In the multiple-choice section, this topic appears in about 3 out of 75 questions.
In the free-response section, this topic appears occasionally.
A nucleus is held together by a nonelectrical, nongravitational force called the nuclear force.
Some nuclei are more stable than others. When a nucleus is unstable, it can attempt to increase its stability by altering its number of neutrons and protons. This is the process of radioactive decay. Elements are naturally radioactive after atomic number 83, Bismuth.
In alpha decay, the nucleus emits a particle that has the same constitution as a helium molecule, with 2 protons and 2 neutrons. Alpha particles are the least penetrating of the products of nuclear decay.
![]()
When a nucleus undergoes alpha decay
![]()
A beta particle is identical to an electron. In beta decay, the nucleus changes a neutron into a proton and an electron and emits the electron.
![]()
When a nucleus undergoes beta decay
![]()
A positron is like an electron with a positive charge. In positron emission, the nucleus changes a proton into a neutron and a positron and emits the positron.
![]()
When a nucleus undergoes positron emission
![]()
In electron capture, the nucleus captures a low energy electron and combines it with a proton to form a neutron.
![]()
When a nucleus undergoes electron capture
![]()
Gamma rays are electromagnetic radiation and have no mass and no charge. Gamma rays usually accompany other forms of nuclear decay, and are the most penetrating of the nuclear decay products.
Nuclei undergo decay to achieve greater stability. You can use the periodic table to predict the kind of decay that an isotope will undergo.
The half-life of a radioactive substance is the time it takes for half of the substance to decay. Most half-life problems can be solved by using a simple chart.
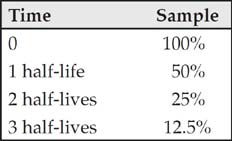
So a sample with a mass of 120 grams and a half-life of 3 years will decay as follows:
(Don’t forget that the chart should start with the time at zero.)
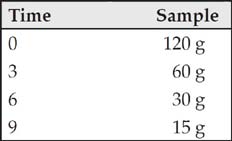
The fact that you’re not allowed to use a calculator for Section I and that you’re not given any half-life formulas for Section II means that you should be able to solve any half-life problem that comes up by using the chart and POE.
When protons and neutrons come together to form a nucleus, the mass of the nucleus is less than the sum of the masses of its constituent protons and neutrons. This difference in mass is called the mass defect. The mass lost in this process is released in the form of energy. If we reverse the process this is the same amount of energy, called the binding energy, required to decompose the nucleus back into protons and neutrons. The relationship between mass and energy is given by Einstein’s famous equation.
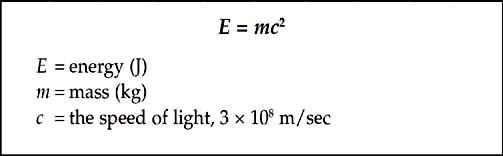
You can see that because c2 is such a large number, a very small change in mass results in a very large change in energy.
Questions 1–4
(A) Alpha decay
(B) Beta (β−) decay
(C) Electron capture
(D) Gamma radiation
(E) Mass defect
1. In this process the number of protons in a nuclide is increased while the mass number remains constant.
2. In this process a nuclide releases a particle that is the equivalent of a helium nucleus.
3. This is the difference between the mass of a nucleus and the sum of its component nucleons.
4. In this process the number of protons in a nuclide is decreased while the mass number remains constant.
Questions 5–8
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
(E) ![]()
5. This pair could be the only two elements present in a sample undergoing alpha decay.
6. This pair could be the only two elements present in a sample undergoing decay.
7. This pair are isotopes of each other.
8. This pair could be the only two elements present in a sample undergoing electron capture.
9. The nuclide ![]() is unstable. Which of the following decay types would
is unstable. Which of the following decay types would ![]() be expected to undergo?
be expected to undergo?
I.
Beta decay (β−) to increase the ratio of protons to neutrons.
II. Positron emission (β+) to decrease the ratio of protons to neutrons.
III. Electron capture to decrease the ratio of protons to neutrons.
(A) I only
(B) II only
(C) I and III only
(D) II and III only
(E) I, II, and III
10. Which of the following statements is true regarding the mass and magnitude of charge of alpha particles and beta particles?
(A) Alpha particles are more highly charged and have greater mass than beta particles.
(B) Beta particles are more highly charged and have greater mass than alpha particles.
(C) Alpha particles are more highly charged than beta particles, but beta particles have greater mass.
(D) Beta particles are more highly charged than alpha particles, but alpha particles have greater mass.
(E) Alpha particles are more highly charged than beta particles, but the masses of the two types of particles are the same.
11. Strontium-90 decays through the emission of beta particles. It has a half-life of 29 years. How long does it take for 80 percent of a sample of strontium-90 to decay?
(A) 9.3 years
(B) 21 years
(C) 38 years
(D) 67 years
(E) 96 years
12. A sample of radioactive material undergoing nuclear decay is found to contain only potassium and calcium. The sample could be undergoing which of the following decay processes?
I. Beta (β−) decay
II. Alpha decay
III. Electron capture
(A) I only
(B) II only
(C) I and III only
(D) II and III only
(E) I, II, and III
13. A sample of radioactive material undergoing nuclear decay is found to contain only 84Po and 82Pb. The sample could be undergoing which of the following decay processes?
I. Beta (β−) decay
II. Alpha decay
III. Electron capture
(A) I only
(B) II only
(C) I and III only
(D) II and III only
(E) I, II, and III
14. A ![]() nuclide emits two alpha particles and two beta (β−) particles. The resulting nuclide is
nuclide emits two alpha particles and two beta (β−) particles. The resulting nuclide is
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
(E) ![]()
15. After 44 minutes, a sample of is found to have decayed to 25 percent of the original amount present. What is the half-life of?
(A) 11 minutes
(B) 22 minutes
(C) 44 minutes
(D) 66 minutes
(E) 88 minutes
1. Use the principles of nuclear chemistry to explain each of the following:
(a) Gamma radiation penetrates farther into the human body than either alpha or beta radiation.
(b) A nucleus weighs less than the sum of the weights of its neutrons and protons.
(c) Arsenic-81 is unstable. What form of radioactive decay would it be expected to undergo?
(d) Potassium-38 decays by electron capture. Write the balanced nuclear reaction for this process.
(e) In a nuclear explosion, a small mass has enormous destructive power.
(a) Write the balanced nuclear reaction for sodium-21.
(b) Write the balanced nuclear reaction for sodium-26.
(c) A sample of sodium-25 is observed to decay for 5 minutes. After this time, no change in the mass of the sample is detected. Explain.
(d) Describe alpha, beta (β−), and gamma radiation in terms of mass and charge.
3. Neon-23 is an unstable nuclide with a half-life of 38 seconds.
(a) What form of decay would neon-23 be expected to undergo? Write the balanced nuclear reaction for that process.
(b) Explain why the nucleus of a neon-23 atom weighs less than the sum of its constituent protons and neutrons.
(c) Describe the change that a sample of neon-23 undergoes in 38 seconds.
(d) Describe alpha, beta (–), and gamma radiation in terms of mass and charge.
(e) The decay of neon-23 is shown on the graph below.
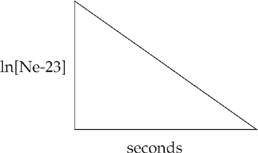
(i) What is the order of the nuclear reaction?
(ii) What is represented by the slope of the graph?
(iii) What are the units of the rate constant for this reaction?
1. B In beta decay, a nuclide releases a β− particle (which is the same as an electron) and converts a neutron to a proton.
![]()
2.
A An alpha particle (![]() ) has two protons and two neutrons, the same as a helium nucleus.
) has two protons and two neutrons, the same as a helium nucleus.
3. E Mass defect is the mass that seems to disappear from neutrons and protons when they are brought together to form a nucleus.
Mass defect is the m in Einstein’s famous equation for the equivalence of mass and energy, E = mc2.
4. C In electron capture, a nuclide absorbs one of its low energy electrons and combines it with a proton to form a neutron.
![]()
5. C In alpha decay, a particle with the mass (4) and charge (+2) of a helium nucleus is given off, so the two nuclides must differ by a mass of 4 and a charge of 2.
![]()
6. A In decay, the mass number remains constant and the proton number decreases by one, so the two nuclides must have the same mass number and differ by one in their proton numbers.
![]()
7. B Isotopes are atoms of the same element (same number of protons) with differing mass numbers.
8. A In electron capture, the mass number remains constant and the proton number decreases by one, so the two nuclides must have the same mass number and differ by one in their proton numbers.
![]()
9. A From the periodic table, we can see that Sn has an atomic mass of 118.71, so 128 is a very large mass number for Sn. The nuclide will decay to increase its proton to neutron ratio, thereby making itself more stable.
10. A An alpha particle is a helium nucleus, so it has a mass of 4 g/mol and a charge of +2.
A beta particle is an electron, so its mass is very small compared with the masses of the protons and neutrons in the alpha particle. The charge on a beta particle is the same as the charge on an electron, –1.
11. D Make a chart. Always start at time = 0. We’re looking for the time it takes for 80 percent of the stuff to decay. That’s the time when 20 percent of the stuff remains.
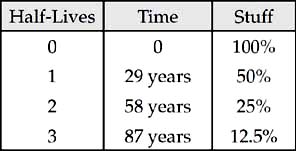
It takes between 2 and 3 half-lives to get to the point when 20 percent remains. The correct answer is the only choice between 58 years and 87 years.
12. C Since decay is occurring and only potassium and calcium are present, one must be decaying to produce the other. Potassium and calcium differ by one proton, so the decay process must either add or subtract a proton.
Beta decay (I) is possible.
19K → 20Ca + -1β
Electron capture (III) is possible.
20Ca + -1e → 19K
Alpha decay (II) causes the loss of two protons, so it can’t be occurring if only K and Ca are present.
13. B Since decay is occurring and only Po and Pb are present, one must be decaying to produce the other. Po has 84 protons and Pb has 82. The only way that a decay process can involve only these two elements is if Po undergoes alpha decay to produce Pb.
Alpha decay (II)
![]()
If beta decay (I) or electron capture (III) were occurring, the proton count would be changing one at a time and 83Bi would also be present in the sample.
14. D Let’s do the math; you set it up like this:
![]()
For the mass number we have
214 – 4 – 4 = 206
For the proton number we have
84 – 2 – 2 – (–1) – (–1) = 84 – 4 + 2 = 82
So the answer is ![]() .
.
15. B Make a chart. Always start at time = 0.

It takes two half-lives for the amount of ![]() to decrease to 25 percent. If two half-lives takes 44 minutes, one half-life must be 22 minutes.
to decrease to 25 percent. If two half-lives takes 44 minutes, one half-life must be 22 minutes.
1. (a) Alpha and beta particles have mass and volume, so the surface of the body can often stop or slow them down. Gamma rays are electromagnetic radiation, which doesn’t undergo collisions and can travel farther into the body.
(b) Some of the mass of the neutrons and protons is lost to nuclear binding energy, which is released by the stable nucleus.
(c) Stable arsenic has an atomic weight of 75, so arsenic-81 has too many neutrons. It will undergo β− decay, which converts a neutron into a proton.
![]()
(d) In electron capture, the nucleus captures an electron from a lower energy level and combines it with a proton to form a neutron.
![]()
(e) Remember Einstein’s equation E = mc2.
This means that the energy released by a nuclear process is 9 × 1016 times as large as the mass consumed.
2. (a) In β+ decay, a proton is converted into a neutron and a positron is emitted.
![]()
(b) In β– decay, a neutron is converted into a proton, and a β– particle (electron) is emitted.
![]()
(c) Over the course of the decay process, only β– particles are emitted. Neutrons are converted into protons, but there is no change in the mass numbers of the nuclides undergoing decay. Beta particles are virtually massless, so the loss of beta particles may not be detected.
(d) Alpha particles have the mass (4) and charge (+2) of a helium nucleus.
Beta (β–) particles have the mass (insignificant) and charge (–1) of an electron.
Gamma radiation has neither mass nor charge. Gamma radiation is electromagnetic radiation.
3. (a) Stable neon has an atomic weight of 20, so neon-23 has too many neutrons. It will undergo β– decay, which converts a neutron to a proton.
![]()
(b) Some of the mass of the protons and neutrons is converted to nuclear binding energy (E = mc2), which is given off when the nucleus forms.
(c) The half-life of neon-23 is 38 seconds. That means that half of any sample of neon-23 will be converted into sodium-23 after 38 seconds.
(d) Alpha particles have the mass (4) and the charge (+2) of a helium nucleus.
Beta (β–) particles have insignificant mass and the charge (–1) of an electron.
Gamma radiation has neither mass nor charge. Gamma radiation is electromagnetic radiation.
(e) (i) This nuclear reaction is first order. The graph of ln[Ne-23] versus time is linear.
(ii) The slope is the negative of the rate constant, k.
(iii) Units are sec–1.