Colleen Loo, Scott Aaronson, and Paul E. Holtzheimer
Psychotropic medications and psychotherapy are effective for many patients with psychiatric disorders. However, a substantial number of patients either fail to achieve or fail to sustain full remission with these treatments. Alternative interventions, especially for those patients who do not respond to or tolerate standard treatments, include a variety of neurostimulation approaches. Electroconvulsive therapy (ECT) predated antidepressant and anti-psychotic medications by nearly two decades (Lisanby, 2007) and remains one of the most effective treatments in psychiatry. More recently, a number of other neurostimulation techniques have been investigated for the treatment of patients with neuropsychiatric disorders. These include repetitive transcranial magnetic stimulation (rTMS), magnetic seizure therapy (MST), transcranial direct current stimulation (tDCS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). These interventions vary significantly in terms of method for stimulating neural tissue, initial mechanism of action, and patient populations most likely to respond. This chapter reviews the various neurostimulation interventions either in use or in active development for the treatment of patients with psychiatric disorders.
ECT is a widely recommended treatment for depression that is pharmacotherapy resistant or where there is clinical urgency for rapid improvement (American Psychiatric Association, 2001; Lancet, 2003; NICE Clinical Guidelines, 2009). ECT has been shown to have superior efficacy to pharmacotherapy in depression (Lancet, 2003; Kho, van Vreeswijk, Simpson, & Zwinderman, 2003). Where dose treatment levels are adequate (e.g., excluding low-dose unilateral ECT), remission rates range from 55% to 87% in research samples (Kellner et al., 2010; Petrides et al., 2001; Sackeim et al., 2000; Sackeim et al., 2008) and from 40% to 68% in community samples (Kho, Zwinderman, & Blansjaar, 2005; Prudic, Olfson, Marcus, Fuller, & Sackeim, 2004). The majority of patients in these samples were treatment resistant, having failed several trials (i.e., at least one adequate trial) of antidepressant medication in the current episode (Table 10.1).
ECT is effective in treating bipolar depression, which may respond more quickly than unipolar depression (Daly et al., 2001; Sienaert, Vansteelandt, Demyttenaere, & Peuskens, 2009b), though switching into mania may occur (Loo, Katalinic, Mitchell, & Greenberg, 2011). ECT has efficacy in treating acute psychotic symptoms in schizophrenia and may enhance outcomes when combined with antipsychotic medications (Tharyan & Adams, 2005). ECT is at least as effective as pharmacotherapy in treating acute mania (Loo et al., 2011). It is a highly and rapidly effective treatment for catatonia (Fink, 2001; Gazdag, Ungvari, & Caroff, 2009). Predictors of response to ECT in depression include older age, psychotic features, and lower treatment resistance (de Vreede, Burger, & van Vliet, 2005; Dombrovski et al., 2005; Petrides et al., 2001).
Cognitive side effects are common after ECT and are often time limited (with recovery in the range of weeks to months after a course of ECT). However, persistent retrograde amnesia may occur, the risk being highest with bitemporal ECT (Sackeim et al., 2007). A meta-analysis found that by 15 days after the end of treatment, cognitive function tended to be improved compared with pre-ECT levels, reflecting improved mental state from the treatment (Semkovska & McLoughlin, 2010). The treatment technique used in ECT (e.g., electrode placement, stimulus parameters, pulse width) is an important determinant of the extent and persistence of cognitive side effects (Sackeim et al., 2007). Newer treatment approaches such as reduction of the stimulus pulse width to the “ultrabrief” range (0.3 ms) result in less cognitive disturbance than the brief pulse (1.0–1.5 ms) treatment approach currently in common use (Loo, Sainsbury, Sheehan, & Lyndon, 2008; Sackeim et al., 2008; Sienaert, Vansteelandt, Demyttenaere, & Peuskens, 2010).
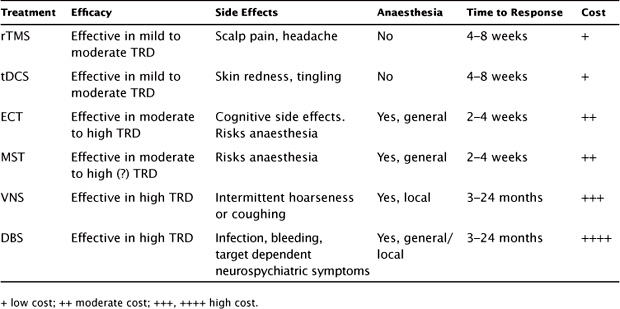
TABLE 10.1 Overview of Key Features of rTMS and Other Brain Stimulation

Acute response to ECT is typically rapid, with up to 90% of depressed patients attaining remission within nine treatments over a 3-week period (Kellner et al., 2010). However, therapeutic gains may rapidly recede within the first few weeks if the ECT course is ceased abruptly (Prudic et al., 2004, 2013), and relapse rates may be as high as 84% over the next 6 months in the absence of any continuation and maintenance treatment (Sackeim, Haskett, et al., 2001). This relapse rate is reduced by optimal pharmacotherapy (Sackeim, Haskett, et al., 2001) and the use of continuation and maintenance ECT (Kellner et al., 2006; Prudic et al., 2013).
A few studies have directly compared the efficacy of ECT and rTMS in depression. Though participants were randomized to receive ECT or rTMS, these studies were not double-blinded due to ethical constraints against the use of sham ECT (with anesthesia). A metaanalysis of these studies found the efficacy of ECT to be superior (Slotema et al., 2011), the difference corresponding to a weighted effect size of 0.47. An open-label pilot study suggested that the superior efficacy of ECT might be particularly evident in psychotic depression (Grunhaus et al., 2000). There is little controversy over superior cognitive outcomes with rTMS (which does not cause cognitive impairment; Moreines, McClintock, & Holtzheimer, 2011) as compared to ECT. There are criticisms of the treatment approach used in these studies for both modalities of treatment including fixed parameters and number of treatments for TMS as well as the potential inadequate dosing of unilateral ECT. Nevertheless, the evidence to date clearly indicates that ECT has superior efficacy, both in speed of response and overall response rates.
ECT remains the most effective proven treatment for depression in widespread clinical use. Response and remission rates are high, and treatment effects occur rapidly, typically within 2–3 weeks. The main limitations of its use are the risk of cognitive side effects, the requirement for general anesthesia, and stigma.
MST is similar to ECT in that it provides a series of treatments, each involving induction of a seizure, over several weeks. MST uses a TMS device to induce seizure; similar to ECT, general anesthesia is used to minimize side effects. The rationale for MST is based on data that suggest that more focal seizure induction in ECT is associated with fewer cognitive side effects (Lisanby, 2007; Sackeim et al., 2008). MST provides a highly focused electrical induction of a generalized seizure, suggesting that it will have efficacy similar to that of ECT but with fewer cognitive side effects (Lisanby, Luber, Finck, Schroeder, & Sackeim, 2001). The risks and potential side effects of MST are similar to those associated with ECT, as both involve seizure induction under general anesthesia.
In several studies, MST has been shown to have a superior cognitive safety profile compared to ECT (Kayser, Bewernick, Axmacher, & Schlaepfer, 2008; Kayser et al., 2011; Kosel, Frick, Lisanby, Fisch, & Schlaepfer, 2003; Lisanby, Luber, Schlaepfer, & Sackeim, 2003; White et al., 2006). An initial controlled trial of MST versus ECT for depression found that ECT was more efficacious (White et al., 2006), but MST required lower doses of anesthetic agents. Another small controlled trial found similar antidepressant effects for MST and ECT. However, recovery of orientation was faster in the MST patients, suggesting that MST may be associated with fewer long-term cognitive side effects (Kayser et al., 2011). An open-label study of high-dose (100 Hz) MST in depressed patients found significant antidepressant effects associated with treatment and no adverse cognitive effects (Fitzgerald et al., 2013). Although the efficacy of MST to date is either similar or inferior to ECT, it is noted that earlier studies often treated at or near seizure threshold. Since more focal induction of a seizure may require higher subsequent treatment parameters (e.g., five to eight times the seizure threshold for right unilateral ECT), it is likely that MST needs to stimulate at several times the seizure threshold in order to be most effective. Newer devices allow this capability, and data from large-scale clinical trials are awaited.
tDCS is a noninvasive technique in which a weak direct electrical current is passed across the scalp. The current flows in one direction (hence “direct” in contrast to an alternating current, which is bidirectional, as in ECT) from the anodal electrode to the cathodal electrode. tDCS can lead to changes in the excitability of neurons in stimulated cortical regions. The stimulation is subconvulsive and given without any need for anesthesia. Weak electrical stimulation has been applied in animal models and humans for many decades and was previously known as “brain polarization.” Animal experiments and neurophysiological studies in humans have shown that anodal stimulation depolarizes the neuronal membrane, whereas cathodal stimulation results in hyperpolarization (Bindman, Lippold, & Redfearn, 1964; Nitsche & Paulus, 2000). More recently, the term “tDCS” has been used to refer to stimulation given with modern, reliable equipment with the potential to deliver higher stimulus intensities (1–3 mA, compared with <1 mA previously). Lasting effects (up to 90 minutes) on neuronal function after a single session of stimulation have been demonstrated (Nitsche & Paulus, 2000). Studies probing the mechanisms of action of tDCS have identified both membrane and synaptic processes (Arul-Anandam & Loo, 2009).
tDCS has been investigated for the treatment of neuropsychiatric disorders, including poststroke rehabilitation (Nitsche et al., 2008) as well as cognitive enhancement (Fox, 2011). In therapeutic applications, treatment sessions are typically repeated on consecutive weekdays over a number of weeks, with empirical data showing cumulative changes in cortical excitability with successive daily sessions (Alonzo, Brassil, Taylor, Martin, & Loo, 2012). From studies to date, the time course for treatment and onset of effects appear to be similar to those of rTMS.
Clinical trials in psychiatry have focused initially on the treatment of depression, with the majority of trials using tDCS technology (since 2000) and reporting positive results. A metaanalysis of the first six randomized, sham-controlled trials (RCTs) post 2000 concluded that tDCS had antidepressant efficacy (effect size of 7.4), though a subsequent metaanalysis of response rates (rather than mean change in depression scores) failed to find a difference between active and sham treatment (Berlim, Van den Eynde, & Daskalakis, 2013). Participants enrolled in these trials ranged from being nontreatment resistant to highly treatment resistant, including having failed ECT. Of the two largest RCTs in depression, Loo et al. (2012) reported a 13% response rate after 3 weeks of tDCS in participants who had failed, on average, two adequate trials of antidepressants (though the response rate increased to 50% after open-label extension to 6 weeks of treatment). In a less treatment-resistant and nonmedicated sample, Brunoni et al. (2013) reported a 43% response rate after 12 sessions of tDCS spaced over 6 weeks (10 sessions over 2 weeks, then 2 additional sessions at fortnightly intervals), with a higher response rate of 63% when tDCS was given in combination with sertraline. Of interest, in this study, which tested the four possible combinations of tDCS or sham stimulation with sertraline or placebo medication, the response to tDCS monotherapy (43%) was higher than the response to monotherapy with sertraline 50 mg (33%). However, inadequate dosing may have contributed to a low response rate in the latter group. Apart from this, there are no data directly comparing the efficacy of tDCS with other antidepressant treatments (including rTMS and ECT).
There is open-label evidence that tDCS may also be effective in bipolar depression (Brunoni et al., 2011), and sham-controlled RCTs of tDCS in bipolar depression are currently in progress. Recently, a sham-controlled RCT found that tDCS reduced the severity of auditory hallucinations in schizophrenia (Brunelin et al., 2012).
In the above studies, tDCS was safe with only minor side effects (most commonly transient skin redness and tingling), though careful attention to treatment technique is necessary, as skin burns may occur if the skin–electrode contact is suboptimal (Loo et al., 2011). Studies suggest that tDCS may actually improve cognitive functioning in the period immediately after stimulation, including data from double-blind, sham-controlled trials in depressed participants (Loo et al., 2012). There are data to suggest that anticonvulsants, which alter ion-channel permeability (e.g., carbamazepine), and benzodiazepines may reduce the effectiveness of tDCS (Brunoni et al., 2012; Nitsche et al., 2003, 2004).
The efficacy and safety profile of tDCS show promise, with early evidence suggesting it may have antidepressant effects that are similar to those of rTMS. However, more studies are needed to confirm efficacy and safety in depression and to investigate its role in treating other psychiatric disorders. tDCS involves inexpensive and portable equipment, which would facilitate large-scale clinical translation. Lack of cognitive impairment and short-term enhancement of cognitive function may be added advantages of treatment.
In the growing universe of neurostimulation techniques, the implantable devices VNS and DBS are in a unique position. From several standpoints, the paradigm of treatment differs dramatically from all other mental health treatment strategies. This is because of the high cost (approximately $35,000 for VNS and $250,000 for DBS), the surgically invasive nature of the procedure, the target population of the most treatment-refractory patients, and a cumulative response pattern, which grows over months and years rather than weeks. The above necessitate a shift in how we study these devices and how we incorporate them into clinical care. We need to create new research instruments or new perspectives as to what constitutes a treatment response on existing scales (perhaps the standard 50% drop in depression rating scale score as a marker for response is too high a barrier for these persistently ill patients). Also, when the time to see significant response is 6 months or longer, sham-controlled blinded studies may be ethically inadvisable.
VNS was first developed and approved by the US Food and Drug Administration (FDA) in 1997 for the treatment of intractable epilepsy. Observations made by investigators and clinicians supported the notion of mood improvement in implanted seizure patients (Elger, Hoppe, Falkai, Rush, & Elger, 2000; Harden et al., 2000). This led to a series of studies in patients with treatment-resistant depression (TRD) and eventual clearance by the FDA for use in depression when at least four treatments had failed (Aaronson et al., 2012; Rush, Marangell, et al., 2005; Sackeim, Rush, et al., 2001). The current FDA-approved device (Cyberonics, Inc., Houston, Texas, US) consists of a titanium-encased lithium battery that is implanted under the skin in the upper chest wall. The battery is connected by a lead wire that is tunneled under the skin to pig-tailed electrodes wrapped around the left vagus nerve. The device is implanted using two incisions under general or local anesthesia in 1 to 2 hours. Two weeks following the surgery, the device is activated and stimulation parameters are set by a wand connected to a handheld programming device. The telemetric programming wand sets the following four stimulation parameters for the device: the current (0.25–3.0 mA), the frequency of stimulation (20–50 Hz), the pulse width (130–500 ms), and the duty cycle (adjustable from the usual settings of 30 seconds on and 5 minutes off). The initial settings are gradually titrated up, usually over the first 2 weeks of treatment as tolerated by the patient. While the mechanism of action is not clear, several animal studies and human neuroimaging studies demonstrate increased limbic activity and changes in neurochemistry after chronic treatment (Carpenter et al., 2004; Conway et al., 2013; Krahl, Senanayake, Pekary, & Sattin, 2004; Neuhaus et al., 2007). VNS demonstrates gradually improving efficacy, which starts about 6 months into treatment and grows over time. Studies have shown improving outcomes at 1 and 2 years (Bajbouj et al., 2010; Nahas et al., 2005; Nierenberg, Alpert, Gardner-Schuster, Seay, & Mischoulon, 2008).
VNS has been studied in highly treatment-resistant populations. The first open-label studies comprised a total of 60 patients including both unipolar and bipolar depression (Rush et al., 2000; Sackeim, Rush, et al., 2001). The average number of adequate failed antidepressant trials in these studies was at least two and averaged between four and five. Response rates at 10 to 12 weeks were between 30% and 40% and remission rates were 14%–17%. Remarkably, naturalistic long-term follow-up on these patients showed continued improvement at 1 and 2 years of chronic treatment with response rates at 44% and 42% and remission rates at 27% and 22%, respectively (Aaronson et al., 2012; Nahas et al., 2005; Rush, Sackeim, et al., 2005).
A subsequent VNS trial was a randomized trial with 235 patients that included a 12-week sham-controlled phase followed by a long-term observational phase (Rush, Marangell, et al., 2005). Patients enrolled in this study had a higher level of treatment resistance, having failed at least four antidepressant trials inclusive of monotherapy and adjunctive agents. At the end of the 12-week sham treatment, there was no significant difference between sham and active treatment groups (15% active versus 10% sham response rates). Follow-up observations again demonstrated a cumulative improvement in response and remission, with response rates between 27% and 34% depending on outcome measure and a remission rate of 16% at 1 year (Rush, Sackeim, et al., 2005). Also, longer-term data support a decline in suicide attempts and psychiatric hospitalizations for depression in patients with VNS compared to patients on medications alone.
A recently published VNS dose-finding study compared three levels as follows: low (0.25 mA and 130-ms pulse width), med (1.0 mA and 250-ms pulse width), and high (1.5 mA and 250-ms pulse width) of double-blinded stimulation in 331 highly treatment-resistant patients (Aaronson et al., 2012). More than 97% had failed at least six previous treatments. Acute-phase treatment lasted 22 weeks, after which output current could be increased by up to 0.75 mA and follow-up was done for up to 50 weeks. While the treatment arms did not show significant separation at 22 weeks, all groups showed significant improvement on the primary outcome measure (a clinician-rated inventory of depressive symptoms [IDS-C]). In the long-term phase, mean change in IDS-C scores showed continued improvement. An analysis of acute-phase responders demonstrated significantly greater durability of response at the med and high doses than at the low dose. At 22 weeks, overall response rates were 20% and remission rates were 10%. At 50 weeks, overall response rates varied from 27% to 53%, depending on the scale and the assigned group, and remission rates ranged from 15% to 23%. The percent of acute-phase responders who continued to respond at 50 weeks varied from 77% to 92% of the med- and high-dose groups compared to 44%–69% of the low-dose group.
There is a large safety-related database given that the majority of VNS implantations are for control of seizures. The device is well tolerated and the retention of patients through these studies has been very high. The major adverse events are related to the surgery for implantation, which are self-limited or associated with the stimulation part of the duty cycle. The electrode for the VNS is typically wrapped around the left vagus nerve near the superior and recurrent laryngeal nerves. During stimulation, this can cause voice alterations, hoarseness, or coughing, which are all frequently seen. Bradyarrhythmias and sleep apnea are rare. The rate of stimulation-induced mania or hypomania is low, and adjustment of treatment parameters is likely to reduce symptoms.
Despite evidence that VNS can be an effective treatment for patients who have failed many adequate medication trials, several difficulties have plagued the development of VNS as a therapy for TRD and hampered large research trials. It takes at least 6 months, possibly more, to see antidepressant effects. As the first major VNS trial had 12 weeks from implantation as its primary outcome measure point, the double-blind phase was likely insufficiently long to demonstrate significant separation. Even the longer 22-week dose-finding study may have been of insufficient length. A question can be raised from an ethical standpoint as to how long should seriously ill patients be treated within a sham-controlled study.
Another complication seen in the dose-finding study is that the low-dose arm (0.25 mA and 125-ms pulse width) was intended to be a surrogate for sham treatment. As it turned out, even this low-dose arm demonstrated significant response compared to the in-group baseline. Thus, primary outcome measures were not met, though patients treated with the low dose had a significantly less durable response than higher-dose groups. This inability to meet primary outcome measures in two large studies has given third-party payers an excuse to deny coverage despite FDA clearance of VNS for use in the severe TRD population.
DBS is an established treatment option for patients with severe, medication-resistant movement disorders (e.g., Parkinson disease, essential tremor, dystonia). DBS of the ventral anterior internal capsule and ventral striatum (VC/VS) has been approved through a Humanitarian Device Exemption from the FDA for the treatment of severe treatment-refractory obsessive-compulsive disorder (OCD; Greenberg et al., 2010). With DBS, one or more electrodes (typically with several individual contacts per electrode) are implanted into a specific brain region using stereotactic neurosurgical techniques. These electrodes are connected to a subcutaneous computer/battery pack (also called an implantable pulse generator [IPG]) through wires that run under the skin from the scalp to the IPG location. The most significant potential adverse events associated with DBS are related to implantation surgery (bleeding, infection, complications from anesthesia). Other adverse events are typically related to specific effects based on the target for stimulation. DBS likely operates through modulation of activity within a broad network of brain regions involved in regulation of a specific behavioral system.
Several studies have tested the safety and efficacy of DBS for TRD. A variety of brain targets have been explored. Individual studies have universally been small (up to 20 patients), uncontrolled, and open label (for long-term effects). However, the patients included represent those who are the most treatment resistant; most studies have included patients with chronic depression (often with a current depressive episode lasting an average of 4 or more years) that have failed a large number of treatments in the current episode (an average of six to seven medications, electroconvulsive therapy, and psychotherapy). Nearly all patients included have become disabled due to their depression.
The DBS target with the earliest published data for use in TRD patients is the subcallosal cingulate white matter (SCCwm). Choice of this target was based on a converging database that implicated this region in the neurobiology of the antidepressant response and TRD (Mayberg, 2009). With chronic SCCwm DBS up to several years, remission rates have been shown to be approximately 40%–60% (Guinjoan et al., 2010; Holtzheimer et al., 2012; Kennedy et al., 2011; Lozano et al., 2008; Mayberg et al., 2005; Puigdemont et al., 2011). Those with depression in the context of bipolar disorder may respond as well as those in the context of major depressive disorder (Holtzheimer et al., 2012). Depressive relapse has been uncommon among remitters. No adverse effects of acute or chronic SCCwm DBS have been identified, including no neurocognitive impairments (Holtzheimer et al., 2012; McNeely, Mayberg, Lozano, & Kennedy, 2008).
Other trials have demonstrated efficacy for DBS of the VC/VS target previously used for OCD. Choice of this target was based on the antidepressant effects seen in OCD patients receiving stimulation at this target (Malone et al., 2009). A similar but smaller target has been the nucleus accumbens, which makes up a significant portion of the ventral striatum (Bewernick et al., 2010; Bewernick, Kayser, Sturm, & Schlaepfer, 2012). An additional rationale for this target was its role in reward processing and potentially anhedonia, which is a prominent symptom in the depressive syndrome (Schlaepfer et al., 2008). Efficacy with each of these DBS targets was similar to that seen with SCCwm DBS. With both of these targets, acute stimulation was associated with a number of side effects, including hypomania (a mild degree of mania), anxiety, perseverative speech (the persistent repetition of a word or phrase), autonomic symptoms, and involuntary facial movements. These effects were reversible with changes in stimulation parameters, and there were no adverse effects of chronic DBS, including no neuropsychological impairments.
The median forebrain bundle (MFB) is a collection of ascending and descending white matter fibers that connect the midbrain and ventral striatum. Based on its presumed role in reward processing as well as connection to other potential targets for DBS for TRD, it was hypothesized that bilateral stimulation of the MFB would have antidepressant efficacy in patients with TRD (Coenen, Panksepp, Hurwitz, Urbach, & Madler, 2012; Schlaepfer, Bewernick, Kayser, Madler, & Coenen, 2013). An initial report of MFB DBS for TRD described clinical response in six of seven patients with at least 12 weeks of open-label stimulation, with four patients achieving remission (Schlaepfer et al., 2013). A number of adverse events were noted, including vision/eye movement changes in all seven patients (related to specific stimulation parameters). No cognitive impairments were noted over the 3 months of stimulation.
Case reports have described antidepressant efficacy for DBS of the inferior thalamic peduncle (Jimenez et al., 2005) and habenula (Sartorius et al., 2010). The rationale for the inferior thalamic peduncle target included its role in the thalamo-cortical network likely involved in mood regulation. The habenula target was chosen based on its role in monoaminergic neurotransmission, especially to prefrontal cortex.
DBS shows early promise as a potential treatment for TRD. However, caution is advised because data to date are based on small open-label trials. These encouraging but preliminary data will need validation in large, randomized, controlled trials. Ideally, these studies will include appropriate sham-controlled designs. As with VNS, the antidepressant effects of DBS appear to take several weeks to months to become apparent. This again raises the ethical and scientific questions of what an appropriate sham-controlled study duration should be.
Key factors to consider when choosing the most appropriate brain stimulation treatment are the level of the patient’s treatment resistance and the relative efficacy of the treatments, predictors of response, clinical urgency and risk, feasibility, tolerability and safety of the treatments, patient preference, and type of depression (bipolar, melancholic, psychotic, catatonic features). At the time of writing (2013), only ECT, rTMS, and VNS are considered FDA-approved treatments for depression. However, the potential roles for MST, tDCS, and DBS are discussed here in light of the research evidence available to date.
For moderately depressed patients who are not significantly treatment refractory and not at immediate clinical risk (such that a rapid response is a priority), rTMS would be the preferred nonpharmacologic somatic treatment. It is nonconvulsive, does not require general anesthesia or a surgical procedure, and is safe and well tolerated. tDCS may also be an option, as evidence to date suggests the time course of treatment and efficacy outcomes are similar to those of rTMS, with arguably a superior safety profile (i.e., no risk of accidental seizures). For patients who are moderately treatment resistant (e.g., have failed one to two adequate courses of pharmacotherapy), appropriate treatment options would be rTMS (or tDCS) and ECT (or MST). The choice of treatment should take into account the severity of the illness, clinical urgency, physical risks (e.g., risk of accidental seizure with rTMS, risk from anesthesia with ECT and MST), and patient preference. ECT is more efficacious than rTMS, induces improvement more quickly, and carries a higher likelihood of full clinical response. This has to be weighed against the higher likelihood of cognitive side effects with ECT, though the risk varies widely with the form of ECT used. MST is likely to have a treatment role in this group, as preliminary studies suggest it may have efficacy that is comparable to that of weaker forms of ECT, with minimal cognitive side effects. Likewise, tDCS may have a role akin to that of rTMS in this group. For more highly treatment-resistant patients (e.g., those who have failed four or more antidepressant treatments, including any trials of rTMS or tDCS), the treatment of choice would be ECT (or MST), VNS, and possibly DBS
Patients who are depressed with psychotic or catatonic features should be given ECT rather than rTMS, as there is insufficient evidence for the efficacy of rTMS in severe depression characterized by psychosis and/or catatonia. Speed of response is an important consideration. In situations of clinical urgency (e.g., high suicide risk, poor oral intake due to severe depression, catatonia, psychotic symptoms), the treatment of choice would be ECT rather than TMS, tDCS, MST, VNS, or DBS.
In patients with extreme TRD who have failed to respond to an adequate trial of ECT, it is unlikely that TMS, tDCS, or MST would be effective. Appropriate treatment options would then be VNS or DBS. Thus, patients who have failed to respond to antidepressant medications (and/or rTMS, tDCS, or MST) should next be offered ECT. If this treatment fails, VNS or DBS could be considered.
For patients successfully treated with ECT who tend to relapse into depression despite adequate pharmacotherapy, maintenance ECT in combination with medications has been shown to reduce the risk of relapse. Likewise, there may be a role for maintenance rTMS in patients who have responded to an acute course of rTMS but who relapse with medication prophylaxis alone. The optimal schedule for maintenance rTMS sessions and the patients most likely to benefit need to be clarified by further research. Whether rTMS has a role substituting for maintenance ECT is unclear (Figure 10.1).
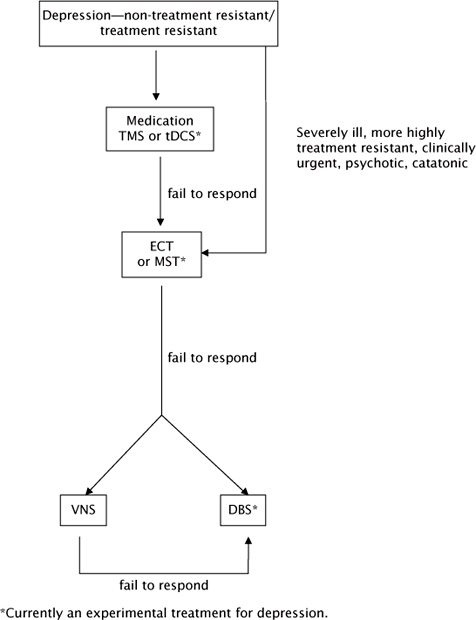
FIGURE 10.1 Flowchart showing the role of rTMS and other therapies in the treatment of depression.
Efficacy has been demonstrated for rTMS in treating auditory hallucinations in schizophrenia, though its effects on other features of the disease are less clear (Matheson, Green, Loo, & Carr, 2010). Thus, for patients with schizophrenia where persistent auditory hallucinations despite treatment with antipsychotic medications are prominent and problematic, a trial of rTMS should be considered. If the problem is of more general acute psychotic symptoms despite treatment with antipsychotic medications, it would be more appropriate to consider ECT rather than rTMS. At present, there are insufficient data to recommend the clinical use of tDCS, MST, VNS, or DBS in schizophrenia.
Aaronson, S. T., Carpenter, L. L., Conway, C. R., Reimherr, F. W., Lisanby, S. H., Schwartz, T. L., … Bunker, M. (2012). Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: Acute and chronic effects. Brain Stimululation, doi:10.1016/j.brs.2012.09.013
Alonzo, A., Brassil, J., Taylor, J. L., Martin, D., & Loo, C. K. (2012). Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimulation, 5(3), 208–213. doi:10.1016/j.brs.2011.04.006
American Psychiatric Association (Ed.). (2001). The practice of electroconvulsive therapy: Recommendations for treatment, training, and privileging (2nd ed.). Washington, DC: American Psychiatric Publishing.
Arul-Anandam, A. P., & Loo, C. (2009). Transcranial direct current stimulation: a new tool for the treatment of depression? Journal Affective Disorders, 117(3), 137–145. doi:10.1016/j.jad.2009.01.016
Bajbouj, M., Merkl, A., Schlaepfer, T. E., Frick, C., Zobel, A., Maier, W., … Heuser, I. (2010). Two-year outcome of vagus nerve stimulation in treatment-resistant depression. Journal Clinical Psychopharmacoogy, 30(3), 273–281. doi:10.1097/JCP.0b013e3181db8831 00004714-201006000-00009 [pii]
Berlim, M. T., Van den Eynde, F., & Daskalakis, Z. J. (2013). Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Journal Psychiatric Research, 47(1), 1–7. doi:10.1016/j.jpsychires.2012.09.025
Bewernick, B. H., Hurlemann, R., Matusch, A., Kayser, S., Grubert, C., Hadrysiewicz, B.,… Schlaepfer, T. E. (2010). Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological Psychiatry, 67(2), 110–116. doi:S0006-3223(09)01094-4 [pii] 10.1016/j.biopsych.2009.09.013 [doi]
Bewernick, B. H., Kayser, S., Sturm, V., & Schlaepfer, T. E. (2012). Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology, 37(9), 1975–1985. doi:10.1038/npp.2012.44
Bindman, L. J., Lippold, O. C., & Redfearn, J. W. (1964). The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. Journal Physiology, 172, 369–382.
Brunelin, J., Mondino, M., Gassab, L., Haesebaert, F., Gaha, L., Suaud-Chagny, M. F., … Poulet, E. (2012). Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. American Journal Psychiatry, 169(7), 719–724. doi:10.1176/appi.ajp.2012.11071091
Brunoni, A. R., Ferrucci, R., Bortolomasi, M., Scelzo, E., Boggio, P. S., Fregni, F., … Priori, A. (2012). Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the Major Depressive Episode: Findings from a naturalistic study. European Psychiatry. doi:10.1016/j.eurpsy.2012.09.001
Brunoni, A. R., Ferrucci, R., Bortolomasi, M., Vergari, M., Tadini, L., Boggio, P. S., … Priori, A. (2011). Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Progress Neuropsychopharmacology Biological Psychiatry, 35(1), 96–101. doi:10.1016/j.pnpbp.2010.09.010
Brunoni, A. R., Valiengo, L., Baccaro, A., Zanao, T. A., de Oliveira, J. F., Goulart, A., … Fregni, F. (2013). The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry, 70(4), 383–391. doi:10.1001/2013.jamapsychiatry.32
Carpenter, L. L., Moreno, F. A., Kling, M. A., Anderson, G. M., Regenold, W. T., Labiner, D. M., & Price, L. H. (2004). Effect of vagus nerve stimulation on cerebrospinal fluid monoamine metabolites, norepinephrine, and gamma-aminobutyric acid concentrations in depressed patients. Biological Psychiatry, 56(0), 418–426.
Coenen, V. A., Panksepp, J., Hurwitz, T. A., Urbach, H., & Madler, B. (2012). Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. Journal Neuropsychiatry Clinical Neuroscience, 24(2), 223–236. doi:10.1176/appi.neuropsych.11080180
Conway, C. R., Chibnall, J. T., Gebara, M. A., Price, J. L., Snyder, A. Z., Mintun, M. A., … Sheline, Y. I. (2013). Association of cerebral metabolic activity changes with vagus nerve stimulation antidepressant response in treatment-resistant depression. Brain Stimululation. doi:10.1016/j.brs.2012.11.006
Daly, J.J., Prudic, J., Devanand, D. P., Nobler, M. S., Lisanby, S. H., Peyser, S., … Sackeim, H. A. (2001). ECT in bipolar and unipolar depression: differences in speed of response. Bipolar Disorders, 3(2), 95–104.
de Vreede, I. M., Burger, H., & van Vliet, I. M. (2005). Prediction of response to ECT with routinely collected data in major depression. Journal Affective Disorders, 86(2–3), 323–327.
Dombrovski, A. Y., Mulsant, B. H., Haskett, R. F., Prudic, J., Begley, A. E., & Sackeim, H. A. (2005). Predictors of remission after electroconvulsive therapy in unipolar major depression. Journal Clinical Psychiatry, 66(8), 1043–1049.
Lancet. (2003). Lancet, 361(9360), 799–808. doi:S0140-6736(03)12705-5 [pii] 10.1016/S0140-6736(03) 12705-5
Elger, G., Hoppe, C., Falkai, P., Rush, A. J., & Elger, C. E. (2000). Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res, 42(2-3), 203–210.
Fink, M. (2001). Convulsive therapy: a review of the first 55 years. Journal Affective Disorders, 63(1-3), 1–15.
Fitzgerald, P. B., Hoy, K. E., Herring, S. E., Clinton, A. M., Downey, G., & Daskalakis, Z. J. (2013). Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depression Anxiety, 30(2), 129–136. doi:10.1002/da.22005
Fox, D. (2011). Neuroscience: Brain buzz. Nature, 472(7342), 156–158. doi:10.1038/472156a
Gazdag, G., Ungvari, G. S., & Caroff, S. N. (2009). Clinical evidence for the efficacy of electroconvulsive therapy in the treatment of catatonia and psychoses. In C. Swartz (Ed.), Electroconvulsive therapy and neuromodulation therapies (pp. 124–148): Cambridge University Press.
Greenberg, B. D., Gabriels, L. A., Malone, D. A., Jr., Rezai, A. R., Friehs, G. M., Okun, M. S.,… Nuttin, B. J. (2010). Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Molecular Psychiatry, 15, 64–79.
Grunhaus, L., Dannon, P. N., Schreiber, S., Dolberg, O. H., Amiaz, R., Ziv, R., & Lefkifker, E. (2000). Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biological Psychiatry, 47(4), 314–324.
Guinjoan, S. M., Mayberg, H. S., Costanzo, E. Y., Fahrer, R. D., Tenca, E., Antico, J., … Nemeroff, C. B. (2010). Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation. J Neuropsychiatry Clinical Neuroscience, 22(3), 265–277. doi:22/3/265 [pii] 10.1176/appi.neuropsych.22.3.265 [doi]
Harden, C. L., Pulver, M. C., Ravdin, L. D., Nikolov, B., Halper, J. P., & Labar, D. R. (2000). A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behavior, 1(2), 93–99.
Holtzheimer, P. E., Kelley, M. E., Gross, R. E., Filkowski, M. M., Garlow, S. J., Barrocas, A., … Mayberg, H. S. (2012). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Archices General Psychiatry, 69(2), 150–158. doi:10.1001/archgenpsychiatry.2011.1456
Jimenez, F., Velasco, F., Salin-Pascual, R., Hernandez, J. A., Velasco, M., Criales, J. L., & Nicolini, H. (2005). A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery, 57(3), 585–593; discussion 585-593.
Kayser, S., Bewernick, B., Axmacher, N., & Schlaepfer, T. E. (2008). Magnetic Seizure Therapy of Treatment-Resistant Depression in a Patient With Bipolar Disorder. Journal of ECT.
Kayser, S., Bewernick, B. H., Grubert, C., Hadrysiewicz, B. L., Axmacher, N., & Schlaepfer, T. E. (2011). Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. Journal Psychiatric Research, 45(5), 569–576. doi:10.1016/j.jpsychires.2010.09.008
Kellner, C. H., Knapp, R. G., Petrides, G., Rummans, T. A., Husain, M. M., Rasmussen, K., … Fink, M. (2006). Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Archives General Psychiatry, 63(12), 1337–1344.
Kellner, C. H., Knapp, R., Husain, M. M., Rasmussen, K., Sampson, S., Cullum, M., … Petrides, G. (2010). Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. British Journal Psychiatry, 196, 226–234. doi:196/3/226 [pii] 10.1192/bjp.bp.109.066183 [doi]
Kennedy, S. H., Giacobbe, P., Rizvi, S. J., Placenza, F. M., Nishikawa, Y., Mayberg, H. S., & Lozano, A. M. (2011). Deep Brain Stimulation for Treatment-Resistant Depression: Follow-Up After 3 to 6 Years. American Journal Psychiatry. doi:appi.ajp.2010.10081187 [pii] 10.1176/appi.ajp.2010.10081187
Kho, K. H., van Vreeswijk, M. F., Simpson, S., & Zwinderman, A. H. (2003). A meta-analysis of electroconvulsive therapy efficacy in depression. Journal of ECT, 19(3), 139–147.
Kho, K. H., Zwinderman, A. H., & Blansjaar, B. A. (2005). Predictors for the efficacy of electroconvulsive therapy: chart review of a naturalistic study. Journal Clinical Psychiatry, 66(7), 894–899.
Kosel, M., Frick, C., Lisanby, S. H., Fisch, H. U., & Schlaepfer, T. E. (2003). Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology, 28(11), 2045–2048.
Krahl, S. E., Senanayake, S. S., Pekary, A. E., & Sattin, A. (2004). Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. Journal Psychiatric Research, 38(3), 237–240.
Lisanby, S. H. (2007). Electroconvulsive therapy for depression. New England Journal Medicine, 357(19), 1939–1945. doi:357/19/1939 [pii] 10.1056/NEJMct075234 [doi]
Lisanby, S. H., Luber, B., Finck, A. D., Schroeder, C., & Sackeim, H. A. (2001). Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Archives General Psychiatry, 58(2), 199–200.
Lisanby, S. H., Luber, B., Schlaepfer, T. E., & Sackeim, H. A. (2003). Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology, 28(10), 1852–1865.
Loo, C. K., Alonzo, A., Martin, D., Mitchell, P. B., Galvez, V., & Sachdev, P. (2012). Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. British Journal Psychiatry, 200(1), 52–59. doi:10.1192/bjp.bp.111.097634
Loo, C. K., Martin, D. M., Alonzo, A., Gandevia, S., Mitchell, P. B., & Sachdev, P. (2011). Avoiding skin burns with transcranial direct current stimulation: preliminary considerations. International Journal Neuropsychopharmacology, 14(3), 425–426. doi:10.1017/S1461145710001197
Loo, C. K., Sainsbury, K., Sheehan, P., & Lyndon, B. (2008). A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. International Journal Neuropsychopharmacology, 11(7), 883–890. doi:10.1017/S1461145708009292
Loo, C., Katalinic, N., Mitchell, P. B., & Greenberg, B. (2011). Physical treatments for bipolar disorder: a review of electroconvulsive therapy, stereotactic surgery and other brain stimulation techniques. Journal Affective Disorders, 132(1-2), 1–13. doi:10.1016/j.jad.2010.08.017
Lozano, A. M., Mayberg, H. S., Giacobbe, P., Hamani, C., Craddock, R. C., & Kennedy, S. H. (2008). Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological Psychiatry, 64(6), 461–467. doi:S0006-3223(08)00703-8 [pii] 10.1016/j.biopsych.2008.05.034
Malone, D. A., Jr., Dougherty, D. D., Rezai, A. R., Carpenter, L. L., Friehs, G. M., Eskandar, E. N.,… Greenberg, B. D. (2009). Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biological Psychiatry, 65(4), 267–275. doi:S0006-3223(08)01083-4 [pii] 10.1016/j.biopsych.2008.08.029
Matheson, S. L., Green, M. J., Loo, C., & Carr, V. J. (2010). Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophrenia Research, 118(1–3), 201–210. doi:10.1016/j.schres.2010.01.002
Mayberg, H. S. (2009). Targeted electrode-based modulation of neural circuits for depression. Journal Clinical Investigation, 119(4), 717–725. doi:38454 [pii] 10.1172/JCI38454
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H. E., Seminowicz, D., Hamani, C., … Kennedy, S. H. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45(5), 651–660.
McNeely, H. E., Mayberg, H. S., Lozano, A. M., & Kennedy, S. H. (2008). Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. Journal Nervous Mental Disease, 196(5), 405–410. doi:10.1097/NMD.0b013e3181710927 00005053-200805000-00007 [pii]
Moreines, J. L., McClintock, S. M., & Holtzheimer, P. E. (2011). Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: a review. Brain Stimulation, 4(1), 17–27.
Nahas, Z., Marangell, L. B., Husain, M. M., Rush, A. J., Sackeim, H. A., Lisanby, S. H., … George, M. S. (2005). Two-Year Outcome of Vagus Nerve Stimulation (VNS) for Treatment of Major Depressive Episodes. Journal Clinical Psychiatry, 66(9), 1097–1104.
Neuhaus, A. H., Luborzewski, A., Rentzsch, J., Brakemeier, E. L., Opgen-Rhein, C., Gallinat, J., & Bajbouj, M. (2007). P300 is enhanced in responders to vagus nerve stimulation for treatment of major depressive disorder. Journal Affective Disorders, 100(1–3), 123–128. doi:10.1016/j.jad.2006.10.005
NICE Clinical Guidelines. (October 2009). The treatment and management of depression in adults. guideline. nice.org.uk/cg90.
Nierenberg, A. A., Alpert, J. E., Gardner-Schuster, E. E., Seay, S., & Mischoulon, D. (2008). Vagus nerve stimulation: 2-year outcomes for bipolar versus unipolar treatment-resistant depression. Biological Psychiatry, 64(6), 455–460. doi:S0006-3223(08)00581-7 [pii] 10.1016/j.biopsych.2008.04.036
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., … Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3), 206–223. doi:S1935-861X(08)00040-5 [pii] 10.1016/j.brs.2008.06.004 [doi]
Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., Lang, N., … Paulus, W. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal Physiology, 553(Pt 1), 293–301. doi:10.1113/jphysiol.2003.049916 [doi] jphysiol.2003.049916 [pii]
Nitsche, M. A., Liebetanz, D., Schlitterlau, A., Henschke, U., Fricke, K., Frommann, K., … Tergau, F. (2004). GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. European Journal Neuroscience, 19(10), 2720–2726. doi:10.1111/j.0953-816X.2004.03398.x [doi] EJN3398 [pii]
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal Physiology, 527 Pt 3, 633–639. doi:PHY_1055 [pii]
Petrides, G., Fink, M., Husain, M. M., Knapp, R. G., Rush, A. J., Mueller, M., … Kellner, C. H. (2001). ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. Journal of ECT, 17(4), 244–253.
Prudic, J., Haskett, R. F., McCall, W. V., Isenberg, K., Cooper, T., Rosenquist, P. B., … Sackeim, H. A. (2013). Pharmacological strategies in the prevention of relapse after electroconvulsive therapy. Journal of ECT, 29(1), 3–12. doi:10.1097/YCT.0b013e31826ea8c4
Prudic, J., Olfson, M., Marcus, S. C., Fuller, R. B., & Sackeim, H. A. (2004). Effectiveness of electroconvulsive therapy in community settings. Biological Psychiatry, 55(3), 301–312. doi:S0006322303010461 [pii]
Puigdemont, D., Perez-Egea, R., Portella, M. J., Molet, J., de Diego-Adelino, J., Gironell, A., … Perez, V. (2011). Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. International Journal Neuropsychopharmacology, 1–13. doi:10.1017/s1461145711001088
Rush, A. J., George, M. S., Sackeim, H. A., Marangell, L. B., Husain, M. M., Giller, C., … Goodman, R. (2000). Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biological Psychiatry, 47(4), 276–286.
Rush, A. J., Marangell, L. B., Sackeim, H. A., George, M. S., Brannan, S. K., Davis, S. M., … Cooke, R. G. (2005). Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biological Psychiatry, 58(5), 347–354.
Rush, A. J., Sackeim, H. A., Marangell, L. B., George, M. S., Brannan, S. K., Davis, S. M., … Barry, J. J. (2005). Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biological Psychiatry, 58(5), 355–363.
Sackeim, H. A., Haskett, R. F., Mulsant, B. H., Thase, M. E., Mann, J. J., Pettinati, H. M., … Prudic, J. (2001). Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. Journal American Medical Association, 285(10), 1299–1307.
Sackeim, H. A., Prudic, J., Devanand, D. P., Nobler, M. S., Lisanby, S. H., Peyser, S., … Clark, J. (2000). A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives General Psychiatry, 57(5), 425–434.
Sackeim, H. A., Prudic, J., Fuller, R., Keilp, J., Lavori, P. W., & Olfson, M. (2007). The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology, 32(1), 244–254.
Sackeim, H. A., Prudic, J., Nobler, M. S., Fitzsimons, L., Lisanby, S. H., Payne, N., … Devanand, D. P. (2008). Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulation, 1(2), 71–83. doi:10.1016/j.brs.2008.03.001 [doi]
Sackeim, H. A., Rush, A. J., George, M. S., Marangell, L. B., Husain, M. M., Nahas, Z., … Goodman, R. R. (2001). Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology, 25(5), 713–728.
Sartorius, A., Kiening, K. L., Kirsch, P., von Gall, C. C., Haberkorn, U., Unterberg, A. W., … Meyer-Lindenberg, A. (2010). Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biological Psychiatry, 67(2), e9-e11. doi:S0006-3223(09)01047-6 [pii] 10.1016/j. biopsych.2009.08.027 [doi]
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Madler, B., & Coenen, V. A. (2013). Rapid Effects of Deep Brain Stimulation for Treatment-Resistant Major Depression. Biological Psychiatry. doi:10.1016/j. biopsych.2013.01.034
Schlaepfer, T. E., Cohen, M. X., Frick, C., Kosel, M., Brodesser, D., Axmacher, N., … Sturm, V. (2008). Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology, 33(2), 368–377.
Semkovska, M., & McLoughlin, D. M. (2010). Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biological Psychiatry, 68(6), 568–577. doi:10.1016/j.biopsych.2010.06.009
Sienaert, P., Vansteelandt, K., Demyttenaere, K., & Peuskens, J. (2009b). Ultra-brief pulse ECT in bipolar and unipolar depressive disorder: differences in speed of response. Bipolar Disorders, 11(4), 418–424. doi:BDI702 [pii] 10.1111/j.1399–5618.2009.00702.x
Sienaert, P., Vansteelandt, K., Demyttenaere, K., & Peuskens, J. (2010). Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: cognitive side-effects. Journal Affective Disorders, 122(1-2), 60–67. doi:S0165-0327(09)00271-7 [pii] 10.1016/j.jad.2009.06.011
Slotema, C. W., Blom, J. D., de Weijer, A. D., Diederen, K. M., Goekoop, R., Looijestijn, J., … Sommer, I. E. (2011). Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biological Psychiatry, 69(5), 450–456. doi:10.1016/j.biopsych.2010.09.051
Tharyan, P., & Adams, C.E. (2005). Electroconvulsive therapy for schizophrenia. Cochrane Database Systemic Reviews, 2(CD000076.).
White, P. F., Amos, Q., Zhang, Y., Stool, L., Husain, M. M., Thornton, L., … Lisanby, S. H. (2006). Anesthetic considerations for magnetic seizure therapy: a novel therapy for severe depression. Anesthesia Analgesia, 103(1), 76–80, table of contents.