• Part A—Questions 1, 2, and 3: 20% each
• Part B—Question 4: 10%
Questions 5 and 6: 15% each
![]()
Total—100%
| 1. | C |
| 2. | A |
| 3. | D |
| 4. | C |
| 5. | D |
| 6. | A |
| 7. | B |
| 8. | D |
| 9. | B |
| 10. | E |
| 11. | B |
| 12. | C |
| 13. | A |
| 14. | D |
| 15. | D |
| 16. | B |
| 17. | E |
| 18. | E |
| 19. | C |
| 20. | C |
| 21. | D |
| 22. | A |
| 23. | D |
| 24. | D |
| 25. | C |
| 26. | E |
| 27. | A |
| 28. | B |
| 29. | A |
| 30. | B |
| 31. | A |
| 32. | C |
| 33. | C |
| 34. | D |
| 35. | B |
| 36. | D |
| 37. | D |
| 38. | A |
| 39. | B |
| 40. | D |
| 41. | B |
| 42. | E |
| 43. | C |
| 44. | C |
| 45. | B |
| 46. | A |
| 47. | E |
| 48. | B |
| 49. | A |
| 50. | E |
| 51. | C |
| 52. | C |
| 53. | C |
| 54. | E |
| 55. | D |
| 56. | D |
| 57. | D |
| 58. | D |
| 59. | B |
| 60. | C |
| 61. | B |
| 62. | B |
| 63. | C |
| 64. | B |
| 65. | A |
| 66. | D |
| 67. | B |
| 68. | E |
| 69. | A |
| 70. | B |
| 71. | D |
| 72. | E |
| 73. | B |
| 74. | D |
| 75. | D |
Number Correct = Multiple-Choice Score
Ignore the questions you left blank. The best possible score on the multiple-choice section is 75.
Roughly speaking
The free-response section is graded more subjectively than the multiple-choice section, so there’s no point in trying to assign a numerical grade to the practice test. But even without a score, you can still get a pretty good idea of how you’re doing from the free-response sections of these practice tests.
Once you’ve finished the free-response sections in the tests in this book, compare your answers with the ones given in the answer key. The answers given in the book are not the only possible answers, but when you compare them with your own answers you should get a sense of whether you would have received credit. The answer key will also provide explanations for questions that you couldn’t answer
Here is a breakdown of the free-response section:
• Part A—Questions 1, 2, and 3: 20% each
• Part B—Question 4: 10%
Questions 5 and 6: 15% each
![]()
Total—100%
The average student answers between ![]() and
and ![]() of the questions that he or she selects on the free-response section correctly. So if you can get more than
of the questions that he or she selects on the free-response section correctly. So if you can get more than ![]() of the questions
on the free-response section of the practice test correct, you’ve got a good chance to get at least a 3 on the actual test.
of the questions
on the free-response section of the practice test correct, you’ve got a good chance to get at least a 3 on the actual test.
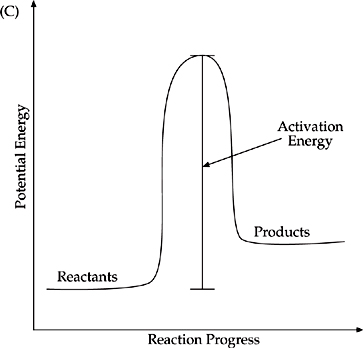
C This reaction has the largest rise from the energy level of the reactants to the peak energy that must be overcome in order for the reaction to proceed.

A This reaction has the largest energy drop from the level of the reactants to the level of the products. That makes it the most exothermic.

D This reaction has the largest rise in energy from the level of the reactants to the level of the products. That makes it the most endothermic and gives it the largest positive value for ∆H.
4. C A paramagnetic element is one whose electrons are not completely spin-paired. For choice (C), sulfur, Hund’s rule says that the first three electrons in the 3p subshell will each occupy an empty orbital. The fourth electron will pair up, leaving two electrons unpaired. All of the other choices have completed subshells, so all the electrons will be spin-paired.
5. D A potassium atom loses an electron when it ionizes; a chlorine atom gains an electron. Both end up with the same ground state electron configuration as an argon atom, given in choice (D).
6. A This atom (neon) has electrons in only two shells. All of the other choices have electrons at higher energy levels, farther away from the nucleus.
7. B The Lewis dot structure for water is shown below.
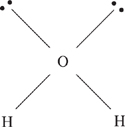
The central oxygen atom forms sp3 hybrid orbitals, resulting in a tetrahedral structure. The central oxygen atom has two unbonded electron pairs, which gives the molecule a bent shape.
8. D NO2 has an odd number (17) of valence electrons, so when we draw the Lewis dot structure, there must be an unpaired electron.

9. B Water has only single bonds or sigma (σ) bonds. All of the other molecules have at least one double bond. The second bond in a double bond is a pi (π) bond.
10. E The oxidation state for an uncombined element is zero.
11. B This is a mixture of a strong acid and a weak base, so at the equivalence point, the mixture will be acidic with a pH less than 7.
12. C This is a mixture of a strong acid and a strong base, so at the equivalence point, they will have completely neutralized each other and only salt water will be left. Therefore, the solution will be neutral, and the pH will be 7.
13. A Both HCl and NaCl dissociate completely, but NaCl will have no effect on the pH of the solution.
Because we are doubling the volume of the HCl solution by adding the salt water, the concentration of HCl will be cut in half, to 0.1-molar. HCl is a strong acid, so [H+] = 0.1-molar and pH = –log[H+] = 1.
14. D This is a mixture of a weak acid and a strong base, so at the equivalence point, the mixture will be basic with a pH greater than 7.
15.
D
Remember: moles = ![]()
So moles of chlorine = ![]() = 2 moles
= 2 moles
If there are 2 moles of chlorine, there must be 2 moles of KClO3.
The molecular weight of KClO3 is 122.5.
So grams of KClO3 = (moles)(MW)
= (2 mol)(122.5 g/mol)
= 245 grams
16. B In distillation, two substances are heated until one of them boils. The gaseous substance is separated from the remaining liquid or solid and condensed in a separate container.
About the other answers:
(A) Titration is used to determine the volume of one solution required to react with a given volume of another solution.
(C) Filtration is used to separate a solid from a liquid by passing the solution through a membrane.
(D) Decantation is used to separate a solid from a liquid by letting the solid settle to the bottom of a container and then pouring off the liquid.
(E) Hydration occurs when ions enter into solution with water.
17. E Aluminum’s valence electrons are in the 3p subshell.
That means that n = 3, l = 1, ml = –1, 0, or 1, and ms = ![]() or –
or –![]() .
.
18. E First, you need to know that C2H6 is an alkane, which has a single C–C bond, and C2H2 is an alkyne, which has a triple C–C bond. Once you realize that, you know that a triple bond will be stronger and shorter than a single bond.
19. C SO32– is oxidized (S4+ → S6+ + 2 e–, LEO), so SO32– acts as the reducing agent.
MnO4– is reduced (Mn7+ + 5 e– → Mn2+, GER), so MnO4– acts as the oxidizing agent.
20. C HSO3– is amphoteric.
It can act as a Brønsted-Lowry acid, giving up a proton to become SO32–.
It can act as a Brønsted-Lowry base, gaining a proton to become H2SO3.
HCl (A) and H2SO4 (B) can only give up protons.
SO42– (D) can only gain protons.
H+ (E) is a proton.
21. D NH3 is the only molecule listed that undergoes hydrogen bonding. In fact, it is the only polar molecule listed.
22.
A
From Dalton’s law, partial pressure of a gas in a sample is directly proportional to its molar quantity, so if ![]() of the gas in the sample is water vapor, then
of the gas in the sample is water vapor, then ![]() of the total pressure will be due to water vapor. So the partial pressure of water vapor is
(780) = 65 mmHg.
of the total pressure will be due to water vapor. So the partial pressure of water vapor is
(780) = 65 mmHg.
The gases are at equilibrium, so the partial pressure of the water vapor will be the same as the vapor pressure of the water.
23. D Backsolve. Start with (C). If there are 5 H+, there can’t be a whole number coefficient for H2O, so (C) is wrong. You should also be able to see that the answer can’t be an odd number, so (A) is also wrong.
Try (D).
If there are 8 H+, then there are 4 H2O.
If there are 4 H2O, then there is 1 MnO4–.
If there is 1 MnO4–, then there is 1 Mn2+.
Mn7+ (in MnO4–) is reduced to Mn2+, so there are 5 e–.
These are the lowest whole number coefficients, so (D) is correct.
24. D Vapor pressure increases with increasing temperature. Water boils when its vapor pressure is equal to the atmospheric pressure. So if the atmospheric pressure is lowered, then water will boil at a lower temperature.
25. C You can find the number of moles of C, H, and O.
Moles = ![]()
Moles of carbon = ![]() = 4 moles
= 4 moles
Moles of hydrogen = ![]() = 8 moles
= 8 moles
Moles of oxygen = ![]() = 4 moles
= 4 moles
The student had 2 moles of the compound, so 1 mole would contain half as much stuff. That’s 2 moles of C, 4 moles of H, and 2 moles of O. That corresponds to CH3COOH, acetic acid. By the way, choice (A), CH2O, is wrong because it’s the empirical formula, not the molecular formula.
26. E The equations given on top give the heats of formation of all the reactants and products (remember, the heat of formation of O2, an element in its most stable form, is zero).
∆H°reaction = ∆H°products – ∆H°reactants
First, the products.
From SO3, we get 2y. That’s it for the products.
Now the reactants.
From SO2, we get 2x. The heat of formation of O2 is defined to be zero, so that’s it for the reactants.
∆H° for the reaction = 2y – 2x
27. A Remember: moles = (molarity)(volume)
The number of moles of HNO3 will remain constant during the dilution.
Moles of HNO3 = (0.60 M)(50.0 ml) = 30 millimoles
Now we can find how much water it will take to create a 0.40 M solution with 30 millimoles of HNO3.
![]()
But 75 ml isn’t the answer. We started with 50 ml of solution and ended up with 75 ml of solution, so we must have added 25 ml of water.
28. B According to Le Châtelier’s law, the equilibrium will shift to counteract any stress that is placed on it.
If the volume is decreased, the equilibrium must shift toward the side with fewer moles of gas. Only choice (B) has fewer moles of gas on the product side (2 moles) than on the reactant side
(3 moles).
29. A Just enough NaOH has been added to neutralize all of the H3C6H5O7 in the reaction shown below.
H3C6H5O7(aq) → H+(aq) + H2C6H5O7–(aq)
So the H3C6H5O7(aq) is gone, and the
H2C6H5O7–(aq) will be present in the greatest concentration because it won’t dissociate much further.
30. B We don’t know how much solution we have, so we can’t find out (I), the mass of the solute, or (III), the volume of the solution.
We can get (II), the molality, from the boiling point elevation with the expression ∆T = kbmx. Because the substance is nonionic, it will not dissociate and x will be equal to 1, so we can leave it out of the calculation.
![]()
31. A From the relationship
∆G° = –RT ln K, we can see that if K is greater than 1, then lnK must be greater than 1, which means that ∆G° must be less than zero.
32. C The formula for boiling-point elevation is ∆T = kbmx, where x is the number of particles into which the solute dissociates. So, the more particles into which the solute dissociates, the greater the boiling point elevation.
The salts in all the answer choices except (C) dissociate into two particles. For choice (C), each Na2SO4 dissociates into three particles, two Na+ and one SO42–.
33.
C Moles = ![]()
Moles of ![]() = 6 moles
= 6 moles
So if 1 mole of hydrate contains 6 moles of H2O, then its formula must be Fe2O3 6 H2O.
34. D In choice (D), the number of moles goes from 1 to 2, and the phase changes from solid to gas, making this a pretty big entropy change. Entropy is also increasing in (A) and (C), but in both cases, the number of moles stays the same and the phase change is only from liquid to gas. The entropy decreases in (B) and stays about the same in (E).
35. B Remember the definition of density.
Density = ![]()
The mass of water doesn’t change as temperature increases, so if the density is decreasing, that must mean that the volume is increasing. Now look at the definition of molarity.
Molarity = ![]()
If the volume is increasing, then the molarity must be decreasing.
Molality is moles per kiligram. Since neither of these is changing, molality is unchanged.
36. D Adding up the atomic numbers in the products we get: 2 + 3 = 5. So the reactant must be boron.
Adding up the atomic masses and keeping in mind that the neutron has a mass of 1, we get
y + 1 = 7 + 4.
So the atomic mass must be 10.
37. D The fact that boiling water maintains a constant temperature of 100°C is useful when a relatively low constant temperature is required. None of the other choices is a true statement.
38. A The addition of a catalyst lowers the activation energy of a reaction, making it easier for the reaction to proceed, so (I) is correct.
Adding a catalyst has no effect on the enthalpy change or equilibrium conditions of a reaction, so (II) and (III) are wrong.
39. B The rate-limiting step in a reaction is the slowest step in the process.
When a reaction occurs slowly, it is because very few collisions among reactant molecules have enough energy to overcome the high activation energy.
40. D Backsolve. Start at (C).
Instead of using 1 as the coefficient for C3H7OH and ![]() for O2, use 2 for C3H7OH and 7 for O2. This won’t change your result, and it will make the math easier.
for O2, use 2 for C3H7OH and 7 for O2. This won’t change your result, and it will make the math easier.
If there are 2 moles of C3H7OH, then there must be 6 moles of CO2 and 8 moles of H2O.
That gives us 16 O’s in the reactants and 20 O’s in the products. So (C) is wrong and we should pick a larger number to put more O’s on the reactant side. Try (D).
There are still 2 moles of C3H7OH, so there must still be 6 moles of CO2 and 8 moles of H2O. Now there are 9 moles of O2. Now we have 20 O’s in the reactants and 20 O’s in the products, so (D) is the correct answer.
41. B The phase change will occur as shown in the diagram below.
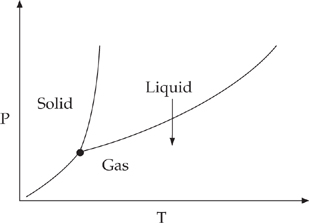
A phase change from liquid to gas is vaporization.
42. E At this point, called the triple point, all the phase change lines converge and all three phases are in equilibrium.
43. C The Heisenberg uncertainty principle states that both the momentum and location of an electron can never be known with absolute certainty. It was Einstein who described the equivalence of mass and energy.
44. C The reaction rate is NOT constant over time, so this is the statement that is not true. In fact, the reaction rate is gradually decreasing as the concentration of reactant A decreases. All of the other statements are true. You can see from the chart that the half-life of A is 20 minutes. The other three statements must be true based on the fact that [A] is decreasing exponentially.
45. B The correct procedures listed here are designed to prevent spattering and spilling—choices (A) and (D)—or contamination of the solution in the bottle—choices (C) and (E). Inserting a pipette directly into the bottle could contaminate the solution if the pipette isn’t perfectly clean.
46. A According to Le Châtelier’s law, the equilibrium will shift to counteract any stress that is placed on it.
If N2 is added, the equilibrium will shift to remove the excess N2. This shift produces more products, and thus, more NH3. So (I) is correct.
Choice (II) is wrong because increasing the volume will cause the reaction to shift toward the side with more moles of gas. In this case the reactant side has more moles of gas (4 moles) than the product side (2 moles). So increasing the volume will decrease the number of moles of NH3.
Choice (III) is wrong because increasing the temperature will favor the endothermic direction, which in this case is the reverse reaction. So once again, the number of moles of NH3 is decreased.
47.
E Moles = ![]()
Moles of CH4 = ![]() = 1 mole
= 1 mole
Moles of O2 = ![]() = 0.5 moles
= 0.5 moles
From the balanced equation, 2 moles of O2 are used up for every mole of CH4.
When all 0.5 moles of O2 are used up, only 0.25 moles of CH4 will be used up, so oxygen is the limiting reagant.
Choices (A), (B), and (C) are wrong because more moles of H2O will be formed and a greater mass of CO2 will be formed.
48.
B From Graham’s law, the rate of effusion of a gas depends on its molecular weight. The larger the molecular weight, the slower the rate of effusion.
For separation of gases by effusion to work, the gases must have different molecular weights, which will cause them to effuse at different rates. N2 and C2H4 have the same molecular weight (28 g/mol), so they can’t be
separated by effusion.
49. A The hydrolysis of the CN– ion is shown by the reaction below.
CN– + H2O ![]() HCN + OH–
HCN + OH–
Putting products over the reactants in the equilibrium expression and omitting water because it is a pure liquid, we get
K = ![]()
50. E For a spontaneous reaction, ∆G is always negative.
From the equation ∆G = ∆H – T ∆S, we can see that the conditions that will make ∆G always negative are when ∆H is negative and ∆S is positive.
51. C Isomers are different molecules that have the same collection of atoms arranged in different ways. Both of the molecules in choice (C) have 2 carbons, 6 hydrogens, and 1 oxygen.
52. C From the ideal gas laws, we know that with volume constant
![]()
Solving for P2, we get
![]()
53.
C An increase in temperature always increases the rate of a reaction, regardless of the change in enthalpy of the reaction.
Temperature change and enthalpy come into play in establishing whether reactants or products are favored at equilibrium according to Le Châtelier’s law, but increasing the temperature will bring the reaction to equilibrium more quickly, regardless of whether the equilibrium favors reactants or products.
54. E Use the formula for Ka.
![]()
We’re trying to figure out [H+]. Since for every HClO that dissociates, you get 1 H+ and 1 ClO–, we can rewrite the expression with
[H+] = [ClO–] = x.
![]()
x2 = (0.12)(3.0 × 10–8) = 0.36 × 10–8
x = 0.60 × 10–4 = 6.0 × 10–5
So the hydrogen ion concentration is 6.0 × 10 –5
M.
55.
D
For every mole of N2 that appears, 2 moles of H2 must disappear, so N2 appears at half the rate that H2 disappears, and (I) is wrong.
For every 2 moles of H2O that appear, 2 moles of NO must disappear, so H2O appears at the same rate that NO disappears, and (II) is correct.
For every 2 moles of NO that
disappear, 2 moles of H2 must disappear, so NO disappears at the same rate that H2 disappears, and (III) is correct.
56. D All of these polyatomic ions have 32 valence electrons distributed in the Lewis dot structure shown below for ClO4–.

In these polyatomic ions, the central atom forms sp3 hybrid orbitals, which have a tetrahedral structure. There are no unshared electron pairs on the central atom, so the geometry is tetrahedral.
57.
D Moles = ![]()
Moles of ZnO = ![]()
For every 2 moles of ZnO produced, 3 moles of O2 are consumed.
So moles of O2 = ![]() (moles of ZnO)
(moles of ZnO)
= ![]()
At STP, volume of gas = (moles)(22.4 L)
So volume of O2 = ![]()
58. D Zn2+ and SO42– are both colorless in solution.
About the other answers
(A) Cu2+ ions are blue in solution.
(B) Ni2+ ions are green in solution.
(C) MnO4– ions are purple in solution.
(E) Fe3+ ions are yellow in solution.
Most salts of transition metals produce colored solutions. That’s because energy is released and absorbed when d subshell electrons change energy levels. This energy is manifested as visible light.
Zn2+ does not produce a colored solution because it has a full 3d subshell. The full subshell means that there are no empty orbitals for the d electrons to jump to. If there are no transitions, there is no light.
59. B The Pb2+ ions and the Cl– ions will combine and precipitate out of the solution, so let’s find out how many of each we have. Remember that each mole of MgCl2 produces 2 moles of Cl–.
Moles = (molarity)(volume)
Moles of Pb2+ = (0.20 M)(0.300 L) = 0.060 mole
Moles of Cl– = (2)(0.20 M)(0.200 L) = 0.080 mole
Since two Cl– ions are required for each Pb2+ ion, subtract half the moles of Cl– (0.040 mole) from the moles of Pb2+ to find the number of moles of Pb2+ left in the solution
0.060 mol – 0.040 mol = 0.020 mol
Now use the formula for molarity to find the concentration of Pb2+ ions. Don’t forget to add the volumes of the two solutions.
Molarity = ![]() = 0.040 M
= 0.040 M
60. C A buffered solution can be prepared by mixing a weak acid or base with an equal amount of its conjugate.
In (I), equal amounts of NH3 (a weak base) and NH4+ (its conjugate acid) are mixed, so (I) creates a buffer.
In (II), equal amounts of H2CO3 (a weak acid) and HCO3– (its conjugate base) are mixed, so (II) also creates a buffer.
In (III), equal amounts of a weak acid and base that are not conjugates are mixed. These two will neutralize each other and will not create a buffered solution.
61. B The reaction will proceed in the reverse direction and produce more H2 when the reaction quotient Q is greater than Kc.
Q takes the form of the equilibrium constant.
![]()
For I: Q = ![]() = 25 Q is less than Kc.
= 25 Q is less than Kc.
For II: Q = ![]() = 100 Q is greater than Kc.
= 100 Q is greater than Kc.
For III: Q = ![]() = 25 Q is less than Kc.
= 25 Q is less than Kc.
So only (II) will produce more H2.
62. B Let’s look at the Nernst equation, which relates cell potential to concentration.
E = E°
![]() logQ at 25°C
logQ at 25°C
The smaller Q becomes, the larger E will become.
Remember, Q is the reaction quotient, which takes the form of Keq, except with initial conditions instead of equilibrium conditions.
In this case ![]() , so increasing [Cu2+]
, so increasing [Cu2+]
decreases Q, which increases E. So (II) is right and (III) is wrong.
The amount of solid has no effect, so (I) is wrong. You can also use Le Châtelier’s law for this one.
63. C The number of nucleons doesn’t change in beta decay, so the mass number must remain at 61.
In beta decay, a neutron is converted to a proton, so the atomic number increases by one.
The balanced nuclear reaction is as follows:
![]()
64.
B Sodium’s valence electron is in the third energy level and potassium’s valence electron is in the fourth energy level, so sodium’s valence electron is closer to the nucleus than potassium’s, so sodium must have a smaller atomic radius.
Also, because sodium’s valence electron is closer to the nucleus, it is more difficult to remove, so sodium will have a higher first ionization energy.
65. A First we have to find the limiting reagent.
Moles = (molarity)(liters)
Moles of HCl = (0.20 M)(0.50 L) = 0.10 moles
Moles of AgNO3 = (0.40 M)(0.50 L) = 0.20 moles
From the balanced equation, the two reactants are used up at equal rates. There is twice as much AgNO3, so when the 0.10 moles of HCl have been used up, there will still be 0.10 moles of AgNO3. So HCl is the limiting reagent.
From the balanced equation, for every mole of HCl consumed, 1 mole of AgCl is produced. So 0.10 moles of AgCl will be produced.
Grams = (moles)(MW)
Grams of AgCl = (0.10 moles)(143 g/mol) = 14 grams
66. D The bond energy is the energy that must be put into a bond to break it.
First let’s figure out how much energy must be put in to the reactants to break their bonds.
To break 1 mole of H–H bonds, it takes 440 kJ.
To break 1 mole of Cl–Cl bonds, it takes 240 kJ.
So to break up the reactants, it takes +680 kJ.
Energy is given off when a bond is formed; that’s the negative of the bond energy.
Now let’s see how much energy is given off when 2 moles of HCl are formed.
2 moles of HCl molecules contain 2 moles of HCl bonds, so (2)(–430) kJ = –860 kJ are given off.
So the value of ∆ H for the reaction is
(–860, E given off) + (680, E put in) = –180 kJ.
67. B Magnesium has two valence electrons in the third shell, so we would expect to see a small jump between the first and second ionization energies.
The third electron must be removed from the second shell, so we would expect to see a much larger jump between the second and third ionization energies.
Choice (B) is the only answer that shows this relationship.
68. E First let’s find out how many moles of electrons we need.
The half-reaction that reduces Na+ to Na(s) is as follows:
Na+ + e– → Na(s)
So it takes 1 mole of electrons to produce 1 mole of Na(s).
Now let’s find out how many coulombs we need.
Moles of electrons = ![]()
So coulombs = (moles of electrons)(96,500) = (1)(96,500) = 96,500 coulombs.
Now we can find how many seconds it takes
Amperes = ![]()
So seconds = ![]() = 96,500 seconds
= 96,500 seconds
69. A For every NaCl in solution, there’s one Cl– ion; and for every KCl we add, we get one Cl– ion.
Let’s find out how many moles of Cl– ions are already in the solution.
Moles = (molarity)(volume)
Moles of Cl– = (0.5 M)(0.2 L) = 0.1 moles
We’re not changing the volume, so to double the concentration of Cl– ions from 0.5-molar to
1.0-molar, we just double the number of moles of Cl– ions. We do that by adding 0.1 moles of KCl.
70. B We can think of the reaction given in the question as the sum of two other reactions.
BaC2O4
![]() Ba2+ + C2O42–
K1 = 2 × 10–7
Ba2+ + C2O42–
K1 = 2 × 10–7
Ni2+ + C2O42–
![]() NiC2O4
K2 =
NiC2O4
K2 = ![]()
Notice that we are using the reverse reaction for the solvation of NiC2O4, so the reactants and products are reversed, and we must take the reciprocal of the solubility product.
When reactions can be added to get another reaction, their equilibrium constants can be multiplied to get the equilibrium constant of the resulting reaction.

71.
D Because they have the same mass number, the mass of ![]() will accumulate at the same rate that the mass of
will accumulate at the same rate that the mass of ![]() disappears.
disappears.
We’re looking for the moment when 10 grams of ![]() remains.
remains.
Make a chart. Start at time = 0.
| Half-Lives | Time | Stuff |
| 0 | 0 days | 100 g |
| 1 | 35 days | 50 g |
| 2 | 70 days | 25 g |
| 3 | 105 days | 12.5 g |
| 4 | 140 days | 6.25 g |
It takes between 3 and 4 half-lives for the amount of ![]() to decrease to 10 grams.
to decrease to 10 grams.
116 days is the only answer choice between 105 days and 140 days.
72. E The solubility of a substance is equal to its maximum concentration in solution.
For every CaF2 in solution, we get one Ca2+ and two F–, so the solubility of CaF2 (let’s call it x) will be the same as [Ca2+].
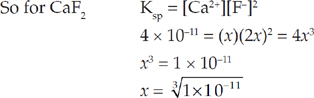
73.
B Moles = ![]()
Moles of Ca(OH)2 = ![]() = 0.10 moles
= 0.10 moles
One mole of CaCl2 must have been consumed for every mole of Ca(OH)2 produced, so there must have been 0.10 moles of CaCl2 in the original solution.
Molarity = ![]()
Molarity of CaCl2 = ![]() = 0.10-molar
= 0.10-molar
74. D The oxidation and reduction half-reactions are as follows:
Cl– is oxidized: 2 Cl– → Cl2 + 2e–
MnO4– is reduced: Mn7+ + 5e– → Mn2+
So Cl– is oxidized and MnO4– is the oxidizing agent.
75. D We subtract the reduction potential for Cu+ (+0.52 V) from the reaction potential for the full reaction (+0.92 V) to get +0.40 V, the oxidation potential for M(s). Remember, you ignore the coefficients in the reaction when you’re calculating reaction potentials. But you’re not done. You want the reduction potential for M2+(aq), the reverse reaction, so you need to change the sign you got for the oxidation potential. So the answer is –0.40 V.
1. (a) Knowing the pH, we can calculate the pOH.
pH + pOH = 14
2.73 + pOH = 14
pOH = 11.27
Knowing the pOH, we can calculate [OH–]
[OH–] = 10–pOH = 10–11.27 = 5.4 × 10–12
(b) Ka = ![]()
Knowing the pH, we can find [H+]. We also know that every HC2H3O2 molecule that dissociates will put 1 H+ ion and 1 C2H3O2 ion in solution.
x = [H+] = [C2H3O2–] = 10–pH = 10–2.73 = 1.86 × 10–3
[HC2H3O2] = 0.200 – x
x is very small, so [HC2H3O2] = 0.200
Now we can solve for Ka.
![]() = 1.7 × 10–5
= 1.7 × 10–5
(c) We can use the Henderson-Hasselbalch expression to find out what value of [C2H3O2] will create a buffer with a pH of 4.
pH = pKa + log ![]()
pH = pKa + log 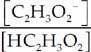
pH = 4.00
pKa = –log(1.73 × 10–5) = 4.76
log 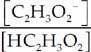 = pH – pKa = 4.00 – 4.76 = –0.76
= pH – pKa = 4.00 – 4.76 = –0.76
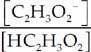 = 10–0.76 = 0.174
= 10–0.76 = 0.174
[HC2H3O2] = 0.200 M
[C2H3O2–] = (0.174)(0.200) = 0.035 M
Moles = (molarity)(volume) = (0.035)(0.500) = 0.018 moles
(d) Because HC2H3O2 dissociates to such a small extent, we can assume that all of the C2H3O2– in the solution came from the NaC2H3O.
Use the base ionization constant for C2H3O2–.
Kb = 
Kb = ![]() = 5.78 × 10–10
= 5.78 × 10–10
At the equivalence point, all of the acetic acid initially present has been converted to acetate ion. So the initial [HC2H3O2] is equal to [C2H3O2] at the equivalence point.
Moles = (molarity)(volume)
Moles of C2H3O2– = (0.400 M)(0.200 L) = 0.080 moles
Molarity = ![]()

[HC2H3O2] = [OH–] = x
Now we can use the Kb equation to find x, the OH– concentration.
We’ll assume that x is much smaller than
0.267 M.
Kb = 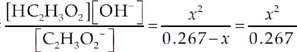
Kb = 5.78 × 10–10 = ![]()
x = [OH–] = 1.24 × 10–5
M
Knowing [OH–], we can calculate pOH, and then pH.
pOH = –log[OH–] = –log(1.24 × 10–5) = 4.91
pH = 14 – pOH
pH = 14 – 4.91 = 9.09
(e) Phenolphthalein, with a pKa of 9, is the best choice. The pH at the equivalence point is about 9, and the indicator should have a pKa that is close to the equivalence point for the titration.
2. (a) From experiments 1 and 2, we can see that when [Cl2] doubles, the rate doubles, so the rate law is first order with respect to Cl2. That is, [Cl2]1.
From experiments 1 and 3, we can see that when [NO] doubles, the rate quadruples, so the rate law is second order with respect to NO. That is, [NO]2.
Rate = k[NO]2[Cl2]
(b) We’ll use experiment 1 for our calculation.
k =  = 180 M–2 min–1
= 180 M–2 min–1
(c) From the balanced equation we can see that for every molecule of Cl2 that disappears, 2 molecules of NOCl appear, so the rate of appearance of NOCl will be twice the rate of disappearance of Cl2.
In experiment 2, the initial rate of disappearance of Cl2 is 1.2 M/min, so the initial rate of appearance of NOCl will be 2.4 M/min.
(d) Use the rate law.
Rate = k[NO]2[Cl2]
Rate = (180 M–2 min–1)(0.25 M)2(0.25 M)
= 2.8 M/min
(e) (i) Use the gas law
P = ![]() = MRT
= MRT
P = (0.15 mol/L)(0.0821L-atm/mol-K)(263K) = 3.2 atm
(ii) Since the initial concentrations of NO and Cl2 are the same for experiment 1, the initial partial pressure due to Cl2 will also be 3.2 atm. From Dalton’s law, the total pressure is equal to the sum of the partial pressures. So 3.2 atm + 3.2 atm = 6.4 atm.
3. (a) ∆H°f° and ∆G°f° for O2(g) are both equal to zero. The enthalpy and free energy of formation of any element in its standard state are equal to zero.
(b) ∆H = Σ∆H°°f (products) – Σ∆H°°f (reactants)
∆H = [(–393.5) + (2)(–285.9)] kJ – [–74.8] kJ
∆H = [–965.3] kJ – [–74.8] kJ
∆H = –890.5 kJ
(c) ∆G° = ∆H° – T ∆S°
∆G° = (–890,500 J) – (298 K)(–242.7 J/K)
∆G° = –818,200 J = –818.2 kJ
(d) ∆S° would become less negative. H2O(g) has more entropy than H2O(l), so the entropy of the products would be increased and the entropy change of the reaction would become more positive (less negative, that is).
(e) (i) First find moles of CH4.
Moles = ![]() =
= ![]() = 1.25 mol
= 1.25 mol
There is a one to one ratio in the balance equation between CH4 and CO2, so 1.25 moles of CO2 are produced.
Now find the grams of CO2.
Grams = (moles)(MW) = (1.25 mol)(44.0 g/mol) = 55 grams
(ii) From (c), the change in enthalpy when one mole of CH4 is consumed is 890.5 kJ. So when 1.25 moles are consumed, ∆H = (890.5 kJ/mol)(1.25 mol) = 1,110 kJ. So about 1,110 kJ were released.
4. (a) (i) SO2 + H2O → H2SO3
(ii) Sulfur dioxide is an acid anhydride, so the hydroxide ion concentration will decrease as the solution becomes more acidic.
(b) (i) 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O
(ii) The enthalpy change for the reaction comes from subtracting the heat of formation of the reactants from the heat of formation of the products, so the heat of formation of the reactants is –3,300 kJ – (–3,100 kJ) = –200 kJ. Because the heat of formation for oxygen gas is zero and there are 2 moles of ethane in the reaction, the heat of formation of ethane is approximately –100 kJ mole.
(c) (i) Cl2 + 2 Br– → 2 Cl– + Br2
(ii) Chlorine gas gains electrons, so it is reduced.
5. (a) Lewis dot structure
for O2 ![]()
Lewis dot structure
for O3 ![]()
(b) O3 is a polar molecule, while O2 is nonpolar, so the dipole–dipole attractions between O3 molecules are stronger than the van der Waals forces between O2 molecules.
If you didn’t recognize the polarity of O3, you may have gotten some partial credit for noting that O3 has more electrons than O2, so O3 will have stronger van der Waals forces between its molecules.
(c) Water molecules are polar. O3 is a polar molecule, while O2 is nonpolar. So water molecules will be more strongly attracted to O3 molecules than they are to the nonpolar O2 molecules.
(d) The bond in O2 is a double bond. Ozone, however, has two resonance forms, each with a single and a double bond, so the two bonds in O3 are each somewhere between a single and a double bond on average. The double bond in O2 is stronger and
shorter than the single/double resonance bonds
in O3.
(e) Oxygen has unpaired electrons in the 2p subshell. This makes oxygen paramagnetic.
(f) The value of the equilibrium constant is very small. This means that the reactants will be far more abundant than the products.
6. (a) (i) The NaC2H3O2 solution will be slightly basic.
The other two solutions will be neutral.
NaC2H3O2 is a salt composed of the conjugate of a strong base and the conjugate of a weak acid, so it will create a basic solution.
Ba(NO3)2 and KCl are salts composed of conjugates of strong acids and bases, so they will create neutral solutions.
(ii) A precipitate will form in the Ba(NO3)2 solution. The other two solutions will show no change.
BaSO4 is insoluble. Na2SO4 and K2SO4 are both soluble.
(iii) The freezing points of the NaC2H3O2 and KCl solutions will be less than 0°C and about the same. The freezing point of the Ba(NO3)2 solution will be lower than the other two.
Freezing-point depression is a colligative property, so it will depend only on the number of particles in solution, not on their identity.
NaC2H3O2 and KCl each dissociate into two ions per molecule, while Ba(NO3)2 dissociates into three ions per unit.
Since the concentrations of all three solutions are the same, the Ba(NO3)2 solution will have the greatest freezing-point depression because it dissociates into the greatest number of particles. By the same reasoning, the freezing points of the NaC2H3O2 and KCl solutions will be about the same.
(iv) Each flame will have a distinct color: Na+ will be yellow, Ba2+ will be green, and K+ will be purple.
(b) (i) CO2, O2, N2, He
All of the gases have the same average kinetic energy, and KE = ![]() mv2, so the larger the molecular weight, the smaller the average velocity. This relationship is given directly by the expression urms =
mv2, so the larger the molecular weight, the smaller the average velocity. This relationship is given directly by the expression urms = ![]() .
.
(ii) Volume increases.
PV = nRT. From the ideal gas law, we can see that when T increases and P and n are held constant, V must increase to maintain equality.
(iii) Decreased temperature and increased pressure would eventually cause deviation from ideal behavior. Carbon dioxide would be most affected.
Deviation from ideal behavior occurs when gas molecules are brought very close together. When gas molecules are packed close together, the weak attractive forces between them become important. Also, in this situation, the volumes occupied by the individual gas molecules can no longer be ignored. Gas molecules are brought close together by low temperatures and high pressures.
Carbon dioxide would be the most affected of the four gases because it is the largest molecule and it has the most electrons. Because of this, the van der Waals forces among CO2 molecules will be stronger than for the other gases.