2 Molecular Breeding
New discoveries in gene splicing made during the 1970s evolved into research applications in plant genetic engineering in the 1980s and eventually to the commercialization of genetically engineered (GE) crops in the 1990s. With the development of libraries of restriction enzymes and DNA ligases, scientists had the basic tools for cutting and splicing DNA sequences from any biological source. The primary methods for molecular breeding1—that is, the creation of plant varieties by introducing into plant cells DNA from foreign sources by laboratory procedures involving recombinant DNA molecule technology—were now in place.
A six-stage process for molecular breeding was widely adopted:
Stage 1: The first stage involves identifying and isolating the foreign gene for transport into a crop, and it can be a long process. The purpose of transplanting a gene from another species into the plant is to endow the plant with a useful phenotype for agriculture, which can include greater efficiency in production, processing, or storage; higher nutritional content; and resistance to environmental threats (such as salt, weeds, and insects). With the development of recombinant DNA techniques, the gene can come from any organism in any species from any biological kingdom. The most popular genes transplanted into food crops have come from bacteria, viruses, or other plants.
Stage 2: In the second stage, the foreign gene is attached to other DNA segments to prepare a transgene complex with components that provide specific instructions to the host cell.
Stage 3: The third stage consists of preparing the host plant cell or tissue to receive the transgene complex in such a way that it can regenerate into whole plants. Generally, this involves removing a tissue piece from a plant, using it to prepare cell cultures, and maintaining those cultures under appropriate hormonal and nutrient conditions.
Stage 4: The fourth stage consists of selecting a delivery system for the transgene complex and its method of transfer into the host cell.
Stage 5: The fifth stage involves selecting the transgenic cells—that is, those cells that have taken up the foreign DNA from other nontransgenic cells within the cell culture and their regeneration into whole plants.
Stage 6: In the sixth and final stage, just prior to the introduction of the GMO into the marketplace, the transgenic plants are evaluated for the desired phenotype with a heritable genetic change and are examined for other characteristics, such as nontoxicity, nonallergenicity, and nutritional equivalence to the parental crop.
In stage 1, the molecular breeder identifies and selects a foreign gene for transfer into the host plant cells. Scientists choose a DNA sequence that encodes a protein from a biological source and that they want to see expressed in the crop plant. It could be a bacterial gene that would afford the plant with a trait like insect or herbicide resistance or a viral gene that would make the plant disease resistant. Other genes investigated include those that would endow the plant with abiotic traits like salt or stress tolerance. The genetically engineered crops that are designed to improve agricultural production by adding traits like insect resistance are termed “first-generation GMOs.” In the first few decades of commercial applications of molecular breeding, scientists focused almost entirely on altering traits that were deemed useful for agricultural production.
After the gene is selected from another organism and the required DNA sequence is isolated (by restriction enzymes), it can be produced in large quantities by polymerase chain reaction (PCR). I use the term transgene to mean the foreign gene that is isolated for transfer to a new biological system.
Stage 2 is the construction of the transgene cassette. After the gene is isolated, it must be attached to several other DNA sequences that make up what is called a transgene cassette. In other words, the transgene is not transferred by itself. Other sequences are added that play important roles in getting the gene to function in its new environment. One of the DNA components added to the transgene is the promoter sequence, which is attached to the desired gene (figure 2.1). It provides the switching (on and off) mechanism to activate and regulate gene expression in the gene’s new environment. A commonly used promoter, known as CAMV35S, comes from the cauliflower mosaic virus. It affords a high degree of expression to the foreign genes transplanted into the plant cells and enables gene activity throughout the life cycle of the plant in most tissues. Thus, a gene for an insecticidal protein will, in all likelihood, be expressed at all stages in the life cycle of the plant.
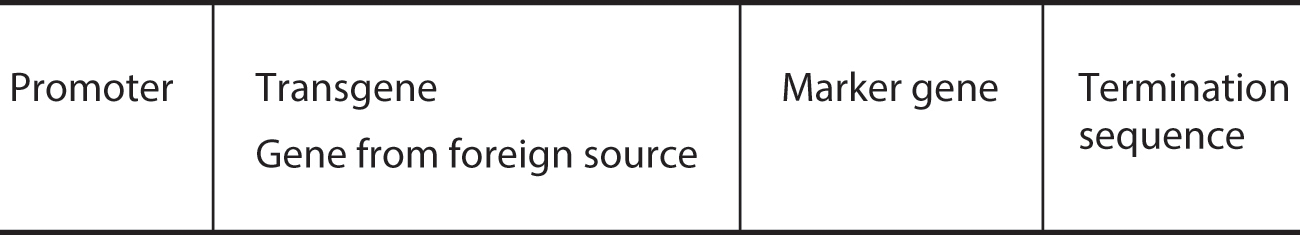
After a gene has been isolated and cloned (amplified in a bacterial vector), it must undergo several modifications before it can be effectively inserted into a plant. This simplified representation of a constructed transgene cassette contains the necessary components for successful integration and expression. The marker gene also has its own promoter and terminator.
Source: http://
A second component in the transgene cassette is the termination sequence (also called a stop codon), which signals to the cellular machinery that the end of the DNA sequence has been reached. The termination codon is a nucleotide triplet that signals a termination of translation into proteins.
A third component in the transgene cassette is the marker gene. Its purpose is to provide the molecular breeder with a mechanism to select the plant cells that have successfully incorporated the transgene cassette. This represents stage 5 in the six-stage process. The marker is a DNA sequence that protects the transgenic plant cells from being destroyed by some external agent that is toxic to the cells without the marker gene. Commonly used markers include genes for herbicide resistance or antibiotic resistance. In the former case, after the transgene cassette has been transmitted to the cells, the cell culture is exposed to an herbicide. Only those cells with the herbicide-resistant marker will be viable. Where antibiotic resistance markers are used, the cells targeted for transformation are exposed to antibiotics, destroying the cells that do not possess the selection marker gene and leaving only the transgenic cells.
In stage 3, plant cells or tissues selected for receiving the transgene cassette are incubated in a medium known as tissue culture. The challenge for plant geneticists is to find the delivery systems to transport foreign genes into the cells or embryos of plants and then to ensure that the transported genes function effectively in their new host plant environment.
For stage 4, two classes of DNA delivery systems, sometimes referred to as indirect and direct, are generally used. Biology-based gene delivery systems, also called vectors, are bacterial plasmids and viruses that naturally carry DNA into plant cells. Although plants have evolved resistance to many types of infectious agents,2 some viral or bacterial agents are invasive to certain plants. They enter the cells of the plant, use its biological machinery to replicate, and sometimes inflict damage to their host. When these vectors carry the transgenes, they are referred to as indirect delivery systems.
There are also nonbiological systems that use chemical and physical processes for transporting foreign DNA directly into plants (direct delivery systems). These systems do not depend on whether the organisms that provide the source of DNA are reproductively compatible (can naturally or even artificially reproduce or hybridize with the recipient organism). The ability of biotechnology to overcome sexual incompatibility, organismal barriers to hybridization, or grafting barriers between two species is what can be termed molecular breeding.
The most widely used biology-based genetic transformation system for plants is Agrobacterium tumefaciens (A. tumefaciens), a soil-dwelling bacterium that infects certain plants and causes crown gall disease. A. tumefaciens, like all bacteria, contains a chromosome that includes most of its genes and additionally a unique plasmid (the Ti plasmid), which is a circular ring of DNA with a smaller number of genes. The Ti plasmid, named for its tumor-inducing properties, contains vir genes and T-DNA (for transferred DNA). The function of the vir genes is to code for proteins that open a channel that allows the T-DNA segment of the Ti plasmid to pass into and infect plant cells. When A. tumefaciens infects plant cells, it deposits the T-DNA segment of its plasmid into the chromosome of the host plant. These take over the machinery of the cell to synthesize sugars that feed the bacteria.
A. tumefaciens cannot enter a healthy plant. But if a plant stem or root is mechanically damaged or biologically wounded, it emits a chemical signal that is picked up by the vir genes of the bacterium. The vir genes activate a series of events resulting in the transfer of the T-DNA sequence from the Ti plasmid to the plant’s chromosome. This remarkable signaling system is exquisitely tuned to the highly evolved bacterial symbiont. The T-DNA sequence of A. tumefaciens initiates a tumor in the plant called crown gall tumorigenesis.3
The discovery of how A. tumefaciens infects plants and transports the T-DNA segment causing crown gall disease was made in 1977 by Mary Chilton and colleagues. After the mechanism of disease transfer was understood and scientists saw that A. tumefaciens can transport its DNA segment into plants, they wondered whether replacing the genes on the bacterial plasmid with ones of their own choosing would allow the bacterium to transport foreign DNA into plant cells. When scientists replaced the genes, they removed the tumor-inducing gene from the Ti plasmid so that it would not cause disease in the plant.
To transport genes (the coding DNA) via the A. tumefaciens bacterial vector, scientists have to go through several steps. First, the target gene for transplantation (the transgene) has to be isolated. Second, a construct has to be developed consisting of four components—a promoter, the coding DNA or transgene itself, a terminator sequence, and a marker gene (as previously described).
The promoter DNA is necessary to activate the correct level of expression of the transgene in the cell. It is able to turn the gene on or off. As mentioned earlier, the most widely used promoter is a DNA sequence from the cauliflower mosaic virus called CaMV35S. It induces a high level of expression for the transgene and is active in almost all circumstances in the cell without stimuli,4 but there are exceptions where the promoter does not work as advertised.5 Finally, the termination sequence signals to the cellular machinery that the end of the gene has been reached.
The most common nonbiological mechanism used to transport foreign genes into plant cells is a projectile system referred to as a gene gun. Tiny gold or tungsten particles that have been coated with transgenic DNA cassette are propelled through the walls of the plant cells into the plant’s chromosomes. The gene gun process is also known as microprojectile bombardment or biolistics. Typically, the projectiles are directed at cell cultures or immature embryos. Particle bombardment was first discussed for transgenic plants in 1987.6
Other direct methods used in transplanting foreign genes include electroporation, which involves the application of a strong electric field across cells and tissue. The electric field makes the cell membrane porous so that purified DNA can be taken up by the cell. Various other methods for opening up the cell wall have been reported in the literature. In silicon carbide mediated transformation (SCMT), silicon carbide fibers puncture the cell walls, allowing DNA to enter. The treatment of plant protoplasts with polyethylene glycol (PEG) also allows foreign DNA to enter the plant cells. And pollen tube pathway (PTP) is another important delivery system for transferring naked DNA into plant ovaries. Although several systems for integrating foreign DNA into the germ plasm of plants have been tried, Agrobacterium tumefaciens and biolistics are the most efficient and widely used systems.7
Molecular breeding, as described here, does not occur in nature. The transgene complexes with promoters, markers, and termination sequences are produced in a laboratory and transferred into plant cells by biological or mechanical vectors. As sophisticated as these breeding methods appear to be, they rarely produce the desired trait in normal plants. It takes many tries. As noted by Jonathan R. Latham, Allison K. Wilson, and Ricarda A. Steinbrecher, “even after selection, there are many reports of apparently normal transgenic plants exhibiting aberrant behavioural or biochemical characteristics … [ranging from] altered nutrient or toxin levels to lower yields.”8 It is likely that transformation—that is, induced mutations or damage to host plant DNA caused by DNA introduction—are behind many of the unanticipated effects of molecular breeding. (Chapter 8 looks at whether unanticipated effects are more or less likely or more or less dangerous in molecular breeding than in traditional breeding.) Given the high rate of untoward effects, it is not surprising that scientists continue to search for more efficient and dependable methods of breeding crops that will reduce the proportion of unanticipated outcomes.
Chapters 8 and 9 will address stage 6, the final stage, where the transgenic crop is evaluated for its agronomic properties and its health and environmental safety.
New Developments in Molecular Breeding
A second wave of biotechnology emerged within ten years after the first commercial products were introduced by the transgenic methods previously described. By 2015, the European Commission was considering seven new genetic engineering techniques to determine whether they would be covered by existing European Union laws and whether they would be classified under the definition of GMO.
The second generation of molecular plant breeding techniques has been said to have one major benefit over transgenesis: they are more highly targeted. But they remain different from both conventional breeding and the first generation of agricultural biotechnology. The new plant breeding techniques are said to be designed “to produce improved crop varieties that are difficult to obtain through traditional breeding methods … but the resulting end products do not contain any foreign genes.”9 This view suggests that the new techniques only edit existing genes in the plant cells; that if they transfer a transgene, they are able to ensure that no other foreign genes are introduced; and that if foreign genes are introduced, they can be bred out. Five of these techniques in molecular breeding include gene editing, oligonucleotide-directed mutagenesis (ODM), cisgenesis and intragenesis, RNA-dependent DNA methylation (RdDM), and synthetic DNA.
Gene Editing
Methods classified as gene editing afford plant breeders the ability to alter the sequence of DNA in a plant cell at predetermined sites in the genome. The method can make small or large insertions or deletions. Two techniques under the category of gene editing are zinc finger nuclease technology and CRISPR/Cas9.
Zinc finger nucleases (ZFNs) are a class of genetically engineered DNA-binding proteins, which are used in the targeted editing of an organism’s genome. The ZFNs contain multiple finger-like protrusions that make contact with their target molecule. ZFNs can create breaks in DNA at a specified location. They have been called custom-designed molecular scissors.
Currently, the most widely used gene editing method is called CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats). Originally discovered in bacteria, which use CRISPR to protect themselves from invading viruses, the DNA-RNA complex consists of DNA repeats, proteins, and RNA molecules that can be tailored to recognize and edit a DNA site in an organism. The bacterial system was the model that was adapted for eukaryotic cells. Thus, CRISPR provides genetically engineered site-specific nucleases that can insert, delete, or replace DNA sequences. It has become an increasingly popular method for gene deletion and gene stacking for transgenic or cisgenic breeding.10 The optimism that CRISPR/Cas9 or its close relatives will be the future of plant breeding is expressed in this prediction in Frontiers in Plant Science: “The CRISPR-Cas9 holds a very promising future in making designer plants by taking only the gene of interest from a wild type species and the gene is then directly interpolated at a precise location, which in turn opens up many avenues for plant breeders for making designer plants.”11 In other words, CRISPR was adapted from its bacterial origins, which protected the bacteria from invading viruses to a form that both edits and replaces genes.
Oligonucleotide-Directed Mutagenesis (ODM)
Oligonucleotide-directed mutagenesis (ODM) is also called site-directed mutagenesis or site-specific mutagenesis.12 First, scientists identify a target gene, and then they construct a short DNA primer of single-stranded nucleic acid, almost identical to the target gene except for one to four nucleotides (which is the mutation). The synthetic primer is introduced into the plant cell and creates a mismatch when it binds to the target gene. The cell, into which ODM is introduced, sometimes preserves the sequence of the introduced oligonucleotide that is replacing the original sequence. Plant cells that have had the ODM introduced are sequenced to determine whether they contain the desired mutation. This type of mutagenesis is considered far more specific and precise than mutagenesis involving radiation or chemicals targeted to plant cells.13
Cisgenesis and Intragenesis
Cisgenesis and intragenesis are variants of transgenic methods, but these techniques differ in the source of the genes used for transfer. Cisgenesis describes the transfer of alleles or genes from a crossable species into a recipient plant cell.14 The transferred cisgenes, as they are called, code for a trait from the targeted crop species or from a sexually compatible donor. Because the plants are sexually compatible, the gene or alleles transferred are part of the conventional breeder’s gene pool. Cisgenesis, which also maintains the regulatory sequences that lie adjacent to it in the donor crop, is a faster method of doing traditional breeding than making many crosses between sexually compatible plants. With intragenesis, the inserted sequences (alleles or genes), including the promoters and the termination sequences, may come from one or more closely related species. This method uses a hybrid DNA complex for transfer to a host, albeit from sexually compatible plants.
RNA-Dependent DNA Methylation (RdDM)
RNA-dependent DNA methylation (RdDM) is a method for changing the trait of a plant without altering its DNA. This form of epigenetics falls into the general category of RNA interference. Small double-stranded RNA molecules can silence a specific gene by closing the switch that allows the gene to express its protein product and its function. The RNA molecules direct the cell to add methyl groups to specific nucleotides along a DNA sequence. The methyl groups can silence (switch off) a gene. Certain traits (such as delayed fruit ripening, enhanced nutrients, or reduced toxins) can be produced or eliminated with RdDM. The question remains of whether altering methylation is heritable. If not, then it would not be breeding by any conventional meaning.
Synthetic DNA
Synthetic DNA is created in a laboratory and transplanted into plant cells. It is sometimes called xenogenic. These DNA sequences cannot be found in any living organism, so conventional breeding could never be used to acquire the genetic transfer.
Proposals for Classifying Techniques
When the new-generation molecular breeding techniques were introduced, Kaare M. Nielsen, a Norwegian professor of medicine, noted a conceptual confusion in the classification of the existing techniques.15 He believed that the proper way to distinguish transgenic techniques was based on the genetic distance of the DNA that could be transferred between species to plants. To address the confusion in public attitudes and regulations, he proposed five categories for organisms designated as transgenic or genetically modified—intragenic (transfers among plants in the same species), famigenic (transfers within species of the same family), linegenic (transfers among species in the same lineage), transgenic (transfers among unrelated species), and xenogenic (transfers from synthetic-laboratory designed genes and a plant host). Under this classification, only intragenic and famigenic transfers can be accomplished by conventional breeding. Nielsen commented that “Current approaches to gene technology-assisted breeding have been called ‘brute force’ in their use of distantly related genes with little consideration for the multiple evolutionary changes that have occurred in the biochemical networks separating species.”16
Other criteria for distinguishing GMOs from non-GMOs have also been cited in the literature. Felix Walter and Holga Puchta argue that a GMO is a plant that can be discriminated from a natural variant (plants that carry an induced mutation of one or a few changed nucleotides) without a transgene inserted and that a non-GMO is a plant that cannot be discriminated from a natural variant. Classical mutagenesis by chemicals and radiation has thus far been classified under traditional breeding. The authors argue that using CRISPR to create mutational changes would not qualify as a GMO crop.17 However, this view is not universally held and would not be considered a way to circumvent GMO regulatory oversight.
The next chapter examines viewpoints about the differences and similarities between traditional and molecular breeding. Of particular concern is whether molecular breeding requires greater attention to untoward plant products and whether methods of breeding should be considered in the evaluation of risks.
Notes
1. I use the term molecular breeding to mean the application of molecular biology tools in plant and animal breeding.
2. Yan Zhang, Thomas Lubberstedt, and Mingliang Xu, “The Genetic and Molecular Basis of Plant Resistance to Pathogens,” Journal of Genetics and Genomics 40, no. 1 (2013): 23–35.
3. M. D. Chilton, M. H. Drummond, D. J. Merlo, D. Sciaky, A. L. Montoya, M. P. Gordon, and E. W. Nester, “Stable Incorporation of Plasmid DNA into Higher Plant Cells: The Molecular Basis of Crown Gall Tumorigenesis,” Cell 11, no. 2 (1985): 263–271.
4. M. Haas, M. Bureau, A. Geldreich, and P. Keller, “Cauliflower Mosaic Virus: Still in the News,” Molecular Plant Pathology 3, no. 6 (2002): 419–429.
5. Nancy Podevin and Patrick du Jardin, “Possible Consequences of the Overlap between the CAMV35S Promoter Regions in Plant Transformation Vectors Used and the Viral Gene VI in Transgenic Plants,” GM Crops and Food 3, no. 4 (2012): 296–300.
6. John C. Sanford, Theodor M. Klein, Edward D. Wolf, and Nelson Allen, “Delivery of Substances into Cells and Tissues: A Projectile Bombardment Process,” Particulate Science Technology 5, no. 1 (2007): 27–37.
7. Behrooz Darbani, Safar Farjnia, Mahmoud Toorchi, Saeed Zakerbostanabad, Shahin Noeparvar, and C. Neal Stewart Jr., “DNA-Delivery Methods to Produce Transgenic Crops,” Biotechnology 7, no. 3 (2008): 385–402.
8. Jonathan R. Latham, Allison K. Wilson, and Ricarda A. Steinbrecher, “The Mutational Consequences of Plant Transformation,” Journal of Biomedicine and Biotechnology 2006 (2006): 1–7.
9. Jan G. Schaart, Clemens C. M. van de Wiel, Lambertus A. P. Lotz, and Marinus J. M. Smulders, “Opportunities for Products of New Plant Breeding Techniques,” Trends in Plant Science 21, no. 5 (2016): 438.
10. Samriti Sharma, Rajinder Kaur, and Anupama Sing, “Recent Advances in CRISPR/Cas Medicated Genome Editing for Crop Improvement,” Plant Biotechnology Reports 11 (2017): 193–207.
11. Leena Arora and Alka Narula, “Gene Editing and Crop Improvement Using CRISPR-Cas9 System,” Frontiers in Plant Science 8, no. 1932 (2017): 1–21.
12. Schaart et al., “Opportunities for Products.”
13. Noel J. Sauer, Jerry Mozoruk, Ryan B. Miller, Zachary J. Warburg, Keith A. Walker, Peter R. Beetham, Christian R. Schöpke, and Greg F. W. Gocal, “Oligonucleotide-Directed Mutagenesis for Precision Gene Editing,” Plant Biotechnology Journal 14 (2016): 496–502.
14. Hongwei Hou, Neslihan Atlihan, and Zhen-Xiang Lu, “New Biotechnology Enhances the Application of Cisgenesis in Plant Breeding,” Plant Genetics and Genomics 5, no. 389 (2014): 1–5.
15. Kaare M. Nelsen, “Transgenic Organisms: Time for Conceptual Diversification,” Nature Biotechnology 21 (2003): 227–228.
16. Nelsen, “Transgenic Organisms,” 228.
17. Felix Wolter and Holga Puchta, “Knocking Out Consumer Concerns and Regulator’s Rules: Efficient Use of CRISPR/Cas Ribonucleoprotein Complexes for Genome Editing in Cereals,” Genome Biology 18, no 1 (2017): 43–45.