Natasha Radhu, Daniel M. Blumberger, Anosha Zanjani, and Zafiris J. Daskalakis
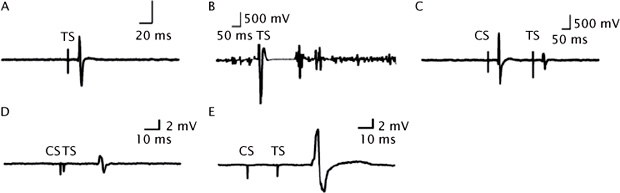
Transcranial magnetic stimulation (TMS) is a cutting-edge noninvasive neurophysiological tool used to investigate the cortex in healthy and disease states (Barker, Jalinous, & Freeston, 1985). Barker and colleagues first demonstrated that a single TMS pulse applied to the motor cortex could activate cortical tissues associated with the hand or leg muscles and that this activation could elicit motor-evoked potentials (MEPs) at the periphery captured through electromyography (EMG) recordings (Figure 8.1A; (Barker, Jalinous, & Freeston, 1985)). TMS is a useful method to further understand the neurobiology of cognitive function, behavior, and emotional processing (McClintock, Freitas, Oberman, Lisanby, & Pascual-Leone, 2011). It involves the generation of a magnetic field through the use of an electromagnetic coil connected to a TMS device, which induces an electrical current in the brain (Wagner, Valero-Cabre, & Pascual-Leone, 2007). TMS is used as an investigational tool as it assesses a variety of cortical phenomena including cortical inhibition (CI), excitation, and plasticity (Kujirai et al., 1993; Classen, Liepert, Wise, Hallett, & Cohen, 1998). Assessing the cortical phenomena using TMS provides valuable insights into the neurophysiological substrates underlying psychiatric and neurological disorders. However, the restriction of such recordings to the motor cortex is of limited interest as the pathophysiology of many neuropsychiatric disorders lies in other areas of the cortex. Thus, evaluating the neurophysiology of brain regions that are more proximal to the underlying phenotype (e.g., the dorsolateral prefrontal cortex [DLPFC]) is essential.
TMS capitalizes on the ability of time-varying magnetic fields to induce eddy currents in biological tissue via Faraday’s principle of electromagnetic induction. TMS fields pass through the scalp unimpeded and noninvasively stimulate brain areas compared to more invasive transcranial electrical stimulation (Hallett, 2000). Conventional approaches to measure cortical neurophysiology involve stimulation of the motor cortex while using MEPs as the primary dependent variable of interest, which is measured in the periphery through EMG. Such approaches have been used to demonstrate important neurophysiological findings in both healthy and disease states, which will be discussed in this chapter.
Applications of TMS
TMS has been used for both therapeutic and diagnostic purposes (Rossini & Rossi, 2007). In relation to its diagnostic application, TMS provides a tool for assessing the timing of cortical processes, cortico-cortical connectivity, CI, facilitation, plasticity, and the interaction between cortical processes (Anand & Hotson, 2002; Chen, 2004; Daskalakis et al., 2004; Di Lazzaro et al., 2004; Pascual-Leone, Walsh, & Rothwell, 2000; Sanger, Garg, & Chen, 2001). In this section, inhibitory and excitatory TMS paradigms are discussed in the context of the underlying neurophysiological mechanisms associated with each method.
TMS can be used as a neurophysiological tool to measure CI, as evidenced by its association with gamma-aminobutyric acid (GABA) inhibitory neurotransmission. These paradigms include the cortical silent period (CSP; Cantello, Gianelli, Civardi, & Mutani, 1992), long-interval cortical inhibition (LICI; Valls-Solé, Pascual-Leone, Wassermann, & Hallett, 1992), short-interval cortical inhibition (SICI; Kujirai et al., 1993), and transcallosal inhibition (TCI; Ferbert et al., 1992).
CSP is a single-pulse paradigm measured by stimulating the contralateral motor cortex of a moderately tonically active muscle (i.e., 20% of maximum contraction) with stimulus intensities of 110%–160% of the resting motor threshold (RMT) resulting in the interruption of voluntary muscle contraction (Cantello et al., 1992; Figure 8.1B). The duration of the CSP is typically measured from MEP onset to the return of any voluntary EMG activity, ending with a deflection in the EMG waveform (Tergau et al., 1999). LICI refers to the pairing of a supra-threshold conditioning stimulus (CS) followed by a suprathreshold test stimulus (TS) at long interstimulus intervals (e.g., 50–100 msec), resulting in inhibition of the MEP produced by the TS in the contralateral muscle (Valls-Solé et al., 1992). LICI is optimal when the CS precedes the TS by 100–150 msec (Sanger et al., 2001; Figure 8.1C). CSP and LICI appear to be assessing GABAB receptor-mediated inhibitory neurotransmission, as evidenced by pharmacological studies (Siebner, Dressnandt, Auer, & Conrad, 1998; McDonnell, Orekhov, & Ziemann, 2006), the time course of the GABAB inhibitory postsynaptic potential (Siebner et al., 1998; McCormick, 1989; Werhahn, Kunesch, Noachtar, Benecke, & Classen, 1999), and the supra-threshold stimulation of the CS (Sanger et al., 2001). For example, administration of baclofen (a GABAB receptor agonist) has been shown to enhance LICI (McDonnell et al., 2006) and CSP (Siebner et al., 1998). Similarly, vigabatrin (a GABA analog) has also been shown to increase LICI and CSP (Pierantozzi et al., 2004). LICI and CSP are associated with high intensities that produce longer periods of inhibition as GABAB receptor-mediated responses have higher activation thresholds and their inhibitory influence is longer (Sanger et al., 2001). Farzan et al. (2010b) found that EMG measures of LICI were significantly correlated with the duration of the CSP. Taken together, this evidence suggests that LICI and CSP are both related to GABAB receptor-mediated inhibitory neurotransmission.
SICI is a paired-pulse inhibitory paradigm that involves a subthreshold CS set at 80% of the RMT that precedes a suprathreshold TS, adjusted to produce an average MEP of 0.5–1.5 mV peak-to-peak amplitude in the contralateral muscle (Kujirai et al., 1993; Figure 8.1D). To measure SICI, conditioning stimuli are applied to the motor cortex before the TS at inter-stimulus intervals between 1 and 4 msec, resulting in inhibition of the MEP response by 50% to 90%. Ziemann et al. (1996a) demonstrated that SICI is increased by medications that facilitate GABAA inhibitory neurotransmission (e.g., lorazepam) in healthy individuals. Using computer simulations, Wang and Buzsaki (1996) showed that the synaptic time constant for GABAA receptors ranges from 10 to 25 msec. This finding demonstrates that SICI is related to GABAA receptor-mediated inhibitory neurotransmission, as evidenced by the similar time course of the GABAA inhibitory postsynaptic potential. SICI is associated with a low-intensity CS, producing shorter periods of inhibition. The GABAA receptor has a lower activation threshold and its inhibitory influence is brief (Sanger et al., 2001).
TCI can be demonstrated by applying a CS to the motor cortex, which inhibits the size of the MEP produced by the TS of the opposite motor cortex (Ferbert et al., 1992; Hanajima et al., 2001). This result is consistent with animal studies which show that stimulation of the motor cortex inhibits the contralateral motor cortex several milliseconds later (Chang, 1953; Asanuma & Okuda, 1962; Matsunami & Hamada, 1984). TCI can be observed at interstimulus intervals between 6 and 50 msec (Ferbert et al., 1992; Gerloff et al., 1998). Daskalakis and colleagues found that similar populations of inhibitory neurons might mediate LICI and TCI (Daskalakis, Christensen, Fitzgerald, Roshan, & Chen, 2002). Therefore, TCI may be related to GABAB activity. This is consistent with the finding that lorazepam increased SICI but did not change TCI, suggesting that TCI is not related to GABAA activity (Pierantozzi et al., 2004).
TMS can also be used to examine cortical excitability, paradigms include MEP amplitude, RMT, and intracortical facilitation (ICF). MEP amplitude is measured as the average response to a series of pulses applied at a consistent TMS intensity or measured as the increasing MEP size produced with increasing TMS intensity (referred to as a MEP response curve; Zaaroor, Pratt, & Starr, 2003). RMT is defined as the minimal intensity that produces a MEP >50 μV in 5 of 10 trials in a relaxed muscle (Rossini et al., 1994). The RMT depends largely on voltage-gated ion channels (Paulus et al., 2008). It has been shown that drugs that block voltage-gated sodium channels, in particular anticonvulsants such as carbamazepine, lamotrigine, and losigamone, increase RMT (Ziemann, Lönnecker, Steinhoff, & Paulus, 1996b). Finally, ICF is a paired-pulse paradigm that can be used to index excitatory activity in the motor cortex. In this paradigm, a CS is applied to the motor cortex before the TS at interstimulus intervals between 7 and 20 msec. This results in an enhanced MEP compared to that produced by the TS alone (Kujirai et al., 1993; Nakamura, Kitagawa, Kawaguchi, & Tsuji, 1997; Figure 8.1E). It has been shown that ICF originates from excitatory postsynaptic potentials transmitted by N-methyl-D-aspartate glutamate receptors (Nakamura et al., 1997). Pharmacological studies have demonstrated a decrease of ICF by N-methyl-D-aspartate receptor antagonists such as dextromethorphan and memantine (Ziemann, Chen, Cohen, & Hallett, 1998). Benzodiazepines such as lorazepam (a GABAA agonist) decreases ICF (Ziemann et al., 1996a) and baclofen (a GABAB agonist) decreases ICF (Ziemann et al., 1996b). However, research has demonstrated that ICF is not exclusively mediated by excitatory interneurons but rather by a net balance between inhibition and excitability (Daskalakis et al., 2004). For a review of the pharmacological effects on inhibitory and excitatory TMS paradigms, see Paulus et al. (2008).
Dysfunction of GABA inhibitory interneurons represents one of the most established neurobiological findings in schizophrenia (SCZ), major depressive disorder (MDD), obsessive-compulsive disorder (OCD), and bipolar disorder. A series of studies have reported that TMS paradigms that generate a functional index of GABA inhibitory neurotransmission from the cortex of healthy human patients have demonstrated a distinct and consistent pattern of deficiency in severe psychiatric disorders. These paradigms show high test–retest reliability and large effect size differences between healthy and patient populations. These tests are relatively easy to perform, inexpensive, and easy to interpret. Several lines of evidence suggest that CI is impaired in these disorders. For example, previous TMS studies have demonstrated deficits in CI assessed from the motor cortex (Figure 8.2) in patients with OCD (Richter et al., 2012; Greenberg et al., 2000; Greenberg, Ziemann, Harmon, Murphy, & Wassermann, 1998), MDD (Levinson et al., 2010; Lefaucheur et al., 2008; Bajbouj et al., 2006; Fitzgerald et al., 2004), SCZ (Wobrock et al., 2008, 2009, 2010; Liu, Fitzgerald, Daigle, Chen, & Daskalakis, 2009; Daskalakis et al., 2002, 2008; Fitzgerald et al., 2003; Fitzgerald, Brown, Daskalakis, Kulkarni, 2002; Fitzgerald, Brown, Daskalakis, deCastella, & Kulkarni, 2002), and bipolar disorder (Levinson, Young, Fitzgerald, & Daskalakis, 2007). Furthermore, a recent metaanalysis (Radhu et al., 2013) found significant effect sizes for decreased SICI, enhanced ICF, and reduced CSP within the OCD population. For MDD, the significant effect sizes were found for decreased CSP and SICI. Significant deficits in SICI were shown in SCZ. These findings are in line with previous literature that suggests CI deficits among psychiatric disorders. Collectively, these studies provide evidence to suggest that impairments in GABA inhibitory neurotransmission are a ubiquitous finding in severe psychiatric illnesses.
FIGURE 8.1 Electromyography Recordings Produced by TMS. (A) A single TMS pulse is applied to the motor cortex producing an MEP. (B) The cortical silent period: starts at the onset of the MEP and ends with the return of motor activity. A suprathreshold TMS pulse is applied to the motor cortex while the contralateral hand muscle is tonically activated. (C) LICI: a suprathreshold-conditioning stimulus precedes a suprathreshold test stimulus by 100 ms, inhibiting the MEP produced by the test stimulus. (D) SICI: a subthreshold conditioning stimulus precedes a suprathreshold test stimulus by 2 ms, inhibiting the MEP produced by the test stimulus. (E) ICF: a subthreshold conditioning stimulus precedes a suprathreshold test stimulus by 20 ms, facilitating the MEP produced by the test stimulus.
Source: Reprinted from Radhu, N., Ravindran, L. N., Levinson, A. J., & Daskalakis, Z. J., Inhibition of the Cortex in Psychiatric Disorders using Transcranial Magnetic Stimulation: Current and Future Directions, Journal of Psychiatry and Neuroscience, Figure. S1: Surface electromyography recordings from a right hand muscle Canadian Medical Association Journal November 2012, 37(6), pages 369–378. © Canadian Medical Association 2012. This work is protected by copyright and the making of this copy was with the permission of the Canadian Medical Association Journal (www.cmaj.ca) and Access Copyright. Any alteration of its content or further copying in any form whatsoever is strictly prohibited unless otherwise permitted by law.
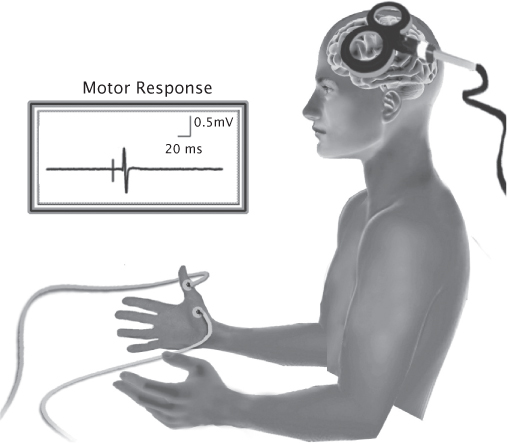
FIGURE 8.2 A single TMS pulse is applied to the motor cortex, activating cortical tissues associated with the abductor pollicis brevis muscle and eliciting an MEP at the periphery captured through electromyography.
GABAergic inhibitory deficits are closely involved in the pathophysiology of SCZ, MDD, OCD, and bipolar disorder. Nevertheless, the overall pattern of these deficits differs among the diseases. TMS paradigms hold potential as biomarkers of psychiatric disorders and treatment response. Biomarker development will lead to strategies that prevent manifestation of the illness and increase our understanding of the underlying neurobiological mechanisms. However, further replication of findings is required. The use of TMS to establish molecular engagement of novel psychopharmacological and somatic treatments (i.e., electroconvulsive therapy [ECT], repetitive TMS, magnetic seizure therapy, transcranial direct current stimulation, or cognitive behavior therapy), particularly within the GABA and glutamate circuits, are other potential biomarker roles for these tests. Conceivably TMS measures of GABAergic and glutamatergic functioning could be used as biological markers of novel treatments that are aimed at enhancing inhibition or decreasing facilitation in the cortex.
In the 1920s, the psychiatrist Hans Berger recorded brain waves from the surface of the human scalp and coined the technique as electroencephalography (EEG; Swartz & Goldensohn, 1998; Buzsaki, 2006). Specifically in EEG, electrical activity of the cortex is monitored by placing multiple electrodes along the scalp; these electrodes record electrical signals that are primarily generated by coordinated output of neurons from the scalpal surface (Nunez & Srinivasan, 2006). Cortical potentials recorded through EEG represent the oscillatory activity of underlying neuronal activity (Nunez & Srinivasan, 2006). Such recordings at rest can be used clinically to diagnose tumors, seizures, encephalopathies, and brain death and can be used as biological markers of neuropsychiatric illnesses (Sponheim, Clementz, Iacono, & Beiser, 2000; Tot, Ozge, Comelekoglu, Yazici, & Bal, 2002; Venables, Bernat, & Sponheim, 2009; Babiloni et al., 2011). By contrast, when sensory stimuli are presented to patients, evoked activity that is of greater electrical power is produced and recorded at the scalp surface when compared to resting EEG recordings. Such activity can be used to evaluate the neurophysiological mechanisms involved in the processing of emotional or cognitive stimuli.
The past decade has seen significant developments in the concurrent use of TMS and EEG to directly assess cortical network properties such as CI, excitability, and connectivity. Simultaneous EEG recording during TMS stimulation was previously unattainable because of the technological shortcomings of EEG amplifiers that would saturate for a long duration due to the large artifact produced by the magnetic stimulation. For example, application of a single TMS pulse would result in artifact lasting for several seconds after the pulse. Such long-lasting artifact blocks the window of time during which neurophysiological processes such as CI occur. Through advances in EEG amplifier technology, researchers have conducted a series of studies to examine TMS paradigms in the motor cortex through simultaneous EEG and EMG as well as in frontal brain regions through EEG recordings.
A significant electromagnetic artifact field is generated by the TMS (at the site of stimulation) and is several-fold larger than that produced by sensory-evoked potentials on EEG recordings (Ilmoniemi et al., 1997). Several developments in EEG amplifier technology have led to a reduction of this artifact. First, Ilmoniemi and colleagues (1997) reported that decoupling of the electrode from the amplifier at or immediately before TMS can markedly reduce the impact of the TMS stimulus artifact on EEG recordings. This was achieved through a sample-and-hold circuit that maintains amplifier output at a constant level during stimulus delivery (Ilmoniemi et al., 1997). They showed that this modification permitted amplifier recovery within 100 μsec after the TMS. Second, in a traditional alternating-current (AC)–coupled EEG amplifier, the typical 500-mV and 50-μsec TMS pulse prevent the signal from returning to zero immediately after the pulse. Rather, the signal that is recorded is followed by a negative deflection that can take seconds to return to its initial state. With the introduction of direct-current (DC)–coupled EEG amplifiers, this prolonged negative swing is eliminated and immediately returns to its linear range after the stimulus stops. DC coupling has become available only in recent years with the introduction of fast 24-bit analogue digital converter (ADC) resolution (i.e., 24 nV/bit) that is superior to the older 16-bit ADC resolution that was limited to 6.1 μV/bit, a resolution that fails to limit the TMS stimulus artifact. The third modification is to record EEG at very high sampling rates (e.g., 20 kHz) to permit full characterization of the TMS pulse and limit the stimulus artifact that is produced on the recordings. By using any of these strategies, EEG recording can become TMS compatible (for a review, see Ilmoniemi & Kicic, 2010). Furthermore, the EEG electrodes used during TMS–EEG should satisfy the physical requirements to operate within the harsh TMS environment. The electrodes must be designed with a small enough diameter to avoid overheating or be affected by the forces that result from the induced TMS currents. Also, the electrodes must be coated with suitable surface material to ensure a proper interface with skin contact (Ilmoniemi and Kicic, 2010). It is suggested that the optimal electrodes to record TMS–EEG are small silver/silver chloride pellet electrodes (e.g., to allow the measurement of the electrical potential on the skin [Roth, Pascual-Leone, Cohen, & Hallett, 1992; Virtanen, Ruohonen, Näätänen, & Ilmoniemi, 1999; Ives, Rotenberg, Poma, Thut, & Pascual-Leone, 2006).
There are several postprocessing procedures for removal of TMS-induced artifact from the EEG recording, as extensively reviewed by Ilmoniemi and Kicic (2010). EEG amplitudes greater than 100 µV and containing large artifacts from electromagnetic residuals, eye blinks, eye movement, or muscle activity should be rejected. Alternatively, there are more superior methods that enable the separation of brain signals from artifacts such as signal-space projection (Ilmoniemi and Kicic, 2010), independent component analysis (Korhonen et al., 2011; Hamidi, Slagter, Tononi, & Postle, 2010), modeling of sources and artifacts (Ilmoniemi and Kicic, 2010), and principal component analysis (Levit-Binnun et al., 2010; Litvak et al., 2007. Offline procedures such as the use of filters to eliminate TMS-related artifact from EEG have also been proposed; these procedures require further investigation (Morbidi et al., 2007). There is one major limitation involved in using these postprocessing techniques, that is, there is no way of verifying that brain activity is not being removed along with the artifact components.
There are four main advantages of using combined TMS–EEG in research studies. First, by using TMS–EEG, investigators can study the mechanisms through which MEPs are generated and modulated. Second, online EEG recording allows for the possibility to evaluate the effects of electromagnetic induction on cortical oscillatory activity to appropriately identify the cortical oscillations that are closely associated with the TMS-induced MEP generation and modulation. A third major advantage of combined TMS–EMG and EEG is the possibility to evaluate the cortico-cortical connectivity between motor cortices. Functional connectivity between cortical regions (e.g., left and right motor cortices) is easily probed by measuring the propagation of TMS-induced cortical responses. TMS–EEG methodologies permit the investigation of the frontal brain areas that are more proximal to the underlying phenotype (nonmotor regions of the cortex). For example, examining LICI in the DLPFC enhances our understanding of the inhibitory mechanisms that underlie a cortical area that is more closely associated with the pathophysiology of psychiatric disorders.
Network oscillations are generated from the rhythmic and synchronized firing of output neurons in the cortex. Oscillations can be recorded from the surface of the cortex through EEG and are represented as five frequency bands. These bands include delta (1–3.5 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (12.5–28 Hz), and gamma (30–50 Hz). Each frequency band is related to different states. For example, the delta and theta bands are greatest during deep sleep and are demonstrated during wakefulness in various pathological conditions (e.g., tumors, Alzheimer’s disease [Huang et al., 2000; Babiloni et al., 2004, 2006; Montez et al., 2009]). Alpha bands are greatest in the incipient stages of sleep, during low arousal periods, and when individuals close their eyes. Beta oscillations show greatest activity during resting wakefulness. Gamma oscillations are associated with the most complex cognitive demands, including information encoding, feature binding, as well as information storage and recall (Tallon-Baudry, Bertrand, Peronnet, & Pernier, 1998; Meltzer et al., 2008). Several studies have suggested that frontal cortical gamma oscillations are necessary for working memory (Howard et al., 2003; Cho, Konecky, & Carter, 2006; Basar-Eroglu et al., 2007; Barr et al., 2009, 2010, 2011). Functionally, gamma oscillatory activity has been suggested to provide the temporal dimension in information encoding, whereby the successful encoding of information depends on its arrival time relative to the gamma cycle (Fries, Nikolic, & Singer, 2007). Interneuron activity mediated by GABA then shapes the time course for prefrontal pyramidal activation (Constantinidis, Williams, & Goldman-Rakic, 2002) that is maximally activated when the fast-spiking interneurons are not firing (Wilson, O’Scalaidhe, & Goldman-Rakic, 1994). GABA receptor activity is also responsible for the generation (GABAA) and modulation (GABAB) of gamma oscillations (Wang & Buzsáki, 1996; Whittington, Traub, & Jefferys, 1995; Traub, Whittington, Colling, Buzsáki, & Jefferys, 1996; Bartos, Vida, & Jonas, 2007; Brown, Davies, & Randall, 2007; Leung & Shen, 2007). Thus, GABA plays a critical role in the generation and modulation of gamma oscillations, which are vital in cognitive tasks.
Ilmoniemi and colleagues were one of the first research groups to use interleaved TMS–EEG to investigate the effect of TMS on cortical excitability (Ilmoniemi et al., 1997). It was demonstrated that TMS applied to the hand representation area of the human motor cortex elicited a cortical response that spread to the adjacent ipsilateral area and to the homologous regions in the opposite hemisphere. It was further shown that the application of TMS to the visual cortex resulted in a similar pattern of signal propagation to the contralateral areas, therefore, providing evidence that the cortical potentials following motor cortex stimulation were less likely to be a result of peripheral sensory activation. This original experiment resulted in a series of studies that further characterized the EEG substrate of cortical excitability, inhibition, plasticity, and connectivity in those who are healthy (Esser et al., 2006; Kähkönen et al., 2001, 2003; Komssi et al., 2002; Komssi & Kähkönen, 2006; Nikulin, Kicić, Kähkönen, & Ilmoniemi, 2003; Paus, Sipila, & Strafella, 2001; Thut et al., 2003).
Daskalakis et al. (2008) and Fitzgerald et al. (2008) were the first to demonstrate that recording LICI (paired-pulse technique) through interleaved TMS–EEG was feasible in both the motor cortex and DLPFC in those who are healthy. In the motor cortex, EEG measures of LICI were represented by the reduction of cortical evoked activity in the electrode C3, which best represents evoked activity in the hand area of motor cortex closest to the optimal site of abductor pollicis brevis activation through TMS (Cui, Huter, Lang, & Deecke, 1999). LICI was defined using the area under rectified unconditioned and conditioned waveforms for averaged EEG recordings between 50 and 150 msec post test stimulus. This interval was chosen because it represents the earliest artifact-free data (i.e., 50 msec post test stimulus) and reflects the duration of GABAB receptor-mediated inhibitory post synaptic potentials (i.e., 250 msec post conditioning stimulus; Deisz, 1999). There was a significant inhibition in mean cortical evoked activity through LICI compared to the test stimulus alone in both the motor cortex and DLPFC (targeted through cortical coregistration methods; Rusjan et al., 2010). Farzan et al. (2010b) has demonstrated the validity, replicability, and test–retest reliability (Cronbach’s α >0.7) of LICI using the TMS–EEG method in both the motor cortex and DLPFC. In this study, a significant correlation was found between MEP suppression and suppression of cortical evoked EEG activity (Farzan et al., 2010b). These results provide compelling evidence to suggest that TMS-induced EEG suppression is related to GABAergic processes (i.e., GABAB inhibition), which mediate EMG measures of LICI (Sanger et al., 2001; Siebner et al., 1998). Similar research was also developed through experiments by Fitzgerald and colleagues (2009) who used equivalent methods and reported maximal inhibition from 50 to 250 msec in DLPFC and from 50 to 175 msec in the parietal lobe. They concluded that LICI might be recorded from several cortical regions with a time course similar to that of known GABAB receptor-mediated inhibition.
More recently, Ferreri et al. (2011) also investigated the ability to record SICI and ICF using TMS–EEG. In these experiments, SICI was recorded using an interstimulus interval of 3 msec, while ICF was recorded using an interstimulus interval of 11 msec. These authors demonstrated that significant inhibition could be reliably recorded in the motor cortex through both EMG and EEG (recorded from the Cz electrode) and that these recordings were correlated, suggesting that such measures are mechanistically related to those recorded from peripheral hand muscles through EMG.
Massimini and colleagues (2005) investigated cortical effective connectivity during wakefulness and sleep using TMS with high-density EEG, evaluating the premotor cortex. They found that during wakefulness, TMS induced a sustained response made of recurrent waves of activity: time-locked high-frequency (20–35 Hz) oscillations followed by a few slower (8–12 Hz) components that persisted until 300 msec. During stage 1 sleep, this TMS-evoked response grew stronger and became shorter in duration. With the onset of non–rapid eye movement (NREM), the TMS-induced brain response changed markedly. The initial wave doubled in amplitude and lasted longer; however, no further TMS-locked activity could be detected following this large wave. Based on these findings, they concluded a breakdown of long-range effective connectivity during NREM sleep. Recently, Massimini et al. (2010) used TMS with high-density EEG over the premotor cortex and found that during REM sleep, the TMS-evoked brain response consisted of a sequence of fast oscillations during the first 150 msec similar to wakefulness. They also found that activity during stage 1 sleep replicated previous findings (Massimini et al., 2005). Using TMS-EEG in sleeping participants, Massimini et al. (2007) demonstrated that TMS evoked a high-amplitude slow wave that originated under the coil and spread over the cortex; this triggered slow waves during sleep was that were state dependent. Regardless of stimulation site and intensity, TMS pulses evoked slow waves during NREM and could not do so during wakefulness. Taken together, these findings suggest that the effects of TMS–EEG are strongly dependent on the state of the activated brain region (i.e., initial level of underlying cortical activity [Silvanto, Cattaneo, Battelli, & Pascual-Leone, 2008; Silvanto, Muggleton, & Walsh, 2008; Silvanto & Muggleton, 2008; Silvanto, Muggleton, Cowey, & Walsh, 2007; Silvanto & Pascual-Leone, 2008]).
Similarly, Ferrarelli and colleagues (2010) indexed TMS-evoked EEG responses in wakefulness compared to induced loss of consciousness using midazolam. Before injection of the anesthetic, TMS pulses to the premotor cortex evoked a complex spatiotemporal pattern of low-amplitude high-frequency activity. Conversely, following midazolam-induced loss of consciousness, TMS pulses gave rise to high-amplitude low-frequency EEG potentials that faded shortly after the stimulation. They concluded that a breakdown of effective cortical connectivity was a key mechanism mediating midazolam-induced loss of consciousness. More recently, using TMS-EEG, Rosonova et al. (2012) evaluated cortical effective connectivity in patients emerging from a coma after a severe brain injury. In patients in a vegetative state who were behaviorally awake (open-eyed) but unresponsive, they found that TMS triggered a simple, local response, indicating a breakdown of effective connectivity similar to that in unconscious, sleeping, or anaesthetized participants. In contrast, in minimally conscious patients (who were nonreflexive), TMS triggered complex long-range activation in distant cortical areas. Taken together, the literature indicates that TMS–EEG, by evaluating effective connectivity, can be used to assess sleep, wakefulness, anesthetized state, and vegetative states.
Several studies have used combined TMS and EEG to examine the pathophysiology of psychiatric disorders. For example, Ferrarelli et al. (2008) stimulated the premotor cortex using combined TMS–EEG and reported reduced TMS-evoked gamma oscillations within the first 100 msec post stimulus in patients with SCZ. These gamma oscillations were significantly attenuated in amplitude and demonstrated less synchrony in the fronto-central regions. The authors concluded that there was an intrinsic dysfunction in frontal thalamocortical circuits in SCZ. Similarly, Farzan et al. (2010a) assessed patients with SCZ, bipolar disorder, and healthy controls using the TMS–EEG paired-pulse technique (i.e., LICI) in both the motor cortex and DLPFC. They found that, overall, LICI (1–50 Hz) in SCZ patients did not differ significantly in any region when compared with bipolar patients and healthy controls. However, when the evoked EEG response was filtered into different frequency bands, they found a significant deficit in the inhibition of gamma oscillations (30–50 Hz) in the DLPFC of SCZ patients relative to patients with bipolar disorder and healthy controls (Figure 8.3). They also found no differences in the inhibition of other oscillatory frequencies in the DLPFC or in the motor cortex between the three groups. The authors concluded that this selective deficit in the inhibition of gamma oscillations demonstrates that the DLPFC is a region in the brain that is closely related to the pathophysiology of SCZ. Furthermore, Frantseva and colleagues (2012) demonstrated an increased TMS-induced cortical activation (in the gamma frequency range) that spread across the cortex, as measured by TMS-EEG in SCZ. However, in healthy controls, this activation faded away soon after stimulation. Recently, Hoppenbrouwers et al. (2013) showed that psychopathic offenders suffer from dysfunctional inhibitory neurotransmission in the DLPFC, as measured through combined TMS and EEG assessing LICI. The authors concluded that the impairments demonstrated in the study might render the psychopath unable to regulate impulses, in turn, subjecting them to a disinhibited, antisocial life. Casarotto et al. (2011) investigated frontal cortex excitability in healthy young and elderly individuals compared to patients with Alzheimer’s disease. They found that TMS-evoked potentials were not affected by physiological aging, unless an abnormal cognitive decline (Alzheimer’s disease) was associated. They demonstrated that frontal cortex excitability, identified as early and local cortical response to TMS, was reduced in elderly patients with Alzheimer’s disease. However, this was not significantly different between healthy young and elderly individuals. Casarotto and colleagues (2013) were the first to evaluate MDD patients using TMS–EEG in order to assess neuroplastic responses before and after their last administration of ECT. They demonstrated that there was a significant increase of frontal cortical excitability (in every patient) after a course of ECT when compared to baseline, suggesting that ECT produces synaptic potentiation. These above-mentioned studies illustrate that TMS–EEG can be used as a clinical tool to characterize the underlying neurobiological dysfunction and to evaluate the neurophysiological effects of treatments over time.
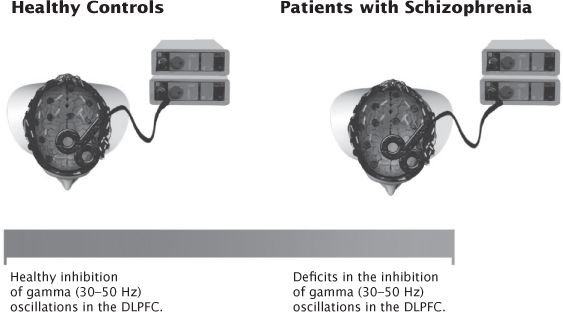
FIGURE 8.3 Patients with schizophrenia have selective deficits in the inhibition of gamma (30–50 Hz) oscillations in the DLPFC compared to healthy controls using interleaved TMS and electroencephalography.
Functional connectivity between cortical regions is easily evaluated by measuring the propagation of TMS-induced cortical responses (via TMS–EEG). Voineskos et al. (2010) evaluated TMS-evoked potentials using single-pulse TMS and studied its effects on inter-hemispherically homologous regions in healthy participants. They found an inverse relationship between microstructural integrity of callosal motor fibers with TMS-induced interhemispheric signal propagation from left to right motor cortex. Also, they demonstrated an inverse relationship between microstructural integrity of the fibers of the genu of the corpus callosum and TMS-induced interhemispheric signal propagation from left to right DLPFC. These findings support a role for the corpus callosum in preservation of functional asymmetry between homologous cortical regions in healthy participants. The authors concluded that delineation of the relationship between corpus callosum microstructure and interhemispheric signal propagation in neuropsychiatric disorders, such as SCZ, might reveal a novel neurobiological mechanism of pathophysiology. Taken together, these studies demonstrate the abnormal functional integration of neuronal systems associated with psychiatric disorders. The above evidence points to important new areas in which TMS–EEG can provide valuable neurophysiological insights in neuropsychiatric disorders.
Advances in cortical stimulation and cortical recording techniques over the past few decades have allowed for the systematic and noninvasive investigation of the neurophysiological processes from the cortex in humans. TMS is a cutting-edge technique that allows for the investigation of the cortical phenomena in both motor and nonmotor regions to further elucidate the pathophysiology of psychiatric disorders. Among such advancements, concurrent TMS and EMG recordings have been instrumental in identifying and probing cortical processes that underlie the generation and modulation of MEPs. Although the evidence is still limited, research to date suggests that disorders such as SCZ, MDD, OCD, and bipolar disorder are characterized by specific deficits in CI and abnormalities in cortical excitability. However, the published studies are not entirely consistent. Factors that may play a role in the discrepant results include small sample sizes, differences in TMS parameters used, use of heterogeneous populations, and presence of comorbid illness. Further, medications may affect outcomes of TMS measures, and it is likely that different classes of psychotropics may do this in unique ways. As such, the inclusion of medicated individuals on various classes of psychotropic agents in these studies is a significant confounder of results. Addressing these issues systematically in future research would allow greater confidence in results and provide a more stable evidence base for elucidating biological markers and mechanisms involved in psychiatric illnesses. The ability to evaluate physiological response profiles of different oscillatory frequencies in response to TMS combined with EEG may ultimately serve as a key technique for evaluating biological markers in psychiatric illnesses. In conclusion, combined TMS and EEG will continue to provide a deeper insight into the neurobiological underpinnings of psychiatric disorders.
This work was supported, in part, by the Canadian Institutes of Health Research (CIHR) Clinician Scientist Award (ZJD), CIHR Fellowship (DMB). This work was funded by an operating award from the Ontario Mental Health Foundation (ZJD), by Constance and Stephen Lieber through a National Alliance for Research on Schizophrenia and Depression Lieber Young Investigator award (ZJD) and Independent Investigator Award (ZJD). NR received funding from the Ontario Graduate Scholarship Program. ZJD received external funding through Neuronetics and Brainsway Inc, Aspect Medical and a travel allowance through Pfizer and Merck. ZJD has also received speaker funding through Sepracor Inc and served on the advisory board for Hoffmann-La Roche Limited. This work was supported by the Grant and Temerty Family through the Centre for Addiction and Mental Health Foundation.
Anand, S., & Hotson, J. (2002). Transcranial magnetic stimulation: neurophysiological applications and safety. Brain Cognition, 50(3), 366–386.
Asanuma, H., & Okuda, O. (1962). Effects of transcallosal volleys on pyramidal tract cell activity of cat. Journal Neurophysiology, 25, 198–208.
Babiloni, C., Binetti, G., Cassetta, E., Cerboneschi, D., Dal Forno, G., Del Percio, C., … Rossini, P. M. (2004). Mapping distributed sources of cortical rhythms in mild Alzheimer’s disease. A multicentric EEG study. Neuroimage, 22(1), 57–67.
Babiloni, C., Cassetta, E., Dal Forno, G., Del Percio, C., Ferreri, F., Ferri, R., … Rossini, P. M. (2006). Donepezil effects on sources of cortical rhythms in mild Alzheimer’s disease: responders vs. non-responders. Neuroimage, 31(4), 1650–1665.
Babiloni, C., Vecchio, F., Lizio, R., Ferri, R., Rodriguez, G., Marzano, N., Frisoni, G., & Rossini, P. (2011). Resting state cortical rhythms in mild cognitive impairment and Alzheimer’s disease: electroencephalographic evidence. Journal Alzheimer’s Disease, 26, 201–214.
Bajbouj, M., Lisanby, S., Lang, U., Danker-Hopfe, H., Heuser, I., & Neu, P. (2006). Evidence for impaired cortical inhibition in patients with unipolar major depression. Biological Psychiatry, 59(5), 395–400.
Barker, A. T., Jalinous R., Freeston I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. The Lancet, 1(8437), 1106–1107.
Barr, M., Farzan, F., Arenovich, T., Chen, R., Fitzgerald, P., & Daskalakis, Z. (2011). The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PloS One, 6(7), e22627.
Barr, M., Farzan, F., Rusjan, P., Chen, R., Fitzgerald, P., & Daskalakis, Z. (2009). Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology, 34(11), 2359–2367.
Barr, M., Farzan, F., Tran, L., Chen, R., Fitzgerald, P., & Daskalakis, Z. (2010). Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophrenia Research, 121(1), 146–152.
Bartos, M., Vida, I., & Jonas, P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience, 8(1), 45–56.
Basar-Eroglu, C., Br, Hildebr, T, H., Karolina Kedzior, K., Mathes, B., & Schmiedt, C. (2007). Working memory related gamma oscillations in schizophrenia patients. International Journal Psychophysiology, 64(1), 39–45.
Brown, J. T., Davies, C. H., & Randall, A. D. (2007). Synaptic activation of GABA(B) receptors regulates neuronal network activity and entrainment. European Journal Neuroscience, 25(10), 2982–2990.
Buzsaki, G. (2006). Rhythms of the brain. New York: Oxford University Press.
Cantello, R., Gianelli, M., Civardi, C., & Mutani, R. (1992). Magnetic brain stimulation The silent period after the motor evoked potential. Neurology, 42(10), 1951–1951.
Casarotto, S., Maatta, S., Herukka, S. K., Pigorini, A., Napolitani, M., Gosseries, O., … Massimini, M. (2011). Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport, 22(12), 592–597.
Casarotto, S., Canali, P., Rosanova, M., Pigorini, A., Fecchio, M., Mariotti, M., … Massimini, M. (2013). Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain topography, 26(2), 326–337.
Chang, H. T. (1953). Cortical response to activity of callosal neurons. Journal Neurophysiology. 16, 117–131. Chen, R. (2004). Interactions between inhibitory and excitatory circuits in the human motor cortex. Experimental Brain Research, 154(1), 1–10.
Cho, R. Y., Konecky, R. O., & Carter, C. S. (2006). Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings National Academy Sciences U S A, 103(52), 19878–19883.
Classen, J., Liepert, J., Wise, S., Hallett, M., & Cohen, L. (1998). Rapid plasticity of human cortical movement representation induced by practice. Journal Neurophysiology, 79(2), 1117–1123.
Constantinidis, C., Williams, G., & Goldman-Rakic, P. (2002). A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nature Neuroscience, 5(2), 175–180.
Cui, R., Huter, D., Lang, W., & Deecke, L. (1999). Neuroimage of voluntary movement: topography of the Bereitschafts potential, a 64-channel DC current source density study. Neuroimage, 9(1), 124–134.
Daskalakis, Z., Christensen, B., Chen, R., Fitzgerald, P., Zipursky, R., & Kapur, S. (2002). Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Archives General Psychiatry, 59(4), p. 347–354.
Daskalakis, Z., Christensen, B., Fitzgerald, P., Moller, B., Fountain, S., & Chen, R. (2008). Increased cortical inhibition in persons with schizophrenia treated with clozapine. Journal Psychopharmacology, 22(2), 203–209. Daskalakis, Z., Christensen, B., Fitzgerald, P., Roshan, L., & Chen, R. (2002). The mechanisms of interhemispheric inhibition in the human motor cortex. Journal Physiology, 543(1), 317–326.
Daskalakis, Z., Farzan, F., Barr, M., Maller, J., Chen, R., & Fitzgerald, P. (2008). Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS–EEG study. Neuropsychopharmacology, 33(12), 2860–2869.
Daskalakis, Z., Paradiso, G., Christensen, B., Fitzgerald, P., Gunraj, C., & Chen, R. (2004). Exploring the connectivity between the cerebellum and motor cortex in humans. Journal Physiology, 557(2), 689–700.
Deisz, R. (1999). GABA(B) receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology, 38(11), 1755–1766.
Di Lazzaro, V., Oliviero, A., Pilato, F., Saturno, E., Dileone, M., Mazzone, P., … Rothwell, J. (2004). The physiological basis of transcranial motor cortex stimulation in conscious humans. Clinical Neurophysiology, 115(2), 255–266.
Esser, S., Huber, R., Massimini, M., Peterson, M., Ferrarelli, F., & Tononi, G. (2006). A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Research Bulletin, 69(1), 86–94.
Farzan, F., Barr, M., Levinson, A., Chen, R., Wong, W., Fitzgerald, P., & Daskalakis, Z. (2010a). Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain, 133(5), 1505–1514.
Farzan, F., Barr, M., Levinson, A., Chen, R., Wong, W., Fitzgerald, P., & Daskalakis, Z. (2010b). Reliability of Long-Interval Cortical Inhibition in Healthy Human Subjects: A TMS–EEG Study. Journal Neurophysiology, 104(3), 1339–1346.
Ferbert, A., Priori, A., Rothwell, J., Day, B., Colebatch, J., & Marsden, C. (1992). Interhemispheric inhibition of the human motor cortex. Journal Physiology, 453(1), 525–546.
Ferrarelli, F., Massimini, M., Peterson, M., Riedner, B., Lazar, M., Murphy, M., … Tononi, G. (2008). Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. American Journal Psychiatry, 165(8), 996–1005.
Ferrarelli, F., Massimini, M., Sarasso, S., Casali, A., Riedner, B., Angelini, G., … Pearce, R. (2010). Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proceedings National Academy Sciences, 107(6), 2681–2686.
Ferreri, F., Pasqualetti, P., Määttä, S., Ponzo, D., Ferrarelli, F., Tononi, G., … Rossini, P. (2011). Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage, 54(1), 90–102.
Fitzgerald, P., Brown, T., Daskalakis, Z., & Kulkarni, J. (2002). A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Research: Neuroimaging, 114(1), 11–22.
Fitzgerald, P., Brown, T., Daskalakis, Z., Decastella, A., & Kulkarni, J. (2002). A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophrenia Research, 56(3), 199–209.
Fitzgerald, P., Brown, T., Marston, N., Daskalakis, Z., De Castella, A., Bradshaw, J., & Kulkarni, J. (2004). Motor cortical excitability and clinical response to rTMS in depression. Journal Affective Disorders, 82(1), 71–76.
Fitzgerald, P., Brown, T., Marston, N., Oxley, T., De Castella, A., Daskalakis, Z., & Kulkarni, J. (2003). A transcranial magnetic stimulation study of abnormal cortical inhibition in schizophrenia. Psychiatry Research, 118(3), 197–207.
Fitzgerald, P., Daskalakis, Z., Hoy, K., Farzan, F., Upton, D., Cooper, N., & Maller, J. (2008). Cortical inhibition in motor and non-motor regions: a combined TMS-EEG study. Clinical EEG Neuroscience, 39(3), 112–117.
Fitzgerald, P., Maller, J., Hoy, K., Farzan, F., & Daskalakis, Z. (2009). GABA and cortical inhibition in motor and non-motor regions using combined TMS–EEG: A time analysis. Clinical Neurophysiology, 120(9), 1706–1710.
Frantseva, M., Cui, J., Farzan, F., Chinta, L., Velazquez, J., & Daskalakis, Z. (2012). Disrupted cortical conductivity in schizophrenia: TMS–EEG study. Cerebral Cortex. 2012 Oct 5. [Epub ahead of print]
Fries, P., Nikolic, D., & Singer, W. (2007). The gamma cycle. Trends Neurosciences, 30(7), 309–316.
Gerloff, C., Cohen, L., Floeter, M., Chen, R., Corwell, B., & Hallett, M. (1998). Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. Journal Physiology, 510(1), 249–259.
Greenberg, B. D., Ziemann, U., Harmon, A., Murphy, D. L., & Wassermann, E. M. (1998). Decreased neuronal inhibition in cerebral cortex in obsessive-compulsive disorder on transcranial magnetic stimulation. Lancet, 352(9131), 881–882.
Greenberg, B., Ziemann, U., Cora-Locatelli, G., Harmon, A., Murphy, D., Keel, J., & Wassermann, E. (2000). Altered cortical excitability in obsessive–compulsive disorder. Neurology, 54(1), 142–147.
Hallett, M. (2000). Transcranial magnetic stimulation and the human brain. Nature, 406(6792), 147–150.
Hamidi, M., Slagter, H., Tononi, G., & Postle, B. (2010). Brain responses evoked by high-frequency repetitive transcranial magnetic stimulation: an event-related potential study. Brain Stimulation, 3(1), 2–14.
Hanajima, R., Ugawa, Y., Machii, K., Mochizuki, H., Terao, Y., Enomoto, H., … Kanazawa, I. (2001). Interhemispheric facilitation of the hand motor area in humans. Journal Physiology, 531(3), 849–859.
Hoppenbrouwers, S., De Jesus, D., Stirpe, T., Fitzgerald, P., Voineskos, A., Schutter, D., & Daskalakis, Z. (2013). Inhibitory deficits in the dorsolateral prefrontal cortex in psychopathic offenders. Cortex. 49(5), 1377–1385.
Howard, M., Rizzuto, D., Caplan, J., Madsen, J., Lisman, J., Aschenbrenner-Scheibe, R., … Kahana, M. (2003). Gamma oscillations correlate with working memory load in humans. Cerebral Cortex, 13(12), 1369–1374.
Huang, C., Wahlund, L., Dierks, T., Julin, P., Winblad, B., & Jelic, V. (2000). Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clinical Neurophysiology, 111(11), 1961–1967.
Ilmoniemi, R. J., Virtanen, J., Ruohonen, J., Karhu, J., Aronen, H. J., Näätänen, R., & Katila, T. (1997). Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport, 8(16), 3537–3540.
Ilmoniemi, R., & Kičić, D. (2010). Methodology for combined TMS and EEG. Brain Topography, 22(4), 233–248.
Ives, J., Rotenberg, A., Poma, R., Thut, G., & Pascual-Leone, A. (2006). Electroencephalographic recording during transcranial magnetic stimulation in humans and animals. Clinical Neurophysiology, 117(8), 1870–1875.
Kähkönen, S., Kesäniemi, M., Nikouline, V. V., Karhu, J., Ollikainen, M., Holi, M., & Ilmoniemi, R. J. (2001). Ethanol modulates cortical activity: direct evidence with combined TMS and EEG. Neuroimage, 14(2), 322–328.
Kähkönen, S., Wilenius, J., Nikulin, V., Ollikainen, M., & Ilmoniemi, R. J. (2003). Alcohol reduces prefrontal cortical excitability in humans: a combined TMS and EEG study. Neuropsychopharmacology, 28(4), 747–754.
Komssi, S., & Kähkönen, S. (2006). The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Research Reviews, 52(1), 183–192.
Komssi, S., Aronen, H., Huttunen, J., Kesäniemi, M., Soinne, L., Nikouline, V., … Ilmoniemi, R. J. (2002). Ipsi-and contralateral EEG reactions to transcranial magnetic stimulation. Clinical Neurophysiology, 113(2), 175–184.
Korhonen, R., Hernandez-Pavon, J., Metsomaa, J., Mäki, H., Ilmoniemi, R., & Sarvas, J. (2011). Removal of large muscle artifacts from transcranial magnetic stimulation-evoked EEG by independent component analysis. Medical Biological Engineering Computing, 49(4), 397–407.
Kujirai, T., Caramia, M., Rothwell, J., Day, B., Thompson, P., Ferbert, A., … Marsden, C. (1993). Cortico-cortical inhibition in human motor cortex. Journal Physiology, 471(1), 501–519.
Lefaucheur, J., Lucas, B., Andraud, F., Hogrel, J., Bellivier, F., Del Cul, A., … Paillere-Martinot, M. (2008). Inter-hemispheric asymmetry of motor corticospinal excitability in major depression studied by transcranial magnetic stimulation. Journal Psychiatric Research, 42(5), 389–398.
Leung, L., & Shen, B. (2007). GABAB receptor blockade enhances theta and gamma rhythms in the hippocampus of behaving rats. Hippocampus, 17(4), 281–291.
Levinson, A. J., Young, L. T., Fitzgerald, P. B., & Daskalakis, Z. J. (2007). Cortical inhibitory dysfunction in bipolar disorder: a study using transcranial magnetic stimulation. Journal Clinical Psychopharmacology, 27(5), 493–497.
Levinson, A., Fitzgerald, P., Favalli, G., Blumberger, D., Daigle, M., & Daskalakis, Z. (2010). Evidence of cortical inhibitory deficits in major depressive disorder. Biological Psychiatry, 67(5), 458–464.
Levit-Binnun, N., Litvak, V., Pratt, H., Moses, E., Zaroor, M., & Peled, A. (2010). Differences in TMS-evoked responses between schizophrenia patients and healthy controls can be observed without a dedicated EEG system. Clinical Neurophysiology, 121(3), 332–339.
Litvak, V., Komssi, S., Scherg, M., Hoechstetter, K., Classen, J., Zaaroor, M., … Kahkonen, S. (2007). Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage, 37(1), 56–70.
Liu, S., Fitzgerald, P., Daigle, M., Chen, R., & Daskalakis, Z. (2009). The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biological Psychiatry, 65(6), 503–509.
Massimini, M., Ferrarelli, F., Esser, S., Riedner, B., Huber, R., Murphy, M., … Tononi, G. (2007). Triggering sleep slow waves by transcranial magnetic stimulation. Proceedings National Academy Sciences, 104(20), 8496–8501.
Massimini, M., Ferrarelli, F., Huber, R., Esser, S., Singh, H., & Tononi, G. (2005). Breakdown of cortical effective connectivity during sleep. Science, 309(5744), 2228–2232.
Massimini, M., Ferrarelli, F., Murphy, M., Huber, R., Riedner, B., Casarotto, S., & Tononi, G. (2010). Cortical reactivity and effective connectivity during REM sleep in humans. Cognitive Neuroscience, 1(3), 176–183.
Matsunami, K., & Hamada, I. (1984). Effects of stimulation of corpus callosum on precentral neuron activity in the awake monkey. Journal Neurophysiology, 52(4), 676–691.
McClintock, S. M., Freitas, C., Oberman, L., Lisanby, S. H., & Pascual-Leone, A. (2011). Transcranial magnetic stimulation: a neuroscientific probe of cortical unction in schizophrenia. Biological Psychiatry, 70(1), 19–27.
Mccormick, D. (1989). GABA as an inhibitory neurotransmitter in human cerebral cortex. Journal Neurophysiology, 62(5), 1018–1027.
McDonnell, M. N., Orekhov, Y., & Ziemann, U. (2006). The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Experimental Brain Research, 173(1), 86–93.
Meltzer, J., Zaveri, H., Goncharova, I., Distasio, M., Papademetris, X., Spencer, S., … Constable, R. (2008). Effects of working memory load on oscillatory power in human intracranial EEG. Cerebral Cortex, 18(8), 1843–1855.
Montez, T., Poil, S. S., Jones, B. F., Manshanden, I., Verbunt, J. P., Van Dijk, B. W., … Linkenkaer-Hansen K. (2009). Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. Proceedings National Academy Sciences, 106(5), 1614–1619.
Morbidi, F., Garulli, A., Prattichizzo, D., Rizzo, C., Manganotti, P., & Rossi, S. (2007). Off-line removal of TMS-induced artifacts on human electroencephalography by Kalman filter. Journal Neuroscience Methods, 162(1), 293–302.
Nakamura, H., Kitagawa, H., Kawaguchi, Y., & Tsuji, H. (1997). Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal Physiology, 498(Pt 3), 817–823.
Nikulin, V. V., Kicić, D., Kähkönen, S., & Ilmoniemi, R. (2003). Modulation of electroencephalographic responses to transcranial magnetic stimulation: evidence for changes in cortical excitability related to movement. European Journal Neuroscience, 18(5), 1206–1212.
Nunez, P., & Srinivasan, R. (2006). A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clinical Neurophysiology, 117(11), 2424–2435.
Pascual-Leone, A., Walsh, V., & Rothwell, J. (2000). Transcranial magnetic stimulation in cognitive neuroscience–virtual lesion, chronometry, and functional connectivity. Current Opinion Neurobiology, 10(2), 232–237.
Paulus, W., Classen, J., Cohen, L., Large, C., Di Lazzaro, V., Nitsche, M., … Ziemann, U. (2008). State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulation, 1(3), 151–163.
Paus, T., Sipila, P., & Strafella, A. (2001). Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. Journal Neurophysiology, 86(4), 1983–1990.
Pierantozzi, M., Grazia Marciani, M., Giuseppina Palmieri, M., Brusa, L., Galati, S., Donatella Caramia, M., … Stanzione, P. (2004). Effect of vigabatrin on motor responses to transcranial magnetic stimulation: an effective tool to investigate in vivo GABAergic cortical inhibition in humans. Brain Research, 1028(1), 1–8.
Radhu, N., De Jesus, D., Ravindran, L., Zanjani, A., Fitzgerald, P., & Daskalakis, Z. (2013). A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clinical Neurophysiology, 124(7), 1309–1320.
Richter, M., De Jesus, D., Hoppenbrouwers, S., Daigle, M., Deluce, J., Ravindran, L., … Daskalakis, Z. (2011). Evidence for cortical inhibitory and excitatory dysfunction in obsessive compulsive disorder. Neuropsychopharmacology, 37(5), 1144–1151.
Rosanova, M., Gosseries, O., Casarotto, S., Boly, M., Casali, A., Bruno, M., … Massimini, M. Others (2012). Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain, 135(4), 1308–1320.
Rossini, P. M., Barker, A. T., & Berardelli A, et al. (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography Clinical Neurophysiology, 91(2), 79–92.
Rossini, P., & Rossi, S. (2007). Transcranial magnetic stimulation Diagnostic, therapeutic, and research potential. Neurology, 68(7), 484–488.
Roth, B., Pascual-Leone, A., Cohen, L., & Hallett, M. (1992). The heating of metal electrodes during rapid-rate magnetic stimulation: a possible safety hazard. Electroencephalography Clinical Neurophysiology/Evoked Potentials Section, 85(2), 116–123.
Rusjan, P., Barr, M., Farzan, F., Arenovich, T., Maller, J., Fitzgerald, P., & Daskalakis, Z. (2010). Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Human Brain Mapping, 31(11), 1643–1652.
Sanger, T., Garg, R., & Chen, R. (2001). Interactions between two different inhibitory systems in the human motor cortex. Journal Physiology, 530(2), 307–317.
Siebner, H., Dressn, T, J., Auer, C., & Conrad, B. (1998). Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve, 21(9), 1209–1212.
Silvanto, J., & Muggleton, N. G. (2008). Testing the validity of the TMS state-dependency approach: targeting functionally distinct motion-selective neural populations in visual areas V1/V2 and V5/MT+. Neuroimage, 40(4), 1841–1848.
Silvanto, J., & Pascual-Leone, A. (2008). State-dependency of transcranial magnetic stimulation. Brain topography, 21(1), 1–10.
Silvanto, J., Cattaneo, Z., Battelli, L., & Pascual-Leone, A. (2008). Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. Journal Neurophysiology, 99(5), 2725–2730.
Silvanto, J., Muggleton, N., & Walsh, V. (2008). State-dependency in brain stimulation studies of perception and cognition. Trends Cognitive Sciences, 12(12), 447–454.
Silvanto, J., Muggleton, N., Cowey, A., & Walsh, V. (2007). Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. European Journal Neuroscience, 25(6), 1874–1881.
Sponheim, S., Clementz, B., Iacono, W., & Beiser, M. (2000). Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biological Psychiatry, 48(11), 1088–1097.
Swartz, B. E., & Goldensohn, E. S. (1998). Timeline of the history of EEG and associated fields. Electroencephalography Clinical Neurophysiology, 106(2), 173–176.
Tallon-Baudry, C., Bertrand, O., Peronnet, F., & Pernier, J. (1998). Induced gamma-band activity during the delay of a visual short-term memory task in humans. Journal Neuroscience, 18(11), 4244–4254.
Tergau, F., Wanschura, V., Canelo, M., Wischer, S., Wassermann, E., Ziemann, U., & Paulus, W. (1999). Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Experimental Brain Research, 124(4), 447–454.
Thut, G., Northoff, G., Ives, J., Kamitani, Y., Pfennig, A., Kampmann, F., … Pascual-Leone, A. (2003). Effects of single-pulse transcranial magnetic stimulation (TMS) on functional brain activity: a combined event-related TMS and evoked potential study. Clinical Neurophysiology, 114(11), 2071–2080.
Tot, S., Ozge, A., Comelekoglu, U., Yazici, K., & Bal, N. (2002). Association of QEEG findings with clinical characteristics of OCD: evidence of left frontotemporal dysfunction. Canadian Journal Psychiatry, 47(6), 538–545.
Traub, R., Whittington, M., Colling, S., Buzsáki, G., & Jefferys, J. (1996). Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. Journal Physiology, 493(Pt 2), 471–484.
Valls-Solé, J., Pascual-Leone, A., Wassermann, E., & Hallett, M. (1992). Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography Clinical Neurophysiology/Evoked Potentials Section, 85(6), 355–364.
Venables, N., Bernat, E., & Sponheim, S. (2009). Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophrenia Bulletin, 35(4), 826–839.
Virtanen, J., Ruohonen, J., Näätänen, R., & Ilmoniemi, R. (1999). Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Medical Biological Engineering Computing, 37(3), 322–326.
Voineskos, A., Farzan, F., Barr, M., Lobaugh, N., Mulsant, B., Chen, R., … Daskalakis, Z. (2010). The role of the corpus callosum in transcranial magnetic stimulation induced interhemispheric signal propagation. Biological Psychiatry, 68(9), 825–831.
Wagner, T., Valero-Cabre, A., & Pascual-Leone, A. (2007). Noninvasive Human Brain Stimulation. Annual Review Biomedical Engineering, 9(1), 527–565.
Wang, X., & Buzsáki, G. (1996). Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. Journal Neuroscience, 16(20), 6402–6413.
Werhahn, K., Kunesch, E., Noachtar, S., Benecke, R., & Classen, J. (1999). Differential effects on motor cortical inhibition induced by blockade of GABA uptake in humans. Journal Physiology, 517(2), 591–597.
Whittington, M., Traub, R., & Jefferys, J. (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature, 373(6515), 612–615.
Wilson, F., O’scalaidhe, S., & Goldman-Rakic, P. (1994). Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proceedings National Academy of Sciences, 91(9), 4009–4013.
Wobrock, T., Hasan, A., Malchow, B., Wolff-Menzler, C., Guse, B., Lang, N., … Falkai, P. (2010). Increased cortical inhibition deficits in first-episode schizophrenia with comorbid cannabis abuse. Psychopharmacology, 208(3), 353–363.
Wobrock, T., Schneider-Axmann, T., Retz, W., Rösler, M., Kadovic, D., Falkai, P., & Schneider, M. (2009). Motor circuit abnormalities in first-episode schizophrenia assessed with transcranial magnetic stimulation. Pharmacopsychiatry, 42(05), 194–201.
Wobrock, T., Schneider, M., Kadovic, D., Schneider-Axmann, T., Ecker, U., Retz, W., … Falkai, P. (2008). Reduced cortical inhibition in first-episode schizophrenia. Schizophrenia Research, 105(1), 252–261.
Zaaroor, M., Pratt, H., & Starr, A. (2003). Time course of motor excitability before and after a task-related movement. Neurophysiologie Clinique/Clinical Neurophysiology, 33(3), 130–137.
Ziemann, U., Chen, R., Cohen, L., & Hallett, M. (1998). Dextromethorphan decreases the excitability of the human motor cortex. Neurology, 51(5), 1320–1324.
Ziemann, U., Lönnecker, S., Steinhoff, B., & Paulus, W. (1996a). The effect of lorazepam on the motor cortical excitability in man. Experimental Brain Research, 109(1), 127–135.
Ziemann, U., Lönnecker, S., Steinhoff, B., & Paulus, W. (1996b). Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Annals Neurology, 40(3), 367–378.