum, and the cerebellum) and, critically, the emotional learning and representational systems that allow the selection of one action over another.
There is only one clinical condition associated with an increased risk for instrumental aggression: conduct disorder (CD) in childhood and antisocial personality disorder (ASPD) in adulthood. However, it is important to remember that youth with CD and adults with ASPD are not considered to be homogeneous groups (Blair, 2001). Specifically, individuals differ according to their level of callous–unemotional (CU) traits (i.e., the degree to which they show reduced guilt and empathy). Level of CU traits in individuals with CD/ASPD have been shown to modulate BOLD responses within the amygdala to social cues (Marsh et al., 2008; White et al., in press) and within the amygdala and ventromedial frontal cortex (vmPFC) during moral reasoning (Marsh et al., 2011). Callous–unemotional traits are inversely related to mood and anxiety symptoms (Patrick, 1994) and it is individuals with elevated CU traits who are at particular risk for instrumental aggression (Frick et al., 2005).
Instrumental aggression can be adaptive. A starving individual who pushes someone over and takes his or her money is not making a poor decision from that person’s own perspective (whatever its value from a societal perspective). The benefit of the soon-to-be purchased food will significantly outweigh the costs of causing (minor) injury to the victim/risking jail. However, an individual who stabs another to death for $80 to purchase a mobile phone is going to be considered, by most individuals, to be making a poor decision. The aggression is maladaptive. The benefits should be represented as significantly less than the costs. An increased risk for (maladaptive) instrumental aggression can emerge following the specific forms of decision-making impairment seen in individuals with elevated CU traits.
There are three clear components necessary to make a good decision. The individual must: (1) learn the value of the options to be chosen among; (2) successfully represent this value information so the options can be chosen among; and (3) successfully choose among the options. Individuals with elevated CU traits appear to have particular difficulties with at least the first two of these components.
Learning the value of options to be chosen among requires stimulus–reinforcement learning. The individual must associate a reinforcement value with the stimulus. A classic measure of stimulus-reinforcement learning is aversive conditioning—the individual learns that a particular stimulus is associated with threat. Individuals with elevated CU traits show marked impairment in stimulus-reinforcement learning. Indeed, an individual’s ability to perform aversive conditioning at 15 years has predictive power regarding whether that individual will display antisocial behavior 14 years later (Raine et al., 1996). Considerable animal and human work shows that the amygdala is critical for aversive conditioning (LeDoux, 2007). Individuals with elevated CU traits show reduced amygdala responses during aversive conditioning (Birbaumer et al., 2005).
A critical cue for reinforcement based learning is the prediction error; that is, the difference between the reinforcement the individual expects to receive and that which he or she does receive. According to learning theory, the greater the prediction error, the faster the learning should occur (Rescorla and Wagner, 1972). Regions implicated in prediction error signaling include the caudate and vmPFC (O’Doherty et al., 2003). Youth with CD and elevated CU traits (requisite studies in adult samples have not been conducted) show indications of impaired prediction error signaling within both the caudate and vmPFC (Finger et al., 2008, 2011).
As the individual learns to associate reinforcement with an object or action, it acquires an expected value. Good decision making by definition involves the selection of an action/stimulus with the highest expected value. VmPFC is critically involved in the representation of expected value (Glascher et al., 2009). Youth with CD show disrupted representation of expected value within vmPFC (Finger et al., 2011).
A critical form of reinforcement for human social interactions involves emotional expressions (Blair, 2003). An individual can show distress by displaying pain, fear, or sadness. To learn to avoid actions that harm others, one must appropriately represent the distress of others. Youth and adults with elevated CU traits show: (1) reduced autonomic responses to the pain of others (Aniskiewicz, 1979); (2) reduced recognition of fearful and sad expressions (for a metaanalysis, see Marsh and Blair, 2008); and (3) reduced amygdala responses to these expressions (Marsh et al., 2008). The amygdala is thought to allow the individual to associate the distress of others with actions/objects that have caused that distress—this is considered the basis for human socialization (Blair, 2007). Indeed, recent animal work has confirmed that the amygdala is critical for this form of learning (Jeon et al., 2010). In line with their expression processing impairments, the presence of CU traits has been shown to interfere with the child’s ability to be socialized using standard parenting techniques (Wootton et al., 1997).
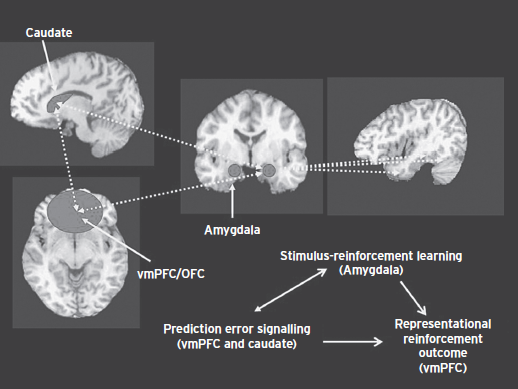
In summary, Figure 85.1 depicts the integrated systems that are dysfunctional in individuals with elevated CU traits and that, through their dysfunction, increase the risk for instrumental aggression. The caudate and vmPFC are involved in prediction error signaling; detecting when a reward or punishment is greater or lesser than expected. Prediction error signals trigger reinforcement learning—they signal to the system that the current reinforcement expectancies are inadequate. If prediction error signaling is disrupted, as data suggest it is in individuals with elevated CU traits, learning about the value of actions and objects will be impaired. The amygdala is involved in stimulus-reinforcement learning allowing the individual to associate reinforcement expectancy values with objects and actions. Again if stimulus-reinforcement learning is disrupted, as data suggest it is in individuals with elevated CU traits, learning about the value of actions and objects will be impaired. A critical type of reinforcement for human social interaction is provided by emotional expressions. To learn to avoid actions that harm others one must associate the aversive qualities of the other individual’s distress with the object/action that caused this distress. The amygdala is critical for this form of stimulus-reinforcement learning, which again appears disrupted in individuals with elevated CU traits. This learning process is critical for socialization; if disrupted, it will interfere with socialization. Individuals with elevated CU traits show significantly less impact of standard socialization practices than typically developing youth. Poorer stimulus-reinforcement learning and responsiveness to the distress of others lead to an individual who represents more poorly the expected value of objects and actions (including the aversiveness of harmful consequences for others). The VmPFC is critical for the representation of expected value. This representation is also disrupted in individuals with elevated CU traits, further contributing to their decision-making impairment. One manifestation of this decision-making impairment is the selection of maladaptive instrumental aggression/antisocial behavior; that is, choosing antisocial behaviors with inadequate representation of both their potential rewards and their potential aversive consequences.

Figure 85.1 The neurobiology of callous–unemotional traits.
REACTIVE AGGRESSION
As noted, reactive aggression is considered to be an automatic response to extreme threat that animal work shows is mediated via a circuit that runs from the medial amygdala downward, largely via the stria terminalis to the medial hypothalamus, and from there to the dorsal half of the PAG (Gregg and Siegel, 2001; Panksepp 1998). This circuitry is assumed to mediate reactive aggression in humans also (Blair, 2001) and to be regulated via frontal cortical regions, particularly vmPFC and potentially regions of anterior cingulate cortex (ACC).
There are two main ways that reactive aggression has been investigated in work with humans, by: (1) examining the pathophysiology of patients at increased risk for reactive aggression; and (2) conducting studies with healthy adults performing putative analogues of reactive aggression.
Several psychiatric conditions show a particularly marked increase in the risk for reactive aggression; for example, intermittent explosive disorder, BPD, and severe mood dysregulation in childhood (Coccaro et al., 2009). In addition, Raine and colleagues have done some work with a population of spouse abusers whose aggression was carefully characterized as reactive rather than instrumental (Lee et al., 2008).
Given the animal literature on reactive aggression, it can be predicted that individuals with a heightened risk for the display of reactive aggression will show heightened responsiveness of regions implicated in reactive aggression to emotional provocation (Blair, 2001); that is, the amygdala, hypothalamus, and PAG (Gregg and Siegel, 2001; Collin et al., 2011; Nelson and Trainor, 2007; Panksepp 1998). In line with this suggestion, patients with intermittent explosive disorder and BPD and reactively aggressive spouse abusers all show increased amygdala responsiveness to threatening stimuli relative to comparison individuals (Coccaro et al., 2007; Lee et al., 2008; New et al., 2009). Patients with BPD have also been found to show an increased amygdala response to interpersonal provocation (New et al., 2009). However, none of these studies reported either increased responsiveness of the hypothalamus or the PAG—although this lack likely reflects methodology, neither region is typically investigated in current functional magnetic resonance imaging work.
What about the regulatory role of frontal cortex? Certainly at least some patients with OFC lesions are at increased risk for reactive aggression (Grafman et al., 1996). This is in line with animal work that shows that lesions of the OFC can increase aggression (Izquierdo et al., 2005).
But what is the nature of this regulatory role? The dominant view is that the OFC inhibits (“puts the brakes on”) the aggressive responses mediated by the amygdala, hypothalamus, and PAG (Heinz et al., 2011; Nelson and Trainor, 2007), but this view is probably wrong. If the OFC was having an inhibitory effect on systems engaged in threat behavior, one might assume that all threat related behavior would be increased following OFC lesions. In contrast, macaques while showing an increase in mild aggression following OFC lesions showed a significantly decreased fear reaction to novel threat stimuli (Izquierdo et al., 2005). Moreover, there are data showing that OFC lesions lead to a reduction in amygdala activity in the context of decision-making paradigms (Schoenbaum and Roesch, 2005).
Work with psychiatric patients at increased risk for reactive aggression is often assumed to support the frontal inhibitory position. It is assumed that patients at risk for reactive aggression will show disruption in the ability to recruit OFC in response to emotional provocation. However, data supporting this hypothesis are notably sparse.
There was a report of reduced activation in spouse abusers proximal to the right ACC and left middle frontal gyrus during the emotional Stroop test (Lee et al., 2008). However, it should be noted that these indications of hypofrontality were not seen in a second study by the same group on this population. Moreover, those from the first study involved a region that was white rather than gray matter and thus must be considered with caution. Similarly, data on patients with BPD have been mixed. For example, although some studies report reduced ACC activity during emotional provocation (New et al., 2009), others do not (Herpertz et al., 2001).
In short, it appears probable that the relationship of vmPFC to the amygdala is not simply suppressive. Animal work clearly demonstrates that lesions of vmPFC do not lead to disinhibited/increased amygdala responding as the “brakes type” regulatory view predicts. Lesions of vmPFC/OFC decrease amygdala responding (Schoenbaum and Roesch, 2005) and decrease fear reactions to novel threat stimuli (Izquierdo et al., 2005). Work with patients does not consistently support the view of decreased vmPFC/OFC activity in response to emotional provocation in patients at risk for heightened levels of aggression.
It is perhaps important to consider a core function of vmPFC/OFC outlined in the preceding section (i.e., its role in the representation of the value of an object or action) and the integrated nature of the role with the functioning of the amygdala and caudate. According to this view of integrated functioning, vmPFC/OFC dysfunction should reduce, not increase, amygdala responsiveness (cf., Schoenbaum and Roesch, 2005) and should consequently lead to a reduction, not an increase, in fear reactions to novel threat stimuli (cf., Izquierdo et al., 2005). Within this view, lesions of vmPFC/OFC will increase reactive aggression not because the aggressive response is dis inhibited but rather because the costs and benefits of engaging in reactive aggression are not properly represented. Of course, this view places an instrumental slant on many occasions of reactive aggression; that is, although reactive aggression may be an automatic response to an extreme threat, it may also be a selected response (as fear reactions to novel threat stimuli are too; Izquierdo et al., 2005). In this regard, it is notable that the aggression shown by primates following OFC lesions correlates highly with the aggression shown to the primate by other primates (Bachevalier et al., 2011). As such, the increased aggression may be just one reflection of poorer behavioral choices in the primate following the OFC lesion. This point is returned to in the following.
The second main ways that reactive aggression has been investigated in humans involves subjects performing putative analogues of reactive aggression; for example, the Taylor Aggression Paradigm (TAP; Taylor, 1967) and the Point Subtraction Aggression Paradigm (PSAP; Cherek et al., 1997). In the TAP, subjects are instructed that they are playing successive competitive reaction time trials against opponents. They are told that whoever lost a trial would be punished by the opponent with aversive thermal stimulation. Opponents can be predetermined, for example, to differ in provocation (i.e., the amount that they punish the subject). The subject’s aggressive responses (retaliatory punishments of the opponent) are a function of provocation level. The similar PSAP examines the subject’s responses to the subtraction of points worth money that he or she is accumulating during a testing session in which losses are attributed to the responding of another person. At each moment, the subject can choose to press button A (pressing 100 times earns money), button B (that will take points away from the fictitious person), or button C (that will protect the subject’s point total for a set number of trials). Work has demonstrated that increases in provocation by the fictitious player increase retaliatory “aggressive” B responses in the subject (Cherek et al., 1997; New et al., 2009).
Relatively little functional imaging studies have been conducted with these paradigms. There have been reports of increased responding within dorsomedial prefrontal cortex, anterior insula cortex (AIC), and caudate to highly provoking confederates relative to less provoking confederates (Kramer et al., 2008). However, in none of this work was amygdala, hypothalamus, or PAG implicated, with the exception of a study investigating the response of individuals with psychopathy (there was no comparison group; Veit et al., 2010). This study reported that inflicting high relative to low punishments to the competitor was associated with increased activity within the AIC, amygdala, and hypothalamus (extending proximal to the PAG).
The literature on the TAP and PSAP shares interesting similarities with the more extensive literature on social exchange paradigms. In social exchange paradigms, a proposer suggests an allocation of resources and typically the subject decides whether or not to accept this allocation and/or punish the proposer for the unfairness of his offer. As such, social exchange paradigms can be considered social provocation paradigms like the TAP and PSAP. Notably unfair offers are associated with anger, and likely aggression, in the receiving party (Sanfey et al., 2003).
Unfair offers by proposers during social exchange paradigms have been found to elicit activity in subjects within both AIC/inferior frontal cortex (IFC), dorsomedial frontal cortex (dmFC), and striatum (Sanfey et al., 2003; King-Ca King-Casas et al., 2008; White et al., in press). There have been suggestions that activity within these regions reflects anger elicited by unfairness to the self (Sanfey et al., 2003) or that they play a critical role in detecting and reacting to social norm violations (King-Casas et al., 2008). Indeed, it has been argued AIC/IFC responds to anger/expectations of anger (including in response to norm violations) and organize a behavioral response (Blair and Cipolotti, 2000). In this regard, it appears that part of this organization involves the recruitment of the PAG (e.g.,White et al., in press). Decisions to reject the proposer’s unfair offers, like decisions to punish another’s provocation on the TAP (Veit et al., 2010), are associated with increased activity within dmFC, AIC/IFC, and striatum as well as the PAG (White et al., 2013).
It is interesting in this regard that dmFC and AIC/IFC also show increased activation following unexpected punishments during, for example, reversal learning tasks (e.g., Budhani et al., 2007). Again the assumption is that dmFC responds to the expectation violation (Alexander and Brown, 2011)—in this case the unexpected punishment—and that AIC/IFC organizes a behavioral response. This might involve a change in behavior but it also, as a definitively frustrating event, might involve the initiation of a frustration based reactive aggression episode.
It was argued in the preceding that reactive aggression can be a selected response and that vmPFC lesions increase reactive aggression because the costs and benefits of engaging in reactive aggression are not properly represented. This is important to remember when considering the literature on the PSAP, TAP, or social exchange literature. These paradigms are not modeling an instinctual response to threat or intruders but rather a planned response to another individual’s provocative behavior. As successful as these models have been of “reactive aggression,” they would appear to be rather more applicable to cases of “instrumental reactive aggression” mentioned in the preceding.
In summary, Figure 85.2 depicts the neural systems involved in the expression of reactive aggression (amygdala, hypothalamus, and PAG) as well as systems implicated in modifying the probability that reactive aggression will be expressed (vmPFC, aIC, and dmFC). Increasing emotional provocation by threat will increase activity in amygdala, hypothalamus, and PAG until, at sufficient strength, reactive aggression will be displayed. Anxiety and mood disorders are associated with an increased risk for reactive aggression because they involve an increase in the underlying responsiveness of at least parts of this circuit. Emotional regulation, even if it does not involve vmPFC placing the brakes on the amygdala, will reduce responsiveness and thus decrease the probability that reactive aggression will be displayed.
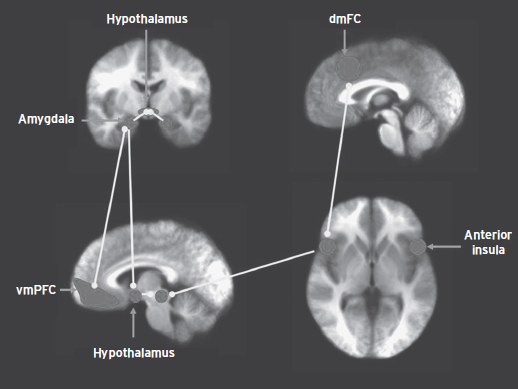
Figure 85.2 The neural systems involved in the expression of reactive aggression.
The dmFC and AIC show increased responses to provocation in the context of reactive aggression paradigms (Kramer et al., 2008) and in response to unfair offers in social exchange paradigms (King-Ca King-Casas et al., 2008; Sanfey et al., 2003; White et al., 2013). They also show increased responses to frustration as initiated by an unexpected punishment for an action expected to result in reward (e.g., Budhani et al., 2007). Retaliatory responses are also associated with dmFC and AIC activity as well as the PAG (White et al., 2013). The suggestion is that the anger initiated by the expectation violation of the other’s inappropriate behavior (delivering shocks or making unfair offers) corresponds to expectation violation signaling and behavioral control mediated by dmFC and AIC. The suggestion would be that this activity could be maintained, or at least reinitiated, whenever the individual considers the provocation and this could potentially lead to an angry aggressive response that will have instrumental components (in that it may be planned; e.g., the person goes home to obtain a weapon before attacking the victim).
The vmPFC allows the representation of the value of an object or an action. The suggestion is that vmPFC’s role in reactive aggression corresponds to its role in action selection. If it is impaired, reactive aggression may be more likely to be expressed. (The individual may have generated anger following provocation but did not represent the aversive consequences of the expression of this anger to themselves or others.)
MOLECULAR MECHANISMS
Some work has indicated that antisocial personality disorder and aggression are heritable. Reviews have suggested that a genetic effect could account for up to 50% of the variance in aggression (Miles and Carey, 1997). Unfortunately, almost all of the literature has failed to consider the heterogeneity within aggressive individuals. There have been indications that CU traits are highly heritable and that aggression only appears to be highly heritable in those with elevated CU traits (the suggestion has been made the aggression of aggressive individuals with low CU traits is more environmentally determined; Viding et al., 2005). However, it is perhaps unlikely that only CU traits, and not the emotional lability underlying the selectively increased risk for reactive aggression, are under genetic influence. Indeed there is molecular evidence that genetic polymorphisms associated with increased emotional reactivity are associated with an increased risk for reactive aggression (Brunner et al., 1993; Heinz et al., 2011). Given this, an attempt will be made here to filter the existing genetic literature though what is known about the neurocognitive architectures mediating different forms of aggression.
SEROTONIN
Serotonin has long been implicated in the regulation of aggression, particularly reactive aggression. Generally, experimental manipulations that increase 5-HT receptor activation have been found to decrease aggression, whereas those that decrease receptor activation have been found to increase aggression (Heinz et al., 2011).
Some of the most dramatic work indicating a relationship between serotonin and aggression has been molecular genetic work. Prominent among this work are studies examining the gene that encodes monoamine oxidase A (MAOA, a catabolic enzyme that breaks down biogenic amines including serotonin). In a seminal study, a single mutation in this gene was associated with criminal/antisocial behavior (Brunner et al., 1993). Although the human functional knockout is rare, there are common polymorphisms in MAOA. The most studied of these is a variable-number tandem repeat (VNTR) polymorphism in the upstream region of the gene, known as the MAOA uVNTR. Certain alleles in this region are associated with higher MAOA expression (MAOA-H alleles), whereas others are associated with lower expression (MAOA-L alleles) (Heinz et al., 2011). MAOA-L subjects (compared with MAOA-H subjects), like other individuals at increased risk for reactive aggression (see the preceding), show heightened amygdala responsiveness to threat stimuli such as angry and fearful faces (Heinz et al., 2011). Moreover, MAOA-L variant subjects are at increased risk for the display of reactive aggression particularly if the individual has been exposed to abuse (Caspi et al., 2002).
Polymorphisms of the 5-HTT gene (5-HTTLPR) also appear to increase risk for reactive aggression particularly if the individual has been exposed to abuse (Reif et al., 2007). Similarly, the common polymorphism of the 5-HTT gene (the 5–HTTLPR S allele) is associated with increased amygdala activation in response to aversive pictures and with a higher risk of experiencing negative mood states when exposed to traumatic life events (Heinz et al., 2011). The suggestion would be that both these MAOA and 5-HTTLPR polymorphisms and abuse increase responsiveness of the basic threat circuitry (amygdala-hypothalamus-PAG). It appears that the effects of these stressors are interactive rather than simply cumulative; genetic load predisposes the individual to be considerably more impacted by stressful events, considerably increasing the risk for reactive aggression.
DOPAMINE
Considerable animal work has shown that dopamine decreases the threshold for aggressive reaction in response to external stimuli, although an excess of these hormones increases the vulnerability and the risk of uncontrolled responses against stress (Volavka et al., 2004). Much of the human work has considered the involvement of the dopaminergic system in impulsivity rather than aggression. However, there has been some particularly interesting work examining the relationship of the catechol-O-methyl transferase (COMT) gene in aggression.
Many studies evaluating the impact of COMT to the genetics of aggression focused on the characterization of the Val158-Met polymorphism. This polymorphism is interesting functionally as there is almost a two fold decrease in the enzymatic activity of the Met158 variant compared to the Val158-encoding allele. In line with animal work showing that heterozygous COMT-deficient male mice exhibited increased aggressive behavior (Heinz et al., 2011), the allele Met158 of COMT has been found to be associated with aggressive personality traits and a propensity for aggression in humans (Rujescu et al., 2003). Importantly, the data again, for the most part, indicate that individuals with the Met allele show increased amygdala responsiveness to emotional provocation (e.g., Smolka et al., 2005).
γ-AMINOBUTYRIC ACID
Alcohol and benzodiazepines have been consistently shown to increase aggression (Fish et al., 2001). Both have an inhibitory effect on cortical activation, by inducing GABA release and stimulating GABA type A (GABAA) and GABAB receptors and their aggression-heightening effects can be potentiated by their co-administration (Fish et al., 2001).
Acute alcohol use is implicated in approximately one-half of all violent crimes and sexual assaults, and also confers risk for intimate partner violence. Treatment with benzodiazepines has been shown to increase the risk of aggression (Gardner and Cowdry, 1985).
It has been suggested that aggression may result from acute alcohol effects that impair prefrontal cortex mediated executive functions and disinhibit limbic processing of threatening stimuli, and elicit reactive aggression (Heinz et al., 2011). However, current data are rather inconsistent with this view—at least with respect to the disinhibition of limbic responsiveness. Thus, administration of alcohol leads to a reduction, rather than an increase, in the amygdala’s response to threatening stimuli (Gilman and Hommer, 2008) as does administration of diazepam (Del-Ben et al., 2012). In other words, acute administration of both alcohol and diazepam inhibits rather than disinhibits limbic responsiveness.
Alcohol is thought to disrupt decision making in that it increases the probability of maladaptive risk-taking behavior, including risky sexual activity, unsafe driving, and aggression (for a review, see Hommer et al., 2011). However, relatively little work has formally demonstrated decision-making deficits following alcohol ingestion. Somewhat more work has examined reward sensitivity in alcoholics, in which there does appear to be reduced reward sensitivity (Hommer et al., 2011).
In summary, there is a growing literature on the molecular mechanisms underpinning aggression, with some of the most provocative data being provided by molecular genetics. Serotonin decreases the risk for reactive aggression, whereas dopamine increases it. Both alter the sensitivity of the basic threat circuitry (particularly the amygdala). Polymorphisms of serotonin (e.g., MAOA and 5-HTTLPR) and dopamine (e.g., COMT) genes that are associated with an increased risk for aggression are also those polymorphisms associated with increased amygdala responsiveness.
The relationship between alcohol (and probably the benzodiazepines) and aggression appears rather different though. Ingestion of either reduces, rather than increases, amygdala responsiveness to emotional stimuli. Moreover, ingestion of alcohol appears to lead to decision-making impairments (although the precise computational basis of these impairments has not yet been well specified). As such, ingestion of alcohol appears to induce a state of increased CU (although the similarities can only be really determined when the details on the decision-making impairment are specified).
CONCLUSIONS
Considerable progress is continuing to be made in understanding the neurobiological basis of human aggression. The neurobiological circuits distinguishing instrumental from reactive aggression continue to be further specified. However, it is becoming clearer that these circuits can overlap in function. Thus, for example, the expression of rage- and frustration-induced reactive aggressive episodes can come under the control of systems involved in the representation of reinforcement value (vmPFC) that are critical for understanding instrumental aggression.
Instrumental aggression is mediated by the same cortical circuits that mediate other forms of instrumental behavior (e.g., premotor cortex and the cerebellum). Individuals may choose to engage in instrumental aggression if their representations of the benefits of the action outweigh their representations of the costs of the action (particularly if no other more beneficial action is available). Instrumental aggression can be considered maladaptive if the individuals have failed to learn the appropriate reinforcements associated with the action; that is, they have an inadequate representation of the distress of the victim, show impairment in stimulus-reinforcement learning, and show impairment in the representation of reinforcement expectancies. These capacities are reliant on the functional integrity of the amygdala, caudate, and vmPFC. Impairments in these capacities are seen in individuals who show elevated CU traits, individuals who are at risk for increased levels of instrumental aggression.
Reactive aggression involves a motor response driven by the amygdala, hypothalamus, and PAG that is modulated by the vmPFC’s role in the representation of reinforcement value. It represents an ultimate response to threat and is displayed to high level threats and (in humans) also to frustration. If the threat or frustration is sufficiently intense the reactive aggression may be relative automatic. However, it is becoming clearer that in humans and other primates that reactive aggression is under considerable modulation by vmPFC. The individual, anticipating displaying reactive aggression, will represent likely future expected reinforcement values of this action; for example, the value associated with the satisfaction of retaliating to the provoker versus that associated with potential jail time.
Although various neurochemical systems are implicated in the expression and modulation of reactive aggression, the roles of serotonin, dopamine, and GABA are perhaps the best understood. Polymorphisms of serotonin (e.g., MAOA and 5-HTTLPR) and dopamine (e.g., COMT) genes that are associated with an increased risk for aggression are also those polymorphisms associated with increased amygdala responsiveness to threat; that is, they increase the responsiveness of systems involved in the expression of reactive aggression. The ingestion of alcohol, in contrast, reduces amygdala responsiveness to distress and leads to decision-making impairments; that is, it proximally induces a state of increased CU.
These are the neural systems that are dysfunctional in individuals with elevated CU traits and that, through their dysfunction, increase the risk for instrumental aggression. The caudate and vmPFC are involved in prediction error signaling,; detecting when a reward or punishment is greater or lesser than expected. Prediction error signals trigger reinforcement learning—they signal to the system that the current reinforcement expectancies are inadequate. If prediction error signaling is disrupted, as data suggest it is in individuals with elevated CU traits, learning about the value of actions and objects will be impaired. The amygdala is involved in stimulus-reinforcement learning allowing the individual to associate reinforcement expectancy values with objects and actions. Again if stimulus-reinforcement learning is disrupted, as data suggest it is in individuals with elevated CU traits, learning about the value of actions and objects will be impaired. A critical type of reinforcement for human social interaction is provided by emotional expressions. To learn to avoid actions that harm others one must associate the aversive qualities of the other individual’s distress with the object or action that caused this distress. The amygdala is critical for this form of stimulus-reinforcement learning, which again appears disrupted in individuals with elevated CU traits. This learning process is critical for socialization and if disrupted will interfere with socialization. Individuals with elevated CU traits show significantly less impact of standard socialization practices than typically developing youth. Poorer stimulus-reinforcement learning and responsiveness to the distress of others will lead to an individual who represents more poorly the expected value of objects and actions, including the aversiveness of harmful consequences for others. The VmPFC is critical for the representation of expected value. This representation is also disrupted in individuals with elevated CU traits, further contributing to their decision-making impairment. One manifestation of this decision-making impairment is the selection of maladaptive instrumental aggression/antisocial behavior; that is, choosing antisocial behaviors with inadequate representation of both their potential rewards and their potential aversive consequences.
These neural systems include those responsible for the basic response to threat (amygdala, hypothalamus, and PAG), which when sufficiently activated by sufficient threat will initiate reactive aggression. In addition, they include the dmFC and AIC. The DmFC responds to expectation violations, whether the expectation violation involves another’s provocative behavior or the failure to receive an expected reward following task completion. The suggestion is that it organizes potential behavioral responses through AIC, including reactive aggression expressed through the PAG. Finally, they also include vmPFC. The VmPFC allows the representation of the value of an object or an action. The suggestion is that the vmPFC’s role in reactive aggression corresponds to its role in action selection. If it is impaired, reactive aggression may be more likely to be expressed. The individual may have generated anger following provocation or experiencing a frustrating event but not represent the aversive consequences of the expression of this anger to him- or herself or others.
DISCLOSURE
Dr. Blair is supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health under grant number 1-ZIA-MH002860-08. He has no financial relationships to disclose.
REFERENCES
Alexander, W.H., and Brown, J.W. (2011). Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14(10):1338–1344.
Aniskiewicz, A.S. (1979). Autonomic components of vicarious conditioning and psychopathy. J. Clin. Psychol. 35:60–67.
Bachevalier, J., Machado, C.J., et al. (2011). Behavioral outcomes of late-onset or early-onset orbital frontal cortex (areas 11/13) lesions in rhesus monkeys. Ann. NY Acad. Sci. 1239:71–86.
Berkowitz, L. (1993). Aggression: Its Causes, Consequences, and Control. Philadelphia, PA: Temple University Press.
Birbaumer, N., Veit, R., et al. (2005). Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch. Gen. Psychiatry 62(7):799–805.
Blair, R.J.R. (2001). Neuro-cognitive models of aggression: the antisocial personality disorders and psychopathy. J. Neurol. Neurosurg. Psychiatry 71:727–731.
Blair, R.J.R. (2003). Facial expressions, their communicatory functions and neuro-cognitive substrates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358(1431):561–572.
Blair, R.J.R. (2007). The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn. Sci. 11(9):387–392.
Blair, R.J.R., and Cipolotti, L. (2000). Impaired social response reversal: a case of “acquired sociopathy.” Brain 123:1122–1141.
Blanchard, R.J., Blanchard, D.C., et al. (1977). Attack and defensive behaviour in the albino rat. Anim. Behav. 25:197–224.
Brunner, H.G., Nelen, M., et al. (1993). Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262(5133):578–580.
Budhani, S., Marsh, A.A., et al. (2007). Neural correlates of response reversal: considering acquisition. NeuroImage 34(4):1754–1765.
Bushman, B.J., and Anderson, C.A. (2001). Is it time to pull the plug on the hostile versus instrumental aggression dichotomy? Psychol. Rev. 108(1):273–279.
Caspi, A., McClay, J., et al. (2002). Role of genotype in the cycle of violence in maltreated children. Science 297(5582):851–854.
Cherek, D.R., Moeller, F.G., et al. (1997). Studies of violent and nonviolent male parolees: I. Laboratory and psychometric measurements of aggression. Biol. Psychiatry 41:514–522.
Coccaro, E.F., McCloskey, M.S., et al. (2007). Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry 62(2):168–178.
Crick, N.R., and Dodge, K.A. (1996). Social information-processing mechanisms on reactive and proactive aggression. Child Dev. 67(3):993–1002.
Del-Ben, C.M., Ferreira, C.A., et al. (2012). Effects of diazepam on BOLD activation during the processing of aversive faces. J. Psychopharmacol. 26(4):443–451.
Dodge, K.A., Lochman, J.E., et al. (1997). Reactive and proactive aggression in school children and psychiatrically impaired chronically assaultive youth. J. Abnorm. Psychol. 106(1):37–51.
Finger, E.C., Marsh, A.A., et al. (2008). Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch. Gen. Psychiatry 65(5):586–594.
Finger, E.C., Marsh, A.A., et al. (2011). Disrupted reinforcement signaling in the orbital frontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am. J. Psychiatry 168(2):834–841.
Fish, E.W., Faccidomo, S., et al. (2001). Alcohol, allopregnanolone and aggression in mice. Psychopharmacol. (Berl.) 153(4):473–483.
Frick, P.J., Stickle, T.R., et al. (2005). Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. J. Abnorm. Child Psychology 33:471–487.
Gardner, D.L., and Cowdry, R.W. (1985). Alprazolam-induced dyscontrol in borderline personality disorder. Am. J. Psychiatry 146:98–100.
Gilman, J.M., and Hommer, D. (2008). Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict. Biol. 13:423–434.
Glascher, J., Hampton, A.N., et al. (2009). Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward related decision making. Cerebral Cortex 19:483–495.
Grafman, J., Schwab, K., et al. (1996). Frontal lobe injuries, violence, and aggression: a report of the Vietnam head injury study. Neurology 46:1231–1238.
Gregg, T.R., and Siegel, A. (2001). Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog. Neuropsychopharmacol. Biol. Psychiatry 25(1):91–140.
Heinz, A.J., Beck, A., et al. (2011). Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat. Rev. Neurosci. 12(7):400–413.
Herpertz, S.C., Dietrich, T.M., et al. (2001). Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol. Psychiatry 50(4):292–298.
Hommer, D.W., Bjork, J.M., et al. (2011). Imaging brain response to reward in addictive disorders. Ann. NY Acad. Sci. 1216:50–61.
Izquierdo, A., Suda, R.K., et al. (2005). Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J. Neurosci. 25(37):8534–8542.
Jeon, D., Kim, S., et al. (2010). Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13(4):482–488.
King-Casas, B., Sharp, C., et al. (2008). The rupture and repair of cooperation in borderline personality disorder. Science 321(5890):806–810.
Kramer, U.M., Buttner, S., et al. (2008). Trait aggressiveness modulates neurophysiological correlates of laboratory-induced reactive aggression in humans. J. Cogn. Neurosci. 20(8):1464–1477.
LeDoux, J.E. (2007). The amygdala. Curr. Biol. 17(20):R868–R874.
Lee, T.M.C., Chan, S.-C., et al. (2008). Strong limbic and weak frontal activation to aggressive stimuli in spouse abusers. Mol. Psychiatry 13(7):655–656.
Lin, D., Boyle, M.P., et al. (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470(7333):221–226.
Marsh, A.A., and Blair, R.J.R. (2008). Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci. Behav. Rev. 32(3):454–465.
Marsh, A.A., Finger, E.C., et al. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 165(6):712–720.
Marsh, A.A., Finger, E.C., et al. (2011). Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Res. 194(3):279–286.
Miles, D.R., and Carey, G. (1997). Genetic and environmental architecture of human aggression. J. Pers. Soc. Psychol. 72(1):207–217.
Nelson, R.J., and Trainor, B.C. (2007). Neural mechanisms of aggression. Nat. Rev. Neurosci. 8:536–546.
New, A.S., Hazlett, E.A., et al. (2009). Laboratory induced aggression: a positron emission tomograpy study of aggressive individuals with borderline personality disorder. Biol. Psychiatry 66:1107–1114.
O’Doherty, J.P., Dayan, P., et al. (2003). Temporal difference models and reward-related learning in the human brain. Neuron 38(2):329–337.
Panksepp, J. (1998). Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press.
Patrick, C.J. (1994). Emotion and psychopathy: startling new insights. Psychophysiology 31:319–330.
Raine, A., Venables, P.H., et al. (1996). Better autonomic conditioning and faster electrodermal half-recovery time at age 15 years as possible protective factors against crime at age 29 years. Dev. Psychol. 32:624–630.
Reif, A., Rosler, M., et al. (2007). Nature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environment. Neuropsychopharmacology 32(11):2375–2383.
Rescorla, R.A., and Wagner, A.R. (1972). A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black, A.H., and Prokasym, W.F., eds. Classical Conditioning II. New York: Appleton-Century-Crofts, pp. 64–99.
Rujescu, D., Giegling, I., et al. (2003). A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol. Psychiatry 54(1):34–39.
Sanfey, A.G., Rilling, J.K., et al. (2003). The neural basis of economic decision-making in the ultimatum game. Science 300(5626):1755–1758.
Schoenbaum, G., and Roesch, M. (2005). Orbitofrontal cortex, associative learning, and expectancies. Neuron 47(5):633–636.
Smolka, M.N., Schumann, G., et al. (2005). Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J. Neurosci. 25(4):836–842.
Taylor, S.P. (1967). Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J. Personality 35:297–310.
Veit, R., Lotze, M., et al. (2010). Aberrant social and cerebral responding in a competitive reaction time paradigm in criminal psychopaths. NeuroImage 49(4):3365–3372.
Viding, E., Blair, R.J.R., et al. (2005). Evidence for substantial genetic risk for psychopathy in 7-year-olds. J. Child Psychol. Psychiatry 46:592–597.
Volavka, J., Bilder, R., et al. (2004). Catecholamines and aggression: the role of COMT and MAO polymorphisms. Ann. NY Acad. Sci. 1036:393–398.
White, S.F., Brislin, S.J., et al. (2012). Callous-unemotional traits modulate the neural response associated with punishing another individual during social exchange: a preliminary investigation. J. Pers. Disord. 27(1):99–112.
White, S.F., Marsh, A.A., et al. (2013). Reduced amygdala responding in youth with disruptive behavior disorder and psychopathic traits reflects a reduced emotional response not increased top-down attention to non-emotional features. Am. J. Psychiatry 169(7):750–758.
White, S.F., Pope, K., et al. (2013). Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am. J. Psychiatry 170(3):315–23.
Wootton, J.M., Frick, P.J., et al. (1997). Ineffective parenting and childhood conduct problems: the moderating role of callous-unemotional traits. J. Consult. Clin. Psychol. 65:301–308.