The fundamental concepts behind transcranial magnetic stimulation (TMS) are more than a century old. James Clerk Maxwell’s laws of electromagnetism were codified in the 1860s, and the electrical nature of brain activity was understood by the following decade (Caton, 1875). For many years, however, attempts at noninvasive electromagnetic brain stimulation were frustrated by the need to switch enormous currents on and off at speeds measured in microseconds. The first successful device was built by Anthony Barker at the University of Sheffield in the 1980s (Barker, Jalinous, and Freeston, 1985) and came to be designated as “magnetic” stimulation to distinguish it from older methods involving direct electrical contact, such as electronconvulsive therapy.
The stimulation coil on the head is, in many ways, the central element of the entire TMS system. As the part in closest contact with the subject, its design and construction are fundamental to patient safety. The coil determines the distribution of induced electric currents within the brain, is a core component of the basic excitation circuit, and is the most important determinant of overall stimulator efficiency.
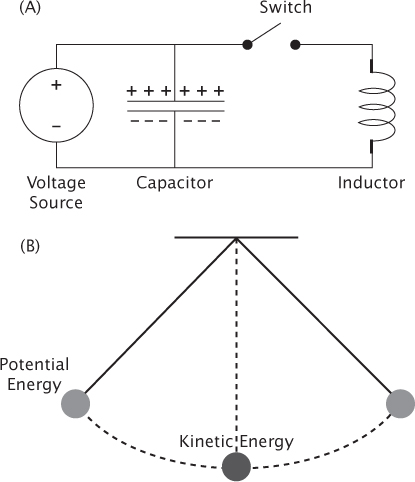
Many magnetic stimulators are based on circuit 1, shown in Figure 2.1A, and include the following components: a source of high-voltage electric current, a capacitor that receives the current and stores energy in the form of electric charge, an induction coil through which that charge will flow to produce a magnetic field, and an electronic switch that allows current to rapidly surge from one to the other.
The combination of capacitor and coil is known to engineers as a resonant circuit and can be thought of as the electrical equivalent of a pendulum (Figure 2.1B). The electric charge stored in the capacitor represents potential energy, just as a pendulum lifted to one side holds the potential energy of the gravitational pull on the weight. When the pendulum is released and swings into a vertical position, all of its potential energy has been transformed into the kinetic energy of the moving weight. As the pendulum swings fully to the other side, the kinetic energy is again converted to potential energy. The idea of kinetic energy in a moving weight is simple and intuitive, whereas the concept of kinetic energy in electric current passing through a coil of wire is less obvious. However, closing the switch in Figure 2.1A does the same thing as releasing the pendulum in Figure 2.1B, that is, the current will flow back and forth indefinitely between the capacitor and the coil until dissipated by friction (which for electricity is termed “resistance”). Both the resonant circuit and the pendulum oscillate at specific frequencies determined by their components. Unlike a pendulum, however, the current in magnetic stimulators is allowed to swing through only a single cycle before the oscillation is terminated. (Instantly stopping thousands of amperes is the tricky part of this particular design, but will not be discussed here.) The result of one full current cycle is one TMS pulse, with the added benefit that most of the current ends up back in the capacitor rather than being lost as heat.
Circuit 2 in Figure 2.2 was more commonly used in the early development of TMS. It looks more complicated than circuit 1 but is easier and cheaper to build. It includes a diode, which diverts current coming out of the coil when the current direction begins to reverse; this is the equivalent of a pendulum beginning to swing in the other direction. The entire current is then dissipated into a large resistor, which has the same effect as putting a brake on the pendulum. This type of circuit is quite wasteful of electricity. The energy cost is tolerable for single pulses or with brief trains of slow, repetitive stimulation. However, for fast, repetitive stimulation, the power requirements and large amounts of heat produced by circuit 2 can easily become unacceptable. Other, more complex but more adjustable driving circuits have been devised; however, these circuits have not been widely adopted (Peterchev, Jalinous, and Lisanby, 2008).

FIGURE 2.1 (A) Circuit 1 is the most basic type of TMS circuit. It includes a voltage source, a capacitor, a switch to release the current, and an induction coil. (B) A simple pendulum, which is analogous to the basic TMS circuit. When the pendulum is raised to one side, it stores potential energy, similar to a charged capacitor. Once the pendulum is released and reaches bottom, all of the energy is kinetic, just as all the energy of the TMS circuit is in the coil when the current is maximum. The pendulum and the TMS circuit can swing back and forth repeatedly between states of stored and kinetic energy.
FIGURE 2.2 Circuit 2 is another type of TMS circuit in which the current is shunted into a resistor halfway through a single oscillation, similar to braking a pendulum after just half a cycle.
The electric current moving through the coil generates a magnetic field, making that coil an electromagnet. Like the permanent magnets stuck to a refrigerator, an electromagnet can attract iron objects nearby. However, it will not necessarily produce TMS. Only a changing magnetic field can induce electric current in nearby electrical conductors, for example, the brain, and thereby depolarize neurons. To stimulate neurons with a varying magnetic field, the voltage, current, and rate at which they change must all attain impressively large values. Typically, the capacitor is charged to a few thousand volts, the peak current through the coil reaches several thousand amperes, and the current oscillates through an entire cycle in well under 1/1000 of a second. For comparison, in North America an old-fashioned 100-watt incandescent light bulb runs on 120 volts and less than 1 ampere. During the tiny fraction of a second in which TMS coil current is flowing, typical systems are operating at millions of watts. The peak magnetic field is in the range of 1 Tesla, around 20,000 times the Earth’s magnetic field. All that energy, and the heat it produces, must be safely isolated from the subject’s head!
All TMS coils have the following fundamental design constraints: they must lie directly against the subject’s head, heavy wires must be used to carry the large currents involved, the wires are subject to substantial magnetic forces during every pulse, and the heat those pulses generate must be dissipated while insulating the wires from the patient and operator. Since TMS was first introduced, researchers and engineers have proposed a striking variety of coil configurations, all intended to optimally focus the induced electric current within the brain (Hsu and Durand, 2001; Kraus, Gugino, Levy, Cadwell, and Roth, 1993; Lontis, Voigt, and Struijk, 2006; Ren, Tarjan, and Popovic, 1995; Roth, Zangen, and Hallett, 2002; Ruohonen, Virtanen, and Ilmoniemi, 1997). Recently Deng and colleagues (2013) reported that the final output of these varied designs could be reduced to two fundamental arrangements: a round coil and a double (figure-8) coil. These are illustrated in Figure 2.3.
FIGURE 2.3 Round and figure-8–shaped TMS coils. The area in orange indicates the approximate areas covered by the induced electric fields. The partial red circle and red arrows indicate the direction of the electric currents within the brain.
The essential feature of a single circular coil is that the brain currents it induces are maximal beneath its outer edge, not beneath the center. This behavior is not intuitive, especially since the peak magnetic field occurs at the coil center. Placing the center of the coil over the presumed target in the brain is likely to produce puzzling results, not to mention disappointment in terms of research results and treatment outcomes. Round coils do have advantages, including simple construction, straightforward heat dissipation, stable head contact, and relatively good penetration beneath the scalp surface. However, the near impossibility of aiming the coils toward a single brain region limits their utility in most applications.
The double coil consists of two round coils placed side by side to form a shape variously described as a figure-8 or a double D. A double “cone” coil is a figure-8 bent to an acute angle at less than 180 degrees, which somewhat improves efficiency and penetration (Lontis, Voigt, and Struijk, 2006). The currents in the two coils run parallel at their junction, reinforcing each other to produce the maximum magnetic field and maximum brain current beneath the center where they form an elongated oval running parallel to the coil junction. Thus, coil placement is greatly simplified and the area of stimulation is more compact. For all types of coils, the induced brain currents run parallel to the current in the overlying coil wires but in the opposite direction. Those currents are invariably parallel to the brain surface, another constraint produced by the physics of current flow within a volume conductor. Tilting the coil on the head will alter the distribution of induced brain currents but not its orientation.
The majority of TMS testing and treatment involves positioning the maximum induced current (lying beneath the center of a figure-8 coil) at a specific cortical target. For depression treatment and many other applications, a common target is the left dorsolateral prefrontal cortex (DLPFC). This position may be located by methods as simple as measuring from specific skull landmarks (fiducials) or as sophisticated as infrared neuronavigation, with a model of the induced electric field projected into an image of the patient’s anatomical magnetic resonance image (MRI). Unfortunately, for many applications outside the motor cortex, our knowledge of the exact functional target area in the brain is less precise than our ability to position the coil over individual gyri. Fox and colleagues (2012) proposed targeting depression treatment to specific subregions of the DLPFC, determined through correlation of resting-state functional MRI activity with the subgenual cingulate region and other areas. If successful, such techniques could rationalize coil positioning and improve treatment response.
Larger coils stimulate wider and deeper volumes of the underlying brain. This may be a disadvantage if the target can be precisely localized. If the area of stimulation is larger than the intended target, the overlap might be useful in ensuring that the stimulus hits the target despite localization errors, or it might be counterproductive by producing effects that are different or even contrary to those intended. Synchronous stimulation that extends over larger regions of cortex might possibly increase the risk of seizure as well. Because the vast majority of TMS studies to date have used only a single coil type and size, the data needed to clarify these issues are largely lacking.
The most ambitious goal of TMS coil design has been to focus stimulation on regions deep beneath the superficial cortex. Success at this objective has been quite limited and is likely to remain so. The extensive analysis by Deng and colleagues (2013) indicates that the physics of induced electric currents outweighs magnetic coil geometry and that large alterations in coil shape tend to produce smaller effects on the actual depth of stimulation. Even theoretically, it appears impossible to induce currents that are stronger at depth than at the brain surface (Heller and van Hulsteyn, 1992). To the extent that somewhat deeper brain penetration is possible, it requires larger coils, and larger coils, in turn, mandate larger volumes of brain stimulation. It is not possible to dissociate these two variables. Any claim that a special arrangement of TMS coils can focus stimulation to a small region beneath the brain surface should be assessed with serious skepticism.
As the intensity, frequency, and duration of TMS increase, the difficulty in delivering all that coil current and in removing all the heat it generates increases correspondingly. Doubling the induced current within the brain requires four times the power and generates four times as much heat. Thus, systems designed for treatment using rapid, repetitive TMS tend to be physically large, pull large amounts of power from the electrical mains, and require cooling by air, water, or oil to prevent the coil from overheating during use. Air-cooling appears to be the safest of these methods. To avoid the need for special cooling while substantially improving stimulation efficiency, a figure-8–type coil can be modified with an iron core constructed from specialized magnetic materials (Epstein and Davey, 2002).
The surface temperature of TMS coils and other objects that contact patients is strictly regulated by engineering codes. In the United States, the upper limit is 41ºC. Commercial coils are generally equipped with internal sensors and automatically shut down when this limit is exceeded. At all points, the coil, lead wire, and connectors must be insulated to several times the maximum stimulator voltage.
The magnetic field within the coil generates a mechanical force on the windings that is responsible for the audible click that accompanies every pulse. The coil enclosure must protect against cracking and even fragmentation over prolonged use that includes repeated cycles of heating and cooling. At times, TMS users have attempted to cool their coils by submerging them in ice water—a measure that is liable to accelerate material fatigue and is absolutely contraindicated during clinical use. Internal mechanical forces rise as the coil dimensions are reduced. This is one of several considerations that make very small coils impractical.
The earliest and most obvious effect of TMS was twitching of the limbs when the coil was placed over motor cortex (Barker, Jalinous, and Freeston, 1985). The easiest muscles to activate are those in the hand and distal upper limb due, in part, to the convenient location of the hand motor area in the central convexity of the brain and, in part, to physiology; these muscles have the purest component of corticospinal innervation from the opposite hemisphere. It is surprising, and still unexplained, that TMS can activate many more muscles, more reliably, than can direct electrical stimulation of the cerebral cortex during brain mapping in patients who are awake.
The extent of TMS muscle activation can be recorded with surface electrodes placed over muscles of interest, using the same basic recording technology as used in peripheral nerve conduction studies. The simplest and most easily quantified measure of muscle contraction is motor threshold, that is, the intensity of stimulation that produces the smallest reproducible activation of the tested muscle. A vast number of factors have been shown to influence motor threshold, including genetics, handedness, hormonal variation, sleep deprivation, drugs, neurological disease, prior contraction of the tested muscle, and even thinking about moving the tested muscle. Reduced motor threshold is considered to represent a state of greater cortical excitability, and higher threshold a state of lower excitability. Some of the factors that change motor threshold have intuitive face validity. For example, mental preparation for movement and drugs with proconvulsant effects lower it (Mars, Bestmann, Rothwell, and Haggard, 2007; Mufti et al., 2010), while many drugs used to treat seizures elevate it (Lee, Seob, Cohen, Bagica, and Theodore, 2005; Ziemann, Steinhoff, Tergau, and Paulus, 1998).
Since any baseline muscle contraction must be quantified (and thereby adds another variable), motor threshold is most often estimated in a relaxed state and referred to as the resting motor threshold (RMT). RMT is the most frequent measure applied in the universe of TMS, with a usual target of 50 or 100 microvolts peak-to-peak. Even when it is not actually the measure of interest, RMT is widely used to establish a baseline of brain excitability for individual subjects and thereby to adjust the intensity of stimulation for other measurements or for treatment. Although RMT has turned out to be an imperfect predictor of the threshold to TMS effects in nonmotor regions, its extensive use testifies to the conviction that an imperfect estimate of brain excitability is superior to no predictor at all. In particular, some type of excitability measure is essential for comparing the brain effects produced by many different stimulators, pulse parameters, and coils, primarily for establishing TMS safety limits in order to avoid induction of seizures and other potential adverse events. RMT has been estimated by visual and neurophysiological criteria and quantified by different statistical techniques (Mishory et al., 2004; Rossini et al., 1994). Present evidence suggests that neurophysiological measures may be more reliable than visual ones (Anderson and George, 2009) and that a simple parameter estimation technique is likely to represent the best combination of accuracy and efficiency (Borckardt, Nahas, Koola, and George, 2006).
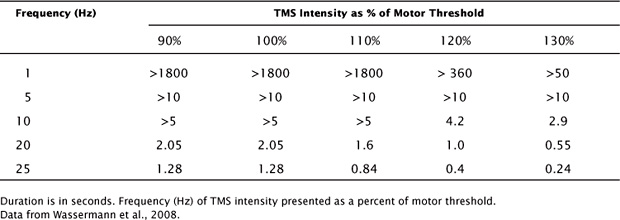
The most widely cited TMS safety data, which were compiled at the US National Institutes of Health (NIH), are given in Table 2.1 (Wassermann, 1998). Additional findings suggest that the intervals between trains of pulses should be 5 seconds or more (Chen et al., 1997). These safety limits have received extensive comment and modification and have become fundamental to the design of TMS research and treatment protocols (Rossi, Hallett, Rossini, and Pascual-Leone, 2009). Exceeding these limits should not be contemplated except under exceptional circumstances. At the same time, remaining within those limits should never be misunderstood as a guarantee of absolute safety. The existing guidelines should, at best, be considered provisional. However, the progressive tightening of regulations related to research on human volunteers means that more comprehensive studies might never be performed.
The fundamental limitations of the NIH safety study include its small number of subjects, all without significant neuropsychiatric deficits, neither very young nor very old, and relatively unstressed. The investigators applied a set of stimulation parameters and coils that were in use at the time. No additional safety data are available regarding the total number of trains and stimuli delivered in a single session or a single day, although many thousands of pulses have been used in 1 day without apparent adverse events (Holtzheimer et al., 2010). It is not possible to extrapolate from the available data to statistical confidence limits for the general population, much less for the extended range of TMS systems and parameters that have been introduced since the original studies. In addition, it has become clear that many individual factors such as genetic susceptibility, sleep deprivation, use of or withdrawal from various medications, and presence of major psychiatric disorders can contribute substantially to seizure risk (Alper, Schwartz, Kolts, and Khan, 2007; Hesdorffer, Hauser, Annegers, and Cascino, 2000; Huber et al., 2013; Kreuzer et al., 2011). New drugs are constantly being introduced. Newer TMS pulse sequences such as “theta burst” cannot be directly compared with older ones. TMS coils intended to produce deep penetration can achieve this only by delivering even stronger stimulation to large volumes of superficial cortex; doing so may alter safety boundaries in ways that are difficult to predict. As a result of all these uncertainties, centers that perform TMS treatment should assume that even with “safe” settings, rare seizures are going to occur, and these centers should be prepared to deal with them appropriately.
TABLE 2.1 Maximum Safe Train Duration as a Function of Intensity
Alper, K., Schwartz, K. A., Kolts, R. L., & Khan, A. (2007). Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biological Psychiatry, 62, 345–354.
Anderson, B. S., & George, M. S. (2009). A review of studies comparing methods for determining transcranial magnetic stimulation motor threshold: observation of movement or electromyography assisted. Journal of the American Psychiatric Nurses Association, 15, 304–313.
Barker, A. T., Jalinous, R., & Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet, 8437, 1106–1107.
Borckardt, J. J., Nahas, Z., Koola, J., & George, M. S. (2006). Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. Journal of ECT, 22, 169–175.
Caton, R. (1875). The electrical currents of the brain. British Medical Journal, 2, 278.
Chen, R., Gerloff, C., Classen, J., Wassermann, E. M., Hallett, M., & Cohen, L. G. (1997). Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalography and Clinical Neurophysiology, 105, 415–421.
Deng, Z.-D., Lisanby, S. L., & Peterchev, A. V. (2013). Electric field depth—focality tradeoff in transcranial magnetic stimulation: comparison of 50 coil designs. Brain Stimulation, 6, 1–13.
Epstein, C. M., & Davey, K. R. (2002). Iron-core coils for transcranial magnetic stimulation. Journal of Clinical Neurophysiology, 19, 376–381.
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., & Pascual-Leone, A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72, 595–603.
Heller, L., & van Hulsteyn, D. B. (1992). Brain stimulation using electromagnetic sources: theoretical aspects. Biophysical Journal, 63, 129–138.
Hesdorffer, D. C., Hauser, W. A., Annegers, J. F., & Cascino, G. (2000). Major depression is a risk factor for seizures in older adults. Annals of Neurology, 47, 246–249.
Holtzheimer, P. E., McDonald, W. M., Mufti, M., Kelley, M. E., Quinn, S., Corso, G., Epstein, C. M. (2010). Accelerated repetitive transcranial magnetic stimulation (aTMS) for treatment-resistant depression. Depression and Anxiety, 27, 960–963.
Hsu, K. H., & Durand, D. M. (2001). A 3-D differential coil design for localized magnetic stimulation. IEEE Transactions on Biomedical Engineering, 48, 1162–1168.
Huber, R., Maki, H., Rosanova, M., Casarotto, S., Canali, P., Casali, A. G., … Massimini, M. (2013). Human cortical excitability increases with time awake. Cerebral Cortex, 23, 1–7.
Kraus, K. H., Gugino, L. D., Levy, W. J., Cadwell, J., & Roth, B. J. (1993). The use of a cap-shaped coil for transcranial magnetic stimulation of the motor cortex. Journal of Clinical Neurophysiology, 10, 353–362.
Kreuzer, P., Langguth, B., Popp, R., Raster, R., Busch, V., Frank, E., … Landgrebe, M. (2011). Reduced intra-cortical inhibition after sleep deprivation: a transcranial magnetic stimulation study. Neuroscience Letters, 493, 63–66.
Lee, H. W., Seob, H. J., Cohen, L. G., Bagica, A., & Theodore, W. H. (2005). Cortical excitability during prolonged antiepileptic drug treatment and drug withdrawal. Clinical Neurophysiology, 116, 1105–1112.
Lontis, E. R., Voigt, M., & Struijk, J. J. (2006). Focality assessment in transcranial magnetic stimulation with double and cone coils. Journal of Clinical Neurophysiology, 23, 463–472.
Mars, R. B., Bestmann, S., Rothwell, J. C., & Haggard, P. (2007). Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Experimental Brain Research, 182, 125–129.
Mishory, A., Molnar, C., Koola, J., Li, X., Kozel, F. A., Myrick, H., … George, M. S. (2004). The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold: using parameter estimation by sequential testing is faster than conventional methods with similar precision. Journal of ECT, 20, 160–165.
Mufti, M. A., Holtzheimer, P. E. 3rd, Epstein, C. M., Quinn, S. C., Vito, N., & McDonald, W. M. (2010). Bupropion decreases resting motor threshold: a case report. Brain Stimulation, 3, 177–180.
Peterchev, A., Jalinous, R., & Lisanby, S. H. (2008). A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS). IEEE Transactions on Biomedical Engineering, 55, 257–266.
Ren, C., Tarjan, P. P., & Popovic, D. B. (1995). A novel electric design for electromagnetic stimulation—the slinky coil. IEEE Transactions on Biomedical Engineering, 42, 918–925.
Rossi, S., Hallett, M., Rossini, P. M., & Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–2039.
Rossini, P. M., Barker, A. T., Berardelli, A., Caramia, M. D., Caruso, G., Cracco, R., … Tomberg, C. (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91, 79–92.
Roth, Y., Zangen, A., & Hallett, M. (2002). A coil design for transcranial magnetic stimulation of deep brain regions. Journal of Clinical Neurophysiology, 19, 361–370.
Ruohonen, J., Virtanen, J., & Ilmoniemi, R. J. (1997). Coil optimization for magnetic brain stimulation. Annals of Biomedical Engineering, 25, 840–849.
Wassermann, E. M. (1998). Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop in the safety of repetitive transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology, 108, 1–16.
Ziemann, U., Steinhoff, B. J., Tergau, F., & Paulus, W. (1998). Transcranial magnetic stimulation: its current role in epilepsy research. Epilepsy Research, 30, 11–30.