In the multiple-choice section, this topic appears in about 7 out of 75 questions. In the free-response section, this topic appears almost every year.
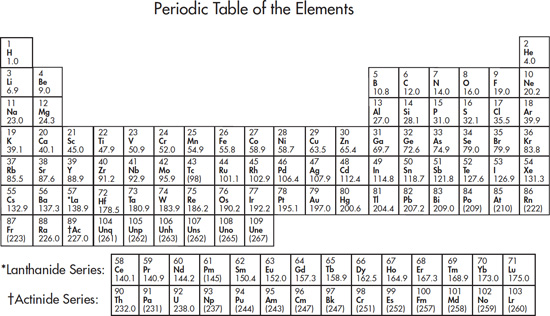
The most important tool you will use on this test is the Periodic Table of the Elements.

Click here to view a larger image
The periodic table gives you very basic but very important information about each element.

The identity of an atom is determined by the number of protons contained in its nucleus. The nucleus of an atom also contains neutrons. The mass number of an atom is the sum of its neutrons and protons. Atoms of an element with different numbers of neutrons are called isotopes; for instance, carbon-12, which contains 6 protons and 6 neutrons, and carbon-14, which contains 6 protons and 8 neutrons, are isotopes of carbon. The molar mass given on the periodic table is the average of the mass numbers of all known isotopes weighted by their percent abundance.
The molar mass of an element will give you a pretty good idea of the most common isotope of that element. For instance, the molar mass of carbon is 12.0 and about 99 percent of the carbon in existence is carbon-12.
The horizontal rows of the periodic table are called periods.
The vertical columns of the periodic table are called groups.
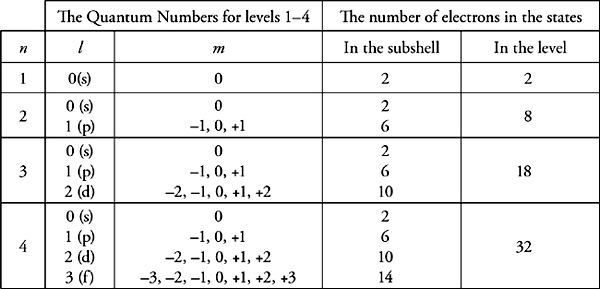
The positions of the electrons in relation to the nucleus are described by their quantum numbers. Each electron has four quantum numbers that apply to its shell, subshell, orbital, and spin.
In a hydrogen atom, the principal quantum number, or shell, of an electron determines its average distance from the nucleus as well as its energy. So electrons in shells with higher values are farther away on average from the nucleus and will have more energy and less stability than electrons in shells with lower values.
The angular momentum quantum number, or subshell, describes the shape of an electron’s orbital.
The orbitals of s subshells are spherical, while the orbitals of p subshells are dumbbell shaped.
The magnetic quantum number, or orbital, describes the orientation of the orbital in space. Roughly, that means it describes whether the path of the electron lies mostly on the x, y, or z axis of a three-dimensional grid.
Each orbital can contain two electrons: one with a positive spin and one with a negative spin.
Here are a couple of graphical ways of looking at quantum numbers.

The Aufbau principle states that when building up the electron configuration of an atom, electrons are placed in orbitals, subshells, and shells in order of increasing energy.
The Pauli exclusion principle states that within an atom, no two electrons can have the same set of quantum numbers. So, each electron in any atom has its own distinct set of four quantum numbers.
You can use the periodic table to tell the first two quantum numbers of the valence electrons of any element. Note that n = principal quantum number.

You can also tell the order in which shells and subshells are filled by following the table from left to right across each period.
You should note that after the third period, the filling of subshells becomes more complicated. Notice, for instance, that the 4s subshell fills before the 3d subshell.
Hund’s rule says that when an electron is added to a subshell, it will always occupy an empty orbital if one is available. Electrons always occupy orbitals singly if possible and pair up only if no empty orbitals are available.
Watch how the 2p subshell fills as we go from boron to neon.

Diamagnetism and paramagnetism are types of magnetism. Diamagnetic elements have all of their electrons spin paired. So, diamagnetic elements are elements with all of their subshells completed.
Some diamagnetic elements are
| Helium | 1s2 |
| Beryllium | 1s2 2s2 |
| Neon | 1s2 2s22p6 |
Most of the elements do not have all of their electrons spin paired and are called paramagnetic elements.
Paramagnetic elements are strongly affected by magnetic fields, whereas diamagnetic elements are not very strongly affected.
Molecules can also be diamagnetic or paramagnetic, depending on the pairing of electrons in their molecular orbitals, but the same basic rule holds: Paramagnetic molecules are affected by magnetic fields, and diamagnetic molecules are not.
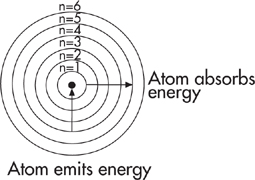
The positively charged nucleus is always pulling at the negatively charged electrons around it, and the electrons have potential energy that increases with their distance from the nucleus. It works the same way that the gravitational potential energy of a brick on the third floor of a building is greater than the gravitational potential energy of a brick nearer to ground level.
The energy of electrons, however, is quantized. That’s important. It means that electrons can only exist at specific energy levels, separated by specific intervals. It’s kind of like if the brick in the building could be placed only on the first, second, or third floor of the building, but not in-between.
The quantized energy of an electron in a hydrogen atom can be found if you know its principal quantum number or shell.
When atoms absorb energy in the form of electromagnetic radiation, electrons jump to higher energy levels. When electrons drop from higher to lower energy levels, atoms give off energy in the form of electromagnetic radiation.
The relationship between the change in energy level of an electron and the electromagnetic radiation absorbed or emitted is given below.

The energy level changes for the electrons of a particular atom are always the same, so atoms can be identified by their emission and absorption spectra.
In the early 1800s, John Dalton presented some basic ideas about atoms that we still use today. He was the first to say that there are many different kinds of atoms, which he called elements. He said that these elements combine to form compounds and that these compounds always contain the same ratios of elements. Water (H2O), for instance, always has two hydrogen atoms for every oxygen atom. He also said that atoms are never created or destroyed in chemical reactions.
In 1869, Dmitri Mendeleev and Lothar Meyer independently proposed arranging the elements into early versions of the periodic table, based on the trends of the known elements.
In the late 1800s, J. J. Thomson watched the deflection of charges in a cathode ray tube and put forth the idea that atoms are composed of positive and negative charges. The negative charges were called electrons, and Thomson guessed that they were sprinkled throughout the positively charged atom like chocolate chips sprinkled throughout a blob of cookie dough.
Robert Millikan was able to calculate the charge on an electron by examining the behavior of charged oil drops in an electric field.

In the early 1900s, Ernest Rutherford fired alpha particles at gold foil and observed how they were scattered. This experiment led him to conclude that all of the positive charge in an atom was concentrated in the center and that an atom is mostly empty space. This led to the idea that an atom has a positively charged nucleus, which contains most of the atom’s mass, and that the tiny, negatively charged electrons travel around this nucleus.

Max Planck figured out that electromagnetic energy is quantized. That is, for a given frequency of radiation (or light), all possible energies are multiples of a certain unit of energy, called a quantum (mathematically, that’s E = hv). So, energy changes do not occur smoothly but rather in small but specific steps.
Neils Bohr took the quantum theory and used it to predict that electrons orbit the nucleus at specific, fixed radii, like planets orbiting the Sun. The Bohr model worked for atoms and ions with one electron but not for more complex atoms.
Werner Heisenberg said that it is impossible to know both the position and momentum of an electron at a particular instant. In terms of atomic structure, this means that electron orbitals do not represent specific orbits like those of planets. Instead, an electron orbital is a probability function describing the possibility that an electron will be found in a region of space.

Louis de Broglie said that all matter has wave characteristics. This is important because sometimes the behavior of electrons is better described in terms of waves than particles.
There is a simple relationship between an electron’s wave and particle characteristics.

De Broglie’s hypothesis is useful for very small particles, such as electrons. For larger particles, the wavelength becomes too small to be of interest.
You can make predictions about certain behavior patterns of an atom and its electrons based on the position of the atom in the periodic table. All the periodic trends can be understood in terms of three basic rules.
The atoms in the left-hand side of the periodic table are called metals. Metals give up electrons when forming bonds. Most of the elements in the table are metals. The elements in the upper right-hand portion of the table are called nonmetals. Nonmetals generally gain electrons when forming bonds. The metallic character of the elements decreases as you move from left to right across the periodic table. The elements in the borderline between metal and nonmetal, such as silicon and arsenic, are called metalloids.
The atomic radius is the approximate distance from the nucleus of an atom to its valence electrons.
Moving from left to right across a period, protons are added to the nucleus, so the valence electrons are more strongly attracted to the nucleus; this decreases the atomic radius. Electrons are also being added, but they are all in the same shell at about the same distance from the nucleus, so there is not much of a shielding effect.
Moving down a group, shells of electrons are added to the nucleus. Each shell shields the more distant shells from the nucleus and the valence electrons get farther away from the nucleus. Protons are also being added, but the shielding effect of the negatively charged electron shells cancels out the added positive charge.
Generally, when electrons are removed from an atom to form a cation, the outer shell is lost, making the cation smaller than the atom. Also, when electrons are removed, electron-electron repulsions are reduced, allowing all of the remaining valence electrons to move closer to the nucleus.
When an electron is added to an atom, forming an anion, electron-electron repulsions increase, causing the valence electrons to move farther apart, which increases the radius.
Electrons are attracted to the nucleus of an atom, so it takes energy to remove an electron. The energy required to remove an electron from an atom is called the first ionization energy. Once an electron has been removed, the atom becomes a positively charged ion. The energy required to remove the next electron from the ion is called the second ionization energy, and so on.
Moving from left to right across a period, protons are added to the nucleus, which increases its positive charge. For this reason, the negatively charged valence electrons are more strongly attracted to the nucleus, which increases the energy required to remove them. Electrons are also being added, and the shielding effect provided by the filling of the s subshell causes a slight deviation in the trend in moving from Group 2A to Group 3A. There is also a slight deviation when the electrons in the p subshell start to pair up, so oxygen has a slightly lower first ionization energy than nitrogen does.
Moving down a group, shells of electrons are added to the nucleus. Each inner shell shields the more distant shells from the nucleus, reducing the pull of the nucleus on the valence electrons and making them easier to remove. Protons are also being added, but the shielding effect of the negatively charged electron shells cancels out the added positive charge.
When an electron has been removed from an atom, electron-electron repulsion decreases and the remaining valence electrons move closer to the nucleus. This increases the attractive force between the electrons and the nucleus, increasing the ionization energy.
Electron affinity is a measure of the change in energy of an atom when an electron is added to it. When the addition of an electron makes the atom more stable, energy is given off. This is true for most of the elements. When the addition of an electron makes the atom less stable, energy must be put in; that’s because the added electron must be placed in a higher energy level, making the element less stable. This is the case for elements with full subshells, like the alkaline earths and the noble gases.
When moving from left to right across a period, the energy given off when an electron is added increases. Electron affinities don’t change very much moving down a group.
Electronegativity refers to how strongly the nucleus of an atom attracts the electrons of other atoms in a bond. Electronegativities of elements are estimated based on ionization energies and electron affinities, and they basically follow the same trends.
The various periodic trends, which don’t include the noble gases, are summarized in the diagram below.

Questions 1− 4
(A) C
(B) N
(C) O
(D) F
(E) Ne
1. This is the most electronegative element.
2. The nuclear decay of an isotope of this element is used to measure the age of archaeological artifacts.
3. All of the electrons in this element are spin-paired.
4. This element, present as a diatomic gas, makes up most of the earth’s atmosphere.
Questions 5−7
(A) Hg
(B) Si
(C) Cu
(D) Zn
(E) Ag
5. This element is commonly used in the manufacture of semiconductors.
6. This element is a liquid at room temperature.
7. After oxygen, this is by far the most common element in the earth’s crust.
8. What is the most likely electron configuration for a sodium ion in its ground state?
(A) 1s2 2s22p5
(B) 1s2 2s22p6
(C) 1s2 2s22p6 3s1
(D) 1s2 2s22p5 3s2
(E) 1s2 2s22p6 3s2
9. Which of the following statements is true regarding sodium and chlorine?
(A) Sodium has greater electronegativity and a larger first ionization energy.
(B) Sodium has a larger first ionization energy and a larger atomic radius.
(C) Chlorine has a larger atomic radius and a greater electronegativity.
(D) Chlorine has greater electronegativity and a larger first ionization energy.
(E) Chlorine has a larger atomic radius and a larger first ionization energy.
10. Which of the following could be the quantum numbers (n, l, ml, ms) for the valence electron in a potassium atom in its ground state?
(A) 3, 0, 0, ![]()
(B) 3, 1, 1, ![]()
(C) 4, 0, 0, ![]()
(D) 4, 1, 1, ![]()
(E) 4, 2, 1, ![]()
12. Which of the following rules states that no two electrons in an atom can have the same set of quantum numbers?
(A) Hund’s rule
(B) The Heisenberg uncertainty principle
(C) The Pauli exclusion principle
(D) The de Broglie hypothesis
(E) The Bohr model
13. Which of the following is true of the alkali metal elements?
(A) They usually take the +2 oxidation state.
(B) They have oxides that act as acid anhydrides.
(C) They form covalent bonds with oxygen.
(D) They are generally found in nature in compounds.
(E) They have relatively large first ionization energies.
14. Which of the following nuclei has 3 more neutrons than protons? (Remember: the number before the symbol indicates atomic mass.)
(A) 11B
(B) 37Cl
(C) 24Mg
(D) 70Ga
(E) 19F
15. Which of the following ions has the smallest ionic radius?
(A) O2−
(B) F−
(C) Na+
(D) Mg2+
(E) Al3+
16. Which of the following is an impossible set of quantum numbers (n, l, ml , ms)?
(A) 4, 0, 0, ![]()
(B) 4, 0, 1, ![]()
(C) 4, 1, 0, ![]()
(D) 4, 1, 1, ![]()
(E) 4, 2, 1, ![]()
17. Which of the following represents the energy of the single electron in a hydrogen atom when it is in the n = 4 state?
(A) 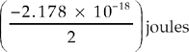
(B) 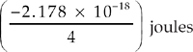
(C) 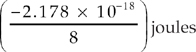
(D) 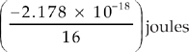
(E) 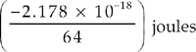
18. When an electron in a hydrogen atom makes the transition from the n = 4 state to the n = 2 state, blue light with a wavelength of 434 nm is emitted. Which of the following expressions gives the energy released by the transition?
(A) 
(B) 
(C) 
(D) 
(E) ![]()
19. The ionization energies for an element are listed in the table below.
| First | Second | Third | Fourth | Fifth |
| 8 eV | 15 eV | 80 eV | 109 eV | 141 eV |
Based on the ionization energy table, the element is most likely to be
(A) sodium.
(B) magnesium.
(C) aluminum.
(D) silicon.
(E) phosphorous.
20. A researcher listed the first five ionization energies for a silicon atom in order from first to fifth. Which of the following lists corresponds to the ionization energies for silicon?
(A) 780 kJ, 13,675 kJ, 14,110 kJ, 15,650 kJ, 16,100 kJ
(B) 780 kJ, 1,575 kJ, 14,110 kJ, 15,650 kJ, 16,100 kJ
(C) 780 kJ, 1,575 kJ, 3,220 kJ, 15,650 kJ, 16,100 kJ
(D) 780 kJ, 1,575 kJ, 3,220 kJ, 4,350 kJ, 16,100 kJ
(E) 780 kJ, 1,575 kJ, 3,220 kJ, 4,350 kJ, 5,340 kJ
1. Explain each of the following in terms of atomic and molecular structures and/or forces.
(a) The first ionization energy for magnesium is greater than the first ionization energy for calcium.
(b) The first and second ionization energies for calcium are comparable, but the third ionization energy is much greater.
(c) Solid sodium conducts electricity, but solid sodium chloride does not.
(d) The first ionization energy for aluminum is lower than the first ionization energy for magnesium.
2. Silicon is a nonmetal with four valence electrons.
(a) Write the ground state electron configuration for silicon.
(b) Which fundamental atomic theory is violated by the following list of quantum numbers representing silicon’s valence electrons?
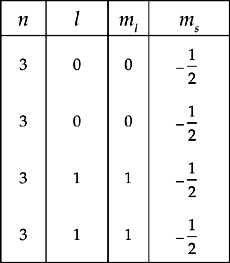
(c) Which fundamental atomic theory is violated by the following list of quantum numbers representing silicon’s valence electrons?
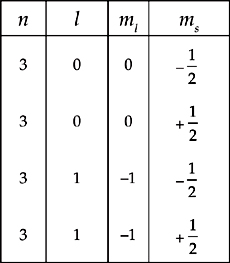
(d) Will a lone silicon atom be diamagnetic or paramagnetic? Justify your answer.
3. Use your knowledge of the periodic table of the elements to answer the following questions.
(a) Explain the trend in electronegativity from P to S to Cl.
(b) Explain the trend in electronegativity from Cl to Br to I.
(c) Explain the trend in atomic radius from Li to Na to K.
(d) Explain the trend in atomic radius from Al to Mg to Na.
4. Use your knowledge of atomic theory to answer the following questions.
(a) State the Heisenberg uncertainty principle.
(b) The absorption spectrum of a hydrogen atom contains dark bands at specific wavelengths. The emission spectrum of a hydrogen atom contains bright bands at the same wavelengths. Explain what causes these bright and dark bands at specific wavelengths.
(c) Explain why the addition of an electron to a chlorine atom is an exothermic process and the addition of an electron to a magnesium atom is an endothermic process.
(d) Explain how the valence electron configuration of sulfur is consistent with the existence of Na2S and SF6.
1. D Fluorine, which needs one electron to complete its outer shell, is the most electronegative element, with an electronegativity of 4.0.
2. A In carbon dating, the ratio of carbon-14 to carbon-12 in an organic artifact is used to determine the age of the artifact.
3. E Neon’s second shell is complete, so all of its electrons are spin-paired.
4. B Nitrogen gas (N2) makes up 78 percent of the earth’s atmosphere. Oxygen is next at 21 percent.
5. B Silicon (Si) is used in semiconductor technology because it has properties that lie in-between metals and nonmetals.
6. A Mercury (Hg) is unusual among metals in that it is a liquid at room temperature. Its melting point is −39 degrees Celsius.
7. B Silicon makes up 26 percent of the earth’s crust by weight (oxygen makes up 50 percent). In fact, compounds including silicon and oxygen make up nearly all rocks and soils.
8. B Neutral sodium in its ground state has the electron configuration shown in choice (C). Sodium forms a bond by giving up its one valence electron and becoming a positively charged ion with the same electron configuration as neon.
9. D As we move from left to right across the periodic table within a single period (from sodium to chlorine), we add protons to the nuclei, which progressively increases the pull of each nucleus on its electrons. So chlorine will have a higher first ionization energy, greater electronegativity, and a smaller atomic radius.
10.
C Potassium’s valence electron is in the 4s subshell. That means that n = 4, l = 0, ml = 0, and ms = ![]() or −
or −![]() .
.
11. C Beryllium’s electrons are paired up in the completed orbitals of the 1s and 2s subshells. Choice (E) is wrong because, according to Hund’s rule, carbon’s two 2p electrons must be placed in different orbitals.
12.
C The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers.
About the other answers:
(A) Hund’s rule states that within a subshell, electrons will be placed in empty orbitals while they are available and will start to pair up in orbitals only when no more empty orbitals are available.
(B) The Heisenberg uncertainty principle states that it is impossible to know with certainty both the position and momentum of a particle at the same moment.
(D) The de Broglie hypothesis relates the wave and particle properties of matter, using the following equation:
Wavelength = ![]()
h is Planck’s constant, 6.63 × 10−34 joule-sec
(E) The Bohr model of the hydrogen atom (which was disproved by the Heisenberg uncertainty principle) states that electrons orbit the nucleus in fixed, quantized circular orbits.
13.
D The alkali metals (Li, Na, K…) are extremely reactive and are found in nature almost exclusively in compounds.
As for the other answers:
Choice (A) is wrong because the alkali metals take the +1 oxidation state. Choice (B) is wrong because alkali metal oxides are basic anhydrides; that is, they form basic solutions in water. Choice (C) is wrong because they form ionic bonds with oxygen. Choice (E) is wrong because they have only one valence
electron, so they have relatively small first ionization energies.
14. B The atomic mass is the sum of the neutrons and protons in any atom’s nucleus. Since atomic number, which indicates the number of protons, is unique to each element we can subtract this from the weight to find the number of neutrons. The atomic number of B is 5, hence the 11B has 6 neutrons, only 1 in excess. The atomic number of Cl is 17, so 37Cl has 20 neutrons, 3 in excess. The atomic number of Mg is 12, so 24Mg has the same number of neutrons as protons. The element Ga has an atomic number of 31, meaning there are 39 neutrons in the given nucleus and the atomic number for F is 9, meaning 10 neutrons in 19F.
15. E All of the ions listed have the same electron configuration as neutral neon. Al3+ has the most protons, so its electrons will experience greater attractive force from the nucleus, resulting in the smallest ions.
16.
B The ml quantum number (orbital) can’t be larger than the l quantum number (subshell), so 4, 0, 1, ![]() is impossible because 1 is greater than 0.
is impossible because 1 is greater than 0.
17.
D When you know the principal quantum number, you must use the formula for the energy of an electron, which is shown below.![]()
The variable n stands for the principal quantum number; in this case, n = 4.
18.
A When you know the wavelength emitted, you must use the formula for the energy of an electron transition, which is shown below. Remember, a nanometer is equal to 10−9 meters.![]()
19. B The ionization energy will show a large jump when enough electrons have been removed to leave a stable shell. In this case, the jump occurs between the second and third electrons removed, so the element is stable after two electrons are removed. Magnesium (Mg) is the only element on the list with exactly two valence electrons.
20. D The ionization energy will show a large jump when enough electrons have been removed to leave a stable shell. Silicon has four valence electrons, so we would expect to see a large jump in ionization energy after the fourth electron has been removed. That’s choice (D).
1. (a) Ionization energy is the energy required to remove an electron from an atom. The outermost electron in Ca is at the 4s energy level. The outermost electron in Mg is at the 3s level. The outermost electron in Ca is at a higher energy level and is more shielded from the nucleus, making it easier to remove.
(b)
Calcium has two electrons in its outer shell. The second ionization energy will be larger than the first but still comparable because both electrons are being removed from the same energy level. The third electron is much more difficult to remove because it is being removed from a lower energy level, so it will have a much higher ionization energy than the other two.
(c) Solid sodium exhibits metallic bonding, in which the positively charged sodium
ions are held together by a sea of mobile, delocalized electrons. These electrons move freely from nucleus to nucleus, making solid sodium a good conductor.
Sodium chloride exhibits ionic bonding, in which positively charged sodium ions and negatively charged chlorine ions hold fixed places in a crystal lattice. The electrons are localized around particular nuclei and are not free to move about the lattice. This makes solid sodium chloride a bad conductor of
electricity.
(d) The valence electron to be removed from magnesium is located in the completed 3s subshell, while the electron to be removed from aluminum is the lone electron in the 3p subshell. It is easier to remove the electron from the higher-energy 3p subshell than from the lower energy (completed) 3s subshell, so the first ionization energy is lower for aluminum.
2. (a) 1s22s22p63s23p2
(b) The Pauli exclusion principle is violated. The Pauli exclusion principle states that no two electrons can have the same set of quantum
numbers.
(c) Hund’s rule is violated. Hund’s rule states that within an energy level, electrons will be placed in empty orbitals while they are available and will only start to pair up in orbitals when no more empty orbitals are available.
(d) A lone silicon atom will be paramagnetic and will be affected by a magnetic field. If all the electrons in an atom are spin-paired, the atom is diamagnetic. If any of the electrons are not spin-paired,
the atom is paramagnetic. Silicon has two electrons in the 3p subshell. They will be placed in different orbitals, so they will not be spin-paired.
3. (a) Electronegativity is the pull of the nucleus of one atom on the electrons of other atoms; it increases from P to S to Cl because nuclear charge increases. This is because as you from move left to right across the periodic table, atomic radii decrease in size. Increasing nuclear charge means that Cl has the most positively charged nucleus
of the three and will exert the greatest pull on the electrons of other atoms.
(b) Electronegativity is the pull of the nucleus of one atom on the electrons of other atoms; it decreases from Cl to Br to I because electron shells are added. The added electron shells shield the nucleus, causing it to have less of an effect on the electrons of other atoms. Therefore, iodine will exert the least pull on the electrons of other atoms.
(c) Atomic radius increases
from Li to Na to K because electrons are being added in higher energy levels, which are farther away from the nucleus; therefore, the K atom is the largest of the three.
(d) Atomic radius increases from Al to Mg to Na because protons are being removed from the nucleus while the energy levels of the valence electrons remains unchanged. If there are fewer positive charges in the nucleus, the electrons of Na will be less attracted to the nucleus and will remain farther
away.
4. (a) A straightforward statement of the Heisenberg uncertainty principle is as follows: It is impossible to know with certainty both the position and momentum of a particle at one moment.
(b) When a hydrogen atom absorbs energy, its electrons jump to higher energy levels. The absorbed energy shows up as a dark area on the absorption
spectrum.
A hydrogen atom gives off energy when its electrons jump back down to lower energy levels. Electromagnetic waves, which are emitted at these jumps, show up as bright areas on the emission spectrum.
In an atom, energy is quantized, which means that electrons can exist only at specific energy levels. When an electron jumps from one energy level to another, it will always emit or absorb exactly the same amount of energy, and because
ΔE = hv, for a particular energy change, radiation of the same frequency will always be emitted or absorbed.
(c) When an electron is added to chlorine, the chlorine ion created has a complete valence shell, which is an extremely stable, low energy configuration. When something becomes more stable, energy is given off, making the process exothermic.
When an electron is added to a magnesium atom, it must be placed by itself in the
3p energy level. Adding an electron in a higher energy level makes the magnesium ion created more energetic and less stable, which means that the process is endothermic.
(d) Sulfur has six valence electrons in its outer shell. In Na2S, sulfur gains two electrons to give its outer shell a complete octet. In SF6 , sulfur uses sp3d2
hybridization to share all six of its valence electrons with fluorine atoms.