Transcranial magnetic stimulation (TMS) represents a paradigm shift for clinical psychiatry. Never before have clinicians been able to focally target the neurocircuitry that underlies brain-based disorders noninvasively. Now our models for the pathophysiology of psychiatric disorders can be tested via an intervention that can both test hypotheses about neurocircuitry as well as deliver therapy that changes the functioning of that circuitry in a lasting fashion.
TMS offers the unprecedented promise of moving beyond what psychotherapy and psychopharmacology can do for patients and providing an effective alternative when these other interventions are unsuccessful or cannot be tolerated due to side effects. It also offers the compelling promise of circuit-guided treatment, by which one can leverage advances in neuroscience regarding the distributed networks underlying the disorder and use these as targets to refine the application of an intervention to correct abnormal functioning in these network. Such an approach offers the dual features of being focused on the underlying neurocircuitry as well as representing a means of tailoring the focus to each individual.
As exciting as these developments are for our field, there remain certain limitations to the present-day practice of clinical TMS in neuropsychiatry. These limitations can be categorized as those relating to the efficacy of TMS (including its therapeutic potency, therapeutic spectrum, approaches to patient selection, and individualization of the treatment), the safety of TMS (including seizure risk and safety in special populations), and clinical trial design/methodologies (including valid sham conditions, effective masking, auditory and somatosensory confounds, and reporting of parameters and procedures to enable valid replication).
In this chapter we review those limitations, present them in the context of the historical development of the first neuromodulation treatment in psychiatry (electroconvulsive therapy [ECT]), and highlight future directions to address those limitations. ECT technique has undergone significant evolution over its seven decades of use. Likewise, we expect TMS technique to evolve with time and lead to future refinements in the technology and its clinical application.
The antidepressant efficacy of TMS, while statistically significant and supported by multiple metaanalyses, is modest and is widely considered to be less effective than the gold standard ECT. The overall effect size of TMS in the pivotal trial sponsored by the manufacturer was 0.55 (confidence interval [CI], 0.10–1.00), which is considered a moderate effect. A key predictor of response was the number of failed adequate antidepressant trials in the current episode. Effect size was 0.83 (CI, 0.20–1.48) for those patients with a single adequate medication trial failure in the current episode and 0.42 (CI, −0.30–1.15) in those patients with more than one adequate medication failure in the current episode (Lisanby et al., 2009). However, it is the more medication-resistant patients who are in the most need of safe and effective alternatives and for whom ECT remains the treatment of choice. The current US Food and Drug Administration (FDA)-labeled indication for TMS is limited to this subset of the depression population with a single failed adequate trial in the current episode. To have a greater clinical impact, broadening this narrow therapeutic spectrum will be important, so that patients who are in the most need of safe and effective alternatives will have options.
Randomized controlled trials and metaanalyses support a higher observed acute response rate with ECT than with TMS (though it is acknowledged that such trials cannot mask the patient-to-treatment condition because one involves anesthesia and seizure induction with the attendant side effects while the other does not; Eranti et al., 2007; Hansen et al., 2011; Keshtkar, Ghanizadeh, & Firoozabadi, 2011; McLoughlin et al., 2007). Studies also report ECT as being more cost effective than TMS (Knapp et al., 2008).
Furthermore, despite descriptions of rapid response after 1 or 2 weeks of treatment in early studies, larger trials support a speed of action that more closely mimics antidepressant medications, with optimal response at 6 weeks (George et al., 2010; O’Reardon et al., 2007). This makes TMS less effective and slower acting than ECT, albeit with a superior safety profile.
Lacking at present is deep knowledge about how best to maintain remission following effective treatment with TMS. Durability of response following remission from TMS appears to be robust (Mantovani et al., 2012), but knowledge of optimal relapse prevention strategies and maintenance TMS schedules is limited.
As with other treatments in psychiatry and the rest of medicine, therapeutic response to TMS is variable. Aside from history of medication resistance, other factors have been identified as contributing to response, such as age, duration of illness, and comorbidity. Lacking at present is knowledge of how to select patients most likely to respond to TMS and how to personalize the intervention to optimize their response. Given the focality of TMS and the fact that there are known individual differences in anatomy, physiology, and likely heterogeneity in the neurocircuitry underlying depression, new tools to inform the design of optimally effective TMS treatment protocols would be helpful. A handful of approaches to enhance the potency of TMS are reviewed here (e.g., novel coils, image guidance, parameter optimization), but there is limited knowledge of how to select among these options for an individual patient or when to abandon the standard treatment protocol of high-frequency TMS to the left dorsolateral prefrontal cortex (DLPFC) in favor of one of these enhanced treatment paradigms.
Of particular concern is optimizing outcomes for geriatric patients, who represent the bulk of those currently receiving ECT. Given the prevalence of cognitive impairment and dementia in the elderly and given their increased risk of suicide, safe and effective alternatives for these patients is a compelling public health priority. Unfortunately, some studies (Pallanti et al., 2012) suggest that the elderly respond less well to TMS than their younger counterparts (Aguirre et al, 2011), though not all studies have found this to be the case (Ciobanu, Girard, Marin, Labrunie, & Malauzat, 2013). While Jorge et al. (2008) found a significant effect of active TMS relative to sham in vascular depression in the elderly, advanced age and frontal gray matter atrophy were associated with worse outcomes.
This purported age effect may be multifactorial, relating to cortical atrophy causing a reduction in the actual delivered dosage of induced electrical current in the brain (If this were the case, it could be counteracted by delivering a distance-adjusted intensity of TMS as proposed in by Nahas et al., 2004.), differences in the etiology of depression in the elderly (e.g., vascular etiology resulting in a disconnection syndrome such that lateral TMS targets are less well connected with transsynaptic limbic regions that may be vital for antidepressant response), and/or aging effects on the neuroplastic action of TMS itself, as has been reported in a preclinical model (Levkovitz & Segal, 2001). There may also be interactions between age and gender, implicating changes in reproductive hormone status as affecting response to TMS (Huang, Wei, Chou, & Su, 2008).
The modest efficacy of TMS as currently practiced calls for further work directed at enhancing efficacy, particularly in the medication-resistant and geriatric populations. The field also needs better and more reliable methods of predicting response and individualizing dosage to optimize efficacy. In addition to enhancing overall efficacy, approaches to accelerate response and guidelines for maintenance schedules to prolong remission and prevent relapse are also needed.
Overall, TMS has an excellent reported safety profile. Common side effects are typically mild (including scalp discomfort and headache), and serious side effects (such as seizure) are rare and have a number of known predisposing factors to help identify patients at greatest risk. Safety guidelines to inform dosage selection to mitigate risk have been published (Wassermann, 1998), and consensus guidelines covering level of medical supervision given specific settings and applications have been developed and widely disseminated (Rossi, Hallett, Rossini, & Pascual-Leone, 2009). These developments have been important to inform the safe application of TMS across research and clinical settings.
While rare, seizure remains the most significant known risk of TMS. Established safety guidelines have gone a long way to informing the safe use of TMS in healthy adult populations and in unmedicated adults who receive treatment with the figure-8 coil as used in the safety studies. These studies enrolled medically and psychiatrically healthy and unmedicated adults. However, when these safety studies are extrapolated to real-world clinical populations and to different coil designs and contexts, there are limits to their ability to inform practice. Of particular concern is when the guidelines are extrapolated to patients on psychotropic medications, which are known to affect seizure risk.
Following FDA approval, there have been a handful of reports of seizures induced in depressed patients receiving TMS with the FDA-approved device at parameters of stimulation that are within the safety guidelines. The FDA Manufacturer and User Facility Device Experience (MAUDE) adverse-event database contains reports of four seizures in patients being treated within the safety guidelines with the FDA-approved device. In all cases, patients were on psychotropic medications with known effects on seizure threshold, and in one case the patient had a history of generalized seizure disorder. Our knowledge of the safety of TMS in the absence of concomitant medications is limited by the sample size of the healthy patients in the safety studies. Therefore, as experience with TMS climbs, a clearer picture of the true incidence of seizure as a complication of TMS will emerge. It is also the case that the lack of safety guidelines to inform TMS parameter selection in patients on psychotropic medications is a significant limitation, as reflected in the postapproval seizures reported to date. The current evidence supports the conclusion that the seizure risk of TMS in patients taking psychotropic medications is likely to be higher than in those patients not taking such medications and that motor threshold determination is not adequate to compensate for that increased risk.
Other side effects reported in the FDA MAUDE database include two cases of retinal detachment (on the side where the TMS coil was placed over prefrontal cortex), two cases of mania, and one hospitalization for suicidal ideation. While suicidal ideation can be expected in a severely depressed sample and mania can occur in response to antidepressant medications and ECT, the report of two cases of retinal detachment (one of which was published; Kung, Ahuja, Iezzi, & Sampson, 2011) is notable given the possibility that TMS could induce contraction of extraocular muscles, causing vibration. A less likely explanation is that currents may be induced in the retina if the coil is sufficiently close to the eye. As clinical experience with TMS increases, such unanticipated and low-incidence side effects may begin to emerge, making field surveillance and voluntary participation in the FDA MAUDE database all the more important.
Given the limits to efficacy and safety concerns of the use of psychotropic medications in children and adolescents and during pregnancy, there is significant interest in the safe use of TMS in these populations. While the few reported studies of TMS in adolescents described good tolerability (as reviewed by D’agati, Bloch, Levkovitz, & Reti, 2010), larger samples will be required to establish rates for low-incidence side effects. As an example, there was a recently reported case of a seizure in a girl aged 15 years who was treated with TMS and concomitant sertraline (Hu et al., 2011). As with adults, children may be more at risk of seizure with concomitant psychotropic medications during TMS. Given that children (individuals under 18 years of age) may have a lower seizure threshold than adults, and that there are no systematic studies of after-discharge threshold with TMS in children/adolescents are were conducted in adults, a higher level of caution in this age group would seem prudent.
It is important to note that safety guidelines were developed for healthy adults who were medication free, receiving stimulation with a figure-8 coil with mono frequencies, and using motor thresholds titrated with electromyography EMG—a noninvasive technique for monitoring muscle activity. Extrapolating to other age groups, concomitant use of seizure threshold altering medications, other coil types, other stimulation paradigms (e.g., theta burst stimulation [TBS]), and intensity adjusted to visual twitch rather than electromyographic-determined motor threshold may affect risk. Indeed, each seizure reported after FDA approval has been attributed to one or a combination of these factors. The fact that real-world clinical samples commonly present with medical comorbidities, concomitant medications, and other features that post challenges to extrapolation, highlights the need for safety studies that address patients with these features, which are typically encountered in clinical practice.
Given the modest efficacy of 10-Hz TMS to the DLPFC with the figure-8 coil, significant interest has been placed in use of deeper-penetrating coils and more potent parameters of stimulation such as TBS. According to the 510K approval letter (K122288, Jan. 7, 2013) for the recently FDA-approved dTMS coil, of 181 patients in the per protocol analysis, one had a seizure. It is expected that the dTMS coil, which stimulates a larger volume of cortex than the figure-8 coil, might have a different side-effect profile than that of the figure-8 coil. A few studies examining TBS in adults and children have described this treatment as generally safe but have also concluded that larger systematic studies are needed in order to establish safety (Oberman, Edwards, Eldaief, & Pascual-Leone, 2011; Wu, Shahana, Huddleston, Lewis, & Gilbert, 2012).
While the side-effect profile has generally been good for TMS, extrapolation to clinical populations, concomitant medications, and novel stimulation paradigms may carry a new set of risks that should be examined.
Keys to effective TMS clinical trial design are the validity and plausibility of the sham condition. A valid sham is key to basic science studies of TMS as well as to studies that evaluate efficacy across a range of disorders. The purpose of sham TMS is to protect clinical trials from bias by masking the patient, TMS operator, and clinical raters to treatment condition and to control for the placebo response by equating the degree of expectancy between the active and sham treatment arms. The ideal sham would be biologically inactive, match the same expectancy as active TMS, and effectively simulate the ancillary aspects of active TMS (including the intensive clinician–patient contact, visual look of the coil, manner of holding the coil, auditory clicking, and somatosenory sensations (scalp sensation, scalp muscle contraction, discomfort/pain, and potential for muscle twitching in a limb depending on coil placement and field distribution).
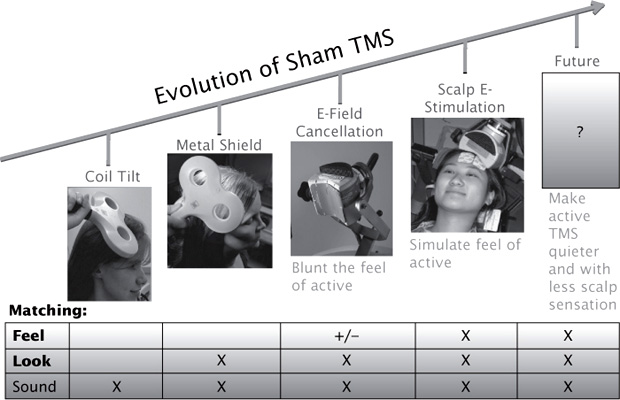
Methods for sham TMS have evolved over time, with significant improvements in validity (Figure 11.1). However, the search for the perfect sham condition to effectively mask randomization, control for the placebo effect, and match ancillary sensations and degree of clinical contact continues.
The original coil tilt sham matched the sound but not the look or feel of active TMS; with inadequate tilt, it could induce significant stimulation in the brain (Lisanby, Gutman, Luber, Schroeder, & Sackeim, 2001). The metal shield sham matched the sound and look of active TMS but lacked the scalp sensation. E-field cancellation at the surface of the scalp was used in the pivotal trial leading to FDA approval (O’Reardon et al., 2007), but it is not known whether this was successful in blunting the sensation of active TMS at the scalp. Other approaches to sham include reversing current flow in the loops of the figure-8 coil such that they cancel at the intersection and use of the sandwich coil with an active coil on one side and a sham on the other. However, both of these approaches result in less scalp sensation, which could cue patients (Sommer et al., 2006; Ruohonen, Ollikainen, Nikouline, Virtanen, & Ilmoniemi, 2000; Hoeft, Wu, Hernandez, Glover, & Shimojo, 2008). Electrical stimulation of the scalp, which was used in the optimization of TMS for the treatment of depression trial, was effective in protecting the trial from bias by matching the degree of scalp sensation between active and sham interventions, in combination with active auditory masking to match ancillary effects (George et al., 2010).
FIGURE 11.1 Evolution of Sham TMS. Over time the approach to sham TMS has evolved, with successively valid emulation of the sound, look, and feel of active TMS. Future developments may achieve improvements through engineering advances that yield quieter coils with less vibration and pulse-shape optimization to reduce scalp sensation.
Future directions for improving sham manipulations may be directed at creating active TMS systems that are quieter and induce less vibration and scalp sensation than conventional TMS. This could be achieved through engineering advances and pulse-shape optimization (Peterchev, Goetz, Westin, Luber, & Lisanby, 2013; Peterchev, Luber, Wagner, Westin, Lisanby, 2008).
Not only is sham important to control for the placebo effect, it is also important to control for ancillary auditory and somatosensory stimulation that could conceivably affect outcome measures independent of their impact on plausibility of the sham condition. For example, new evidence indicates that tetanic auditory or visual stimulation can induce plasticity in the brain (for a review, see Clapp, Hamm, Kirk, & Teyler, 2012).
Addressing the limitations to the efficacy and safety of TMS will rely on emerging evidence in the scientific literature, which makes accurate reporting vital for useful metaanalyses to be able to be conducted and inform the field. Given the infinitely large, multidimensional parameter space, synthesis across studies with divergent methodologies is a challenge. At minimum, accurate reporting of salient aspects of dosage in the scientific literature may enable meaningful dose/response relationships to emerge. Guidelines for this reporting are now available and should further our understanding of what comprises key elements of the dose of brain stimulation relevant for optimizing efficacy and safety (Peterchev et al., 2012).
Our current knowledge of the efficacy and safety of TMS across clinical applications in neuropsychiatry hinges on the validity of the sham condition used. However, studies have used a variety of sham conditions, and only the most recent ones have used a validated sham that matches the scalp sensation of active TMS. This may explain why early trials had higher effect sizes than more recent trials. Advances in validated sham manipulations, innovative approaches to reduce the ancillary effects of active TMS so that it is easier to mask, and increased consistency in the reporting of dosing parameters should enable meaningful metaanalyses of the published literature and inform the optimization of TMS for clinical applications.
In reflecting on the limitations and future directions of clinical TMS, it is useful to consider the historical context of the development of clinical therapeutic interventions in psychiatry. Here, the gold standard, ECT, is used as an example. ECT has undergone considerable modification throughout its 75-year history in active clinical use in psychiatry. It has consistently been considered highly efficacious. However, successful evolution of the technique and refinement of dosimetry has evolved over time, and these changes have ushered in an improved safety profile.
Significant milestones in the history of ECT include the advent of anesthesia (particularly muscle paralysis, which avoided bone fractures), refinement in patient selection (with the recognition that it is more effective for mood disorders than the original application of schizophrenia), and optimization of various aspects of ECT dosing.
Steps in the evolution of ECT dosimetry, which are summarized in Table 11.1, include approaches to individualize the dosage and to optimize the spatial and temporal components of the dose. Our understanding of what is meant by the “dosage” of a neuromodulation treatment has evolved over this time and continues to evolve in response to advancing understanding of how electrical fields interact with brain function (Peterchev et al., 2012; Peterchev, Rosa, Deng, Prudic, & Lisanby, 2010). The dosage of an electrical intervention such as ECT or an electromagnetic intervention such as TMS can be understood in terms of the spatial distribution of the electric field induced in the brain and in terms of the temporal aspects of how the field strength varies over time (Figure 11.2). The temporal aspects have the following two features: the shape and width of each individual pulse and the parameters that describe the train of pulses (frequency, duration, intertrain interval). We now know that each of these aspects exerts independent effects on the brain’s response to stimulation. However, in the ECT field, these parameters are typically combined into a single metric (charge, in millicoulombs), which represents the total area under the curve of the delivered current.
TABLE 11.1 Evolution of ECT Technique
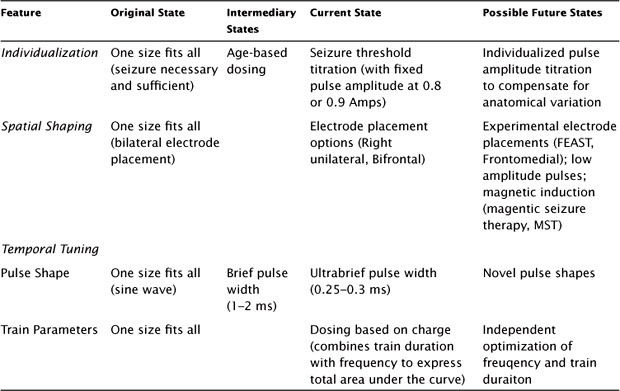
Originally, ECT was a “one-size-fits-all” technique, with limited attention to dosage beyond what was necessary for the induction of a seizure that was thought to be both necessary and sufficient to ensure efficacy. At the outset, all patients received stimulation with bilateral electrode placement and sine wave stimulus, and stimulation was applied until a seizure was induced. Over time it became apparent that individuals differed in their dosage requirements to induce a seizure, leading to approaches for individualizing the dosage, for example, age-based dosing and seizure-threshold titration. A series of randomized, controlled trials provided convincing evidence that dosage exerts a major impact on both efficacy and side effects (Sackeim et al., 2000, 2008). Thus, the current standard is to individualize ECT dosage based on empirical titration of each patient’s seizure threshold. An expanded array of electrode placements is now available in an attempt to target the induced field more precisely and lessen cognitive side effects.
Interestingly, the spatial components of the dosage (electrode placement) and temporal components of ECT dosage (pulse shape and train parameters) interact in determining outcome. For example, ultrabrief pulse width lowers the cognitive side effects of both unilateral and bilateral ECT and lowers the efficacy of bilateral, but not unilateral, ECT (Sackeim et al., 2008).
Future developments in the field of ECT have focused on identifying new ways to improve its side-effect profile through optimization of the approach to individualization, spatial shaping of the field, and temporal tuning (summarized in Table 11.1). New approaches to individualization may involve titration of the pulse amplitude. While the TMS field has long utilized the procedure of motor threshold titration to individualize pulse amplitude, ECT continues to use a fixed high current. However, individualizing pulse amplitude can be done with ECT and represents a potential new way of adjusting for anatomical differences that may explain individual heterogeneity in clinical outcomes (Lee, Lisanby, Laine, & Peterchev, 2012; Lee, Deng, Laine, Lisanby, & Peterchev, (2011; Lee et al., 2010, 2012).
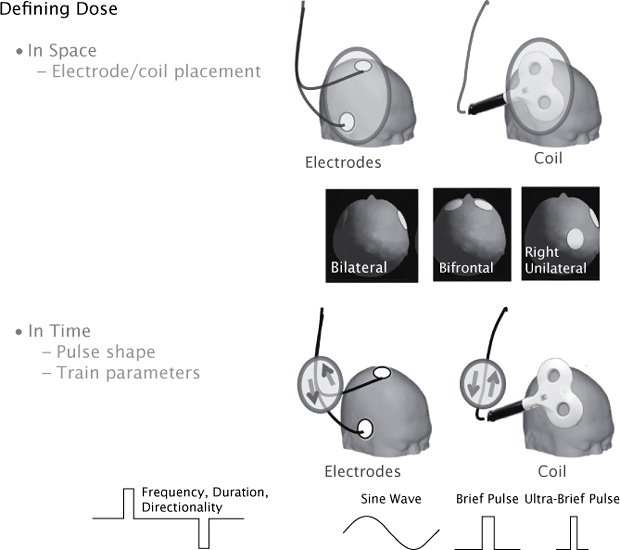
FIGURE 11.2 Defining Dosage of Transcranial Stimulation. The dosage of ECT or TMS can be characterized in terms of how the electric field is distributed in space and how the field strength changes over time. The spatial distribution is controlled by the shape and placement of the electrodes on the head (in the case of ECT) and by the shape and placement of the stimulating coil (in the case of TMS). Typical ECT electrode placements are shown in the insert. The temporal components of the dosage may be characterized in terms of the shape of each pulse (such as sine wave versus square wave), the width of the pulse (brief pulse versus ultrabrief pulse), the amplitude of each pulse, and the parameters that describe the train of pulses (frequency, duration, and directionality).
In terms of spatial optimization, novel electrode placements are under investigation to better focus the field and spare regions of the brain implicated in cognitive side effects (Spellman, Peterchev, & Lisanby, 2009; Rosa, Abdo, Rosa, Lisanby, & Peterchev, 2012). The lowering pulse amplitude also makes the field more focal. The feasibility of low-amplitude ECT has been reported, and the potential benefits are under active study (Rosa, Abdo, Lisanby, & Peterchev, 2011). Finally, inducing the seizure magnetically (magnetic seizure therapy [MST]) allows enhanced control over the site and extent of stimulation due to the lack of impedance from the scalp and skull (Lisanby, Schlaepfer, Fisch, & Sackeim, 2001). Superior cognitive outcomes with MST compared with ECT have been reported (McClintock et al., 2013; Spellman et al., 2008; Lisanby, Luber, Schlaepfer, & Sackeim, 2003). Preliminary reports of efficacy have been encouraging, demonstrating similar response rates between ECT and MST (Kayser et al., 2011).
In terms of temporal optimization, moving to ultrabrief pulse shape has already dramatically lowered cognitive side effects (Sackeim et al., 2008). The potential value of other pulse shapes has been proposed on a theoretical basis but has yet to be explored clinically (Hofmann, Ebert, Tass, & Hauptmann, 2011). Regarding the parameters that describe the train of pulses, it has been reported that lower frequencies, longer durations, and unidirectional trains are more efficient (Spellman et al., 2009; Devanand, Lisanby, Nobler, & Sackeim, 1998).
An unanswered question for the future evolution of ECT includes how best to prevent relapse following remission. Despite its excellent acute efficacy, relapse post ECT remains an important clinical challenge (Kellner et al., 2006) and is the focus of ongoing research (Lisanby et al., 2008).
History has taught us that dosage is key to efficacy and safety and that the approach to dosing should evolve over time as knowledge of mechanisms emerges. The rational design of neuro-modulation therapies, including TMS and ECT, will require optimizing the risk/benefit ratio, which will in turn require discovery of the dose–response relationships that govern how the brain responds to transcranial stimulation. This work will hinge on careful definition, characterization, and optimization of the dose of TMS, including its spatial and temporal aspects, as well as approaches to individualizing dose. History has also taught us that relapse prevention is a critical and as yet unsolved issue, bearing close attention with TMS as well.
We have seen TMS technology evolve considerably since it was first introduced as a tool in neuroscience in 1985 and as an approved treatment in psychiatry in 2008. While the field of ECT has had more than seven decades to mature, the TMS field has had only seven years post FDA approval. Progress has been comparatively rapid, informed by the historical context of ECT’s history as well as modern engineering approaches to device and coil design, coupled with computational modeling and neuroscience discoveries.
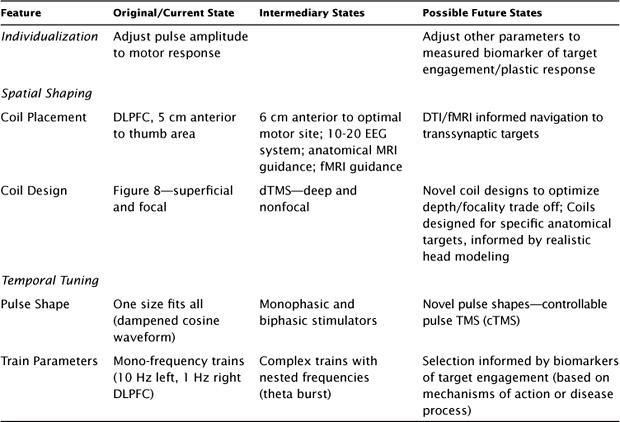
While the field of TMS is young compared with that of ECT, the technology has been rapidly evolving, spurred by considerable engineering innovations and neurosciences advances in understanding of mechanisms. Given the excellent safety profile of TMS, much of the focus for clinical TMS has been on optimizing efficacy. Current and future approaches to optimizing clinical TMS, which are summarized in Table 11.2, involve innovations in individualization of stimulation, shaping the spatial distribution of the induced field, and temporal tuning of pulse and train characteristics.
The current approach to clinical TMS dosing is to individualize pulse amplitude via titration of the motor threshold (usually visual twitch threshold) and to apply a fixed frequency, train duration, intertrain interval, and number of pulses based on the FDA-approved protocol for depression. While this approach may be effective for some patients, there are some inherent limitations that could affect individual response. For example, visual twitch overestimates motor threshold, and safety guidelines were based on EMG-determined MT (as were the clinical trials with TMS for depression). We recently reported that visual twitch MT is 11% higher than EMG-determined MT and that in more than half of patients, use of the visual twitch MT would have resulted in stimulation beyond the safety guidelines (Westin, Bassi, Lisanby, & Luber, 2013). This is an especially salient point when considering the additional confound of concomitant medications, which can further increase risk, making careful MT determination all the more important for clinical populations.
TABLE 11.2 Evolution of TMS Technique
Regardless of how MT is determined, it is a measure of motor cortex response; however, it is not a direct measure of response of the brain area targeted in depression (the DLPFC). While titrating intensity to MT has been established as a means of ensuring safety, it is not clear that this is the optimal means of ensuring efficacy. This highlights the need for an “MT-equivalent” for response of cortical areas outside of the motor cortex, such as the DLPFC. Potentially attractive options worth exploring in this regard include TMS-evoked potential (TMS-EP) and TMS/ functional magnetic resonance imaging (fMRI) interleaving, though the clinical utility of this approach is yet to be determined.
Furthermore, MT only adjusts amplitude based on response to a single pulse. Clinical TMS treatment is given in trains of pulses, and MT does not capture individual variability in response to the cumulative effect of a train of pulses. This highlights the need for a bio-marker for neuroplastic response to a TMS train. Putative markers of such plasticity include TMS-EP, which has revealed cumulative effects of TMS pulses in a train (Hamidi, Slagter, Tononi, & Postle, 2010), and a paired associative stimulation paradigm, which is a marker of heterosynaptic plasticity and has revealed potentiation following TMS trains as well as deficient plasticity in certain patient groups (Player et al., 2013).
Beyond individualization of TMS amplitude, leaving the other parameters fixed fails to take into account all aspects that contribute to dosing. Indeed, emerging literature suggests that other dosing paradigms beyond the current FDA-approved paradigm may be more effective (e.g., bilateral stimulation, right-sided stimulation, increased number of pulses per session, MRI-guidance). However, at present, there is little guidance on how to select among these paradigms for a particular patient or how to individualize the parameters within a particular paradigm.
The spatial distribution of the electrical field that is induced with TMS is driven by coil placement and coil design. The original coil placement for depression used in most studies was the DLPFC, identified as the region 5 cm anterior to the optimal site for the primary motor cortex. While this can be located reliably, there is a high degree of variability in how well this reaches the DLPFC target, and this variability is expected to result in reduced clinical outcomes (Herbsman et al., 2009). Basing placement on the 10–20 electroencephalography (EEG) system is considered a more valid method because it adjusts to differences in head size. Also, this system has improved structural image guidance, which has been reported to improve antidepressant efficacy (Fitzgerald et al., 2009). However, accounting for variation in anatomy only tells part of the story. Differences in functional neuroanatomy should also be considered. fMRI guidance has been particularly useful in improving the effect size of TMS in cognitive neuroscience studies (Luber et al., 2007, 2008, 2012) and has been explored in clinical settings as well (Mantovani, Westin, Hirsch, & Lisanby, 2010). However, more work needs to be done to establish how successful such an approach may be for depression.
Another intriguing approach for optimizing coil placement in order to reach remote targets is connectivity-guided coil placement. Fox and colleagues (2012) reported that the more successful depression targets in the DLPFC demonstrate the greatest anticorrelation with rostral cingulate on resting state fMRI. This is interesting given the emerging importance of connectivity-informed selection of deep brain stimulation (DBS) targeting, using diffusion tensor imaging data (Gutman, Holtzheimer, Behrens, Johansen-Berg, & Mayberg, 2009). Other future directions for spatial optimization of coil placement include identification of cortical targets other than the DLPFC that may be relevant for depression and may have more robust direct connectivity to limbic regions as well as multifocal targeting.
Regarding coil design, the first FDA-approved coil was a focal figure-8 coil. Recently FDA approved a deeper penetrating and less focal coil, the H-coil. This coil is reported to have robust efficacy (Harel et al., 2011; Levkovitz et al., 2009); however, a head-to-head comparison with the figure-8 coil has not been conducted. Deeper penetrating coils are attractive for direct stimulation of deep targets. Nonfocal coils enable the synchronous driving of larger regions of the cortex, increasing the likelihood of reaching the clinically important targets but also increasing the likelihood of inadvertently stimulating nontargets, which may impact its safety profile. As discussed above, changes in coil design can affect seizure risk.
Computational modeling has demonstrated that there is always a depth/focality trade-off such that the deeper penetrating coils are necessarily less focal (Deng, Lisanby, & Peterchev, 2013). Nevertheless, these computational tools can enable the design of coils optimized for specific targets and characterize their degree of focality and spread (Deng, Peterchev, & Lisanby, 2008).
Much of the focus to date has been on the spatial components of dosing (coil location and coil design), with less focus on the temporal components. However, the temporal components are expected to be key to the induction of plasticity. Temporal components of the dosage include the pulse shape and the parameters that describe the pulse train.
Regarding pulse-shape optimization, whereas the original TMS devices induced sinusoidal waveforms that were not amenable to user control, new engineering developments have enabled the design of TMS stimulators that can induce a broadening range of pulse shapes and characteristics (Peterchev, Murphy, & Lisanby, 2010, 2011; Peterchev, Jalinous, & Lisanby, 2008). We recently demonstrated that the width of the TMS pulse is a powerful driver of its effect on cortical excitability (Luber et al., 2012). These new technologies provide researchers with an extended range of pulse shapes and train parameters to chose from in order to optimize the physiological potency of the stimulation for specific neurobiological ends (Goetz et al., 2013).
Regarding the parameters that describe the train of pulses, researchers evaluated whether delivering the total number of pulses in a shorter amount of time could accelerate antidepressant response and had some promising results (Holtzheimer et al., 2010). Accelerating recovery is particularly important for reducing overall morbidity and reducing the period of risk for suicide. Others have examined other aspects of the temporal components of the train, such as individualizing frequency based on EEG (Price, Lee, Garvey, & Gibson, 2010) and use of a nested frequency package such as TBS. This technique has been reported to induce lasting changes in neural oscillations and to induce more potent neuroplasticity than monofrequencies of TMS (Noh, Fuggetta, Manganotti, & Fiaschi, 2012). TBS mimics the natural nesting of frequencies seen in the human brain, with gamma oscillation power modulated by the phase of theta oscillations.
It has been proposed that neural oscillations and coupling across frequencies in the power and phase of these oscillations form an integral part of how the brain accomplishes higher-order cognitive tasks such as working memory and multisensory integration (see, for example, Lee & Jeong, 2013). As such, there is tremendous potential for physiologically informed TMS paradigms to expand the clinical utility of TMS. Indeed, it is the temporal precision of TMS that is a major differentiator of neuromodulation modalities from pharmacology. Whereas pharmacology is dependent on half-lives that are typically in the hour to day to week range, TMS can be given with millisecond precision and in a fashion that is tightly coupled with the dynamics of endogenous brain function.
While studies have long combined TMS with psychopharmacology (typically continuing previously ineffective antidepressant medications and adding on TMS), the targeted use of pharmacology to promote TMS-induced plasticity is a novel future direction worth exploring in order to enhance potency and promote durability of effect. Other strategies include coupling TMS with priming from other devices (such as transcranial direct current stimulation) and with behavioral therapies to promote learning and skill acquisition or to facilitate extinction learning, as recently reported with the combined use of dTMS along with exposure therapy in posttraumatic stress disorder. Indeed, the feasibility of simultaneous cognitive behavioral therapy and TMS for depression treatment has been reported, but there is yet to be a controlled trial evaluating this interesting paradigm Vedeniapin, Cheng, & George, 2010). Given the neurorehabilitation literature that suggests that TMS priming of neurorehabilitation can promote recovery of function (Takeuchi & Izumi, 2012), it stands to reason that a similar approach could be useful in other neurobehavioral conditions.
When taken in the historical context of ECT, the evolution of TMS technique is advancing at a comparatively rapid rate. While in the field of ECT it has taken decades to refine the treatment and discover dose/response relationships, research in the TMS field is rapidly accumulating and shaping the future of this technology. Whether these advances will translate into clinically meaningful results for patients above and beyond what current technology has to offer awaits demonstration in the form of randomized controlled trials. The availability of sophisticated modeling technologies and engineering advances that were not available decades ago bodes well for TMS having a more accelerated clinical optimization than was the case with ECT.
One of the greatest challenges facing clinical TMS is how to optimize the dosage in order to maximize efficacy and how to individualize the treatment. Indeed, these challenges remain for ECT as well, particularly with respect to maximizing safety and sustaining remission. The parameter space of variables that define the dosage of TMS (and ECT) is infinite and multidimensional. This poses a major challenge to optimizing the risk/benefit ratio of the treatment. Among the components of dosage yet to be optimized with TMS are pulse and train parameter selection, coil selection and placement, treatment schedule (number of sessions per day and days per week), factors that may influence response such as concomitant medications, psychotherapies (during TMS or at a separate time), and other pharmacological, device-based, or behavioral approaches to prime the state of the brain at the time of stimulation.
Further compounding this challenge, at present we lack sufficient basic knowledge of how electrical fields interact with the brain to predict dose/response relationships. While the field of psychopharmacology is informed by the basic disciplines of pharmacokinetics and pharmacodynamics, the analog disciplines for electrokinetics and electrodynamics are only now emerging. This calls for a basic discipline at the interface of engineering and psychiatry to inform dosimetry of neuromodulation techniques. Basic research on mechanisms of action of TMS will be key to optimizing efficacy and safety. New tools are available for modeling the E field and the neuronal response to stimulation that are likely to be of significant help in this regard.
Ultimately the clinical utility of focal brain stimulation, whether with TMS, ECT, DBS, or other methodologies yet to be validated, depends on our knowledge of the neuro-circuitry and neurodynamics of neuropsychiatric disorders. The more we know about the pathophysiology of the disorder in question, the better able we will be to exert clinical benefit through focal neuromodulation.
Aguirre, I., Carretero, B., Ibarra, O., Kuhalainen, J., Martínez, J., Ferrer, A., & Garcia-Toro, M. (2011). Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. Journal Affective Disorders, 130(3), 466–469.
Ciobanu, C., Girard, M., Marin, B., Labrunie, A., & Malauzat, D. (2013). rTMS for pharmacoresistant major depression in the clinical setting of a psychiatric hospital: Effectiveness and effects of age. Journal Affective Disorders, 150(2), 677–681.
Clapp, W., Hamm, J., Kirk, I., & Teyler, T. (2012). Translating long-term potentiation from animals to humans: a novel method for noninvasive assessment of cortical plasticity. Biological Psychiatry, 71(6), 496–502.
D’agati, D., Bloch, Y., Levkovitz, Y., & Reti, I. (2010). rTMS for adolescents: Safety and efficacy considerations. Psychiatry Research, 177(3), 280–285.
Deng, Z.-D., Lisanby, S. L., & Peterchev, A. V. (2013). Electric field depth—focality tradeoff in transcranial magnetic stimulation: comparison of 50 coil designs. Brain Stimulation, 6, 1–13.
Deng, Z., Peterchev, A., & Lisanby, S. (2008). Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS). Conference Proceedings IEEE Engineering in Medicine and Biology Society, 5675–5679.
Devanand, D. P., Lisanby, S. H., Nobler, M. S., & Sackeim, H. A. (1998). The relative efficiency of altering pulse frequency or train duration when determining seizure threshold. Journal ECT, 14(4), 227–235.
Eranti, S., Mogg, A., Pluck, G., Landau, S., Purvis, R., Brown, R., … McLoughlin, D. M. (2007). A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. American Journal Psychiatry, 164(1), 73–81.
Fitzgerald, P., Hoy, K., Mcqueen, S., Maller, J., Herring, S., Segrave, R., … Daskalakis, Z. (2009). A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology, 34(5), 1255–1262.
Fox, M., Buckner, R., White, M., Greicius, M., & Pascual-Leone, A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72(7), 595–603.
George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., … Sackeim, H. A. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives General Psychiatry, 67, 507–516.
Goetz, S., Truong, C., Gerhofer, M., Peterchev, A., Herzog, H., & Weyh, T. (2013). Analysis and Optimization of Pulse Dynamics for Magnetic Stimulation. PloS One, 8(3), e55771, 1-12.
Gutman, D., Holtzheimer, P., Behrens, T., Johansen-Berg, H., & Mayberg, H. (2009). A tractography analysis of two deep brain stimulation white matter targets for depression. Biological Psychiatry, 65(4), 276–282.
Hamidi, M., Slagter, H., Tononi, G., & Postle, B. (2010). Brain responses evoked by high-frequency repetitive transcranial magnetic stimulation: an event-related potential study. Brain Stimulation, 3(1), 2–14.
Hansen, P. E., Ravnkilde, B., Videbech, P., Clemmensen, K., Sturlason, R., Reiner, M., … Vestergaard, P. (2011). Low-frequency repetitive transcranial magnetic stimulation inferior to electroconvulsive therapy in treating depression Journal ECT, 27(1), 26–32.
Harel, E., Zangen, A., Roth, Y., Reti, I., Braw, Y., & Levkovitz, Y. (2011). H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World Journal Biological Psychiatry, 12(2), 119–126.
Herbsman, T., Avery, D., Ramsey, D., Holtzheimer, P., Wadjik, C., Hardaway, F., … Nahas, Z. (2009). More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation anti-depressant response. Biological Psychiatry, 66(5), 509–515.
Hoeft, F., Wu, D., Hernandez, A., Glover, G., & Shimojo, S. (2008). Electronically switchable sham transcranial magnetic stimulation (TMS) system. PloS One, 3(4), e1923, 1-10.
Hofmann, L., Ebert, M., Tass, P., & Hauptmann, C. (2011). Modified pulse shapes for effective neural stimulation. Frontiers Neuroengineering, 4, 1-10.
Holtzheimer, P. E., McDonald, W. M., Mufti, M., Kelley, M. E., Quinn, S., Corso, G., & Epstein, C. M. (2010). Accelerated repetitive transcranial magnetic stimulation (aTMS) for treatment-resistant depression. Depression Anxiety, 27, 960–963.
Hu, S., Wang, S., Zhang, M., Wang, J., Hu, J., Huang, M., … Xu, Y. (2011). Repetitive transcranial magnetic stimulation-induced seizure of a patient with adolescent-onset depression: a case report and literature review. Journal International Medical Research, 39(5), 2039–2044.
Huang, C., Wei, I., Chou, Y., & Su, T. (2008). Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology, 33(6), 821–831.
Jorge, R., Moser, D., Acion, L., & Robinson, R. (2008). Treatment of vascular depression using repetitive transcranial magnetic stimulation. Archives General Psychiatry, 65(3), 268–276.
Kayser, S., Bewernick, B., Grubert, C., Hadrysiewicz, B., Axmacher, N., & Schlaepfer, T. (2011). Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. Journal Psychiatric Research, 45(5), 569–576.
Kellner, C., Knapp, R., Petrides, G., Rummans, T., Husain, M., Rasmussen, K.,… Fink, M. (2006). Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Archives General Psychiatry, 63(12), 1337–1344.
Keshtkar, M., Ghanizadeh, A., & Firoozabadi, A. (2011). Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for the treatment of major depressive disorder, a randomized controlled clinical trial. Journal ECT, 27(4), 310-314.
Knapp, M., Romeo, R., Mogg, A., Eranti, S., Pluck, G., Purvis, R., … McLoughlin, D. M. (2008). Cost-effectiveness of transcranial magnetic stimulation vs. electroconvulsive therapy for severe depression: a multi-centre randomised controlled trial. Journal Affective Disorders, 109(3), 273–285.
Kung, S., Ahuja, Y., Iezzi, R., & Sampson, S. (2011). Posterior vitreous detachment and retinal tear after repetitive transcranial magnetic stimulation. Brain Stimulation, 4(4), 218–221.
Lee, W. H., Deng, Z. D., Kim, T. S., Laine, A. F., Lisanby, S. H., & Peterchev, A. V. (2010). Regional electric field induced by electroconvulsive therapy: a finite element simulation study. Conference Proceedings IEEE Engineering in Medicine and Biology Society, 2045–2048.
Lee, W. H., Deng, Z. D., Laine, A. F., Lisanby, S. H., & Peterchev, A. V. (2011). Influence of white matter conductivity anisotropy on electric field strength induced by electroconvulsive therapy. Conference Proceedings IEEE Engineering in Medicine and Biology Society, 5473–5476.
Lee, W. H., Lisanby, S. H., Laine, A. F., & Peterchev, A. V. (2012). Stimulation strength and focality of electroconvulsive therapy with individualized current amplitude: a preclinical study. Conference Proceedings IEEE Engineering in Medicine and Biology Society, 6430-6433.
Lee, J., & Jeong, J. (2013). Correlation of risk-taking propensity with cross-frequency phase–amplitude coupling in the resting EEG. Clinical Neurophysiology, 124(11), 2172-2180.
Lee, W., Deng, Z., Kim, T., Laine, A., Lisanby, S., & Peterchev, A. (2012). Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: Influence of white matter anisotropic conductivity. Neuroimage, 59(3), 2110–2123.
Levkovitz, Y., & Segal, M. (2001). Aging affects transcranial magnetic modulation of hippocampal evoked potentials. Neurobiology Aging, 22(2), 255–263.
Levkovitz, Y., Harel, E., Roth, Y., Braw, Y., Most, D., Katz, L., … Zangen, A. (2009). Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimulation, 2(4), 188–200.
Lisanby, S. H., Husain, M. M., Rosenquist, P. B., Maixner, D., Gutierrez, R., Krystal, A.,… George, M. S. (2009). Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology, 34, 522–534.
Lisanby, S., Gutman, D., Luber, B., Schroeder, C., & Sackeim, H. (2001). Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry, 49(5), 460–463.
Lisanby, S., Luber, B., Schlaepfer, T., & Sackeim, H. (2003). Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology, 28(10), 1852–1865.
Lisanby, S., Sampson, S., Husain, M., Petrides, G., Knapp, R., Mccall, V., … Kellner, C. (2008). Toward individualized post-electroconvulsive therapy care: piloting the Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE) intervention. Journal ECT, 24(3), 179–182.
Lisanby, S., Schlaepfer, T., Fisch, H., & Sackeim, H. (2001). Magnetic seizure therapy of major depression. Archives General Psychiatry, 58(3), 303–305.
Luber, B., Kinnunen, L., Rakitin, B., Ellsasser, R., Stern, Y., & Lisanby, S. (2007). Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency-and time-dependent effects. Brain Research, 1128(1), 120–129.
Luber, B., Stanford, A., Bulow, P., Nguyen, T., Rakitin, B., Habeck, C., … Lisanby, S. (2008). Remediation of Sleep-Deprivation–Induced Working Memory Impairment with fMRI-Guided Transcranial Magnetic Stimulation. Cerebral Cortex, 18(9), 2077–2085
Luber, B., Steffener, J., Tucker, A., Habeck, C., Peterchev, A., Deng, Z., … Lisanby, S. (2012). Extended Remediation of Sleep Deprived-Induced Working Memory Deficits Using fMRI-guided Transcranial Magnetic Stimulation. Sleep, 36(6), 857–871.
Mantovani, A., Westin, G., Hirsch, J., & Lisanby, S. H. (2010). Functional magnetic resonance imaging guided transcranial magnetic stimulation in obsessive-compulsive disorder. Biological Psychiatry, 67(7), e39–40.
Mantovani, A., Pavlicova, M., Avery, D., Nahas, Z., McDonald, W. M., Wajdik, C. D., … Lisanby, S. H. (2012). Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (Tms) in treatment-resistant depression. Depression Anxiety, 29, 883–890.
Mcclintock, S., Dewind, N., Husain, M., Rowny, S., Spellman, T., Terrace, H., & Lisanby, S. (2013). Disruption of component processes of spatial working memory by electroconvulsive shock but not magnetic seizure therapy. International Journal Neuropsychopharmacology, 16(01), 177–187.
McLoughlin, D. M., Mogg, A., Eranti, S., Pluck, G., Purvis, R., Edwards, D., … Knapp, M. (2007). The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis. Health Technology Assess, 11(24), 1–54.
Nahas, Z., Li, X., Kozel, F. A., Mirzki, D., Memon, M., Miller, K., … George, M. S. (2004). Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depression Anxiety, 19(4), 249–256.
Noh, N. A., Fuggetta, G., Manganotti, P., & Fiaschi, A. (2012). Long lasting modulation of cortical oscillations after continuous theta burst transcranial magnetic stimulation. PLoS One, 7(4):e35080, 1-12.
O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., … Sackeim, H. A. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biological Psychiatry, 62, 1208–1216.
Oberman, L., Edwards, D., Eldaief, M. & Pascual-Leone, A. (2011). Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. Journal Clinical Neurophysiology, 28(1), 67–74.
Pallanti, S., Cantisani, A., Grassi, G., Antonini, S., Cecchelli, C., Burian, J., … Quercioli, L. (2012). rTMS age-dependent response in treatment-resistant depressed subjects: a mini-review. CNS Spectrums, 17(01), 24–30.
Peterchev, A. V., Luber, B., Wagner, T. D., Westin, G. G., Lisanby, S. H., Eds. (2008). First human study of controllable pulse shape transcranial magnetic stimulation (cTMS): effect of pulse width on corticospinal response and scalp sensation. American College of Neuropsychopharmacology 2008 Annual Meeting; 2008.
Peterchev, A. V., Murphy, D. L., & Lisanby, S. H. (2010). Repetitive transcranial magnetic stimulator with controllable pulse parameters (cTMS). Conference Proceedings IEEE Engineering in Medicine and Biology Society, 2922–2926.
Peterchev, A. V., Rosa, M. A., Deng, Z. D., Prudic, J., & Lisanby, S. H. (2010). Electroconvulsive therapy stimulus parameters: rethinking dosage. Journal ECT, 26(3), 159–174.
Peterchev, A., Goetz, S., Westin, G., Luber, B., & Lisanby, S. (2013). Pulse width dependence of motor threshold, & input—output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clinical Neurophysiology, 124(7), 1364–1372.
Peterchev, A., Jalinous, R., & Lisanby, S. (2008). A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS). IEEE Transactions Biomedical Engineering, 55(1), 257–266.
Peterchev, A., Murphy, D., & Lisanby, S. (2011). Repetitive transcranial magnetic stimulator with controllable pulse parameters. Journal Neural Engineering, 8(3), e036016, 1-24.
Peterchev, A., Wagner, T., Mir, A. P., Nitsche, M., Paulus, W., Lisanby, S., … Bikson, M. (2012). Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimulation, 5(4), 435–453.
Player, M., Taylor, J., Weickert, C., Alonzo, A., Sachdev, P., Martin, D., … Loo, C. (2013). Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology.
Price, G., Lee, J., Garvey, C., & Gibson, N. (2010). The use of background EEG activity to determine stimulus timing as a means of improving rTMS efficacy in the treatment of depression: a controlled comparison with standard techniques. Brain Stimulation, 3(3), 140–152.
Rosa, M. A., Abdo, G. L., Lisanby, S. H., & Peterchev, A. (2011). Seizure induction with low-amplitude-current (0.5 A) electroconvulsive therapy. Journal ECT, 27(4), 341–342.
Rosa, M. A., Abdo, G. L., Rosa, M. O., Lisanby, S. H., & Peterchev, A. (2012). Frontomedial electrode placement with low current amplitude: a case report. Journal ECT, 28, 144–150.
Rossi, S., Hallett, M., Rossini, P. M., & Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–2039.
Ruohonen, J., Ollikainen, M., Nikouline, V., Virtanen, J., & Ilmoniemi, R. (2000). Coil design for real and sham transcranial magnetic stimulation. IEEE Transactions Biomedical Engineering, 47(2), 145–148.
Sackeim, H. A., Prudic, J., Devanand, D. P., Nobler, M. S., Lisanby, S., Peyser, S., … Clark, J. (2000). A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives General Psychiatry, 57(5), 425–434.
Sackeim, H., Prudic, J., Nobler, M., Fitzsimons, L., Lisanby, S., Payne, N., … Devanand, D. P. (2008). Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulation, 1(2), 71–83.
Sommer, J., Jansen, A., Dräger, B., Steinsträter, O., Breitenstein, C., Deppe, M., & Knecht, S. (2006). Transcranial magnetic stimulation—a sandwich coil design for a better sham. Clinical Neurophysiology, 117(2), 440–446.
Spellman, T., Mcclintock, S., Terrace, H., Luber, B., Husain, M., & Lisanby, S. (2008). Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biological Psychiatry, 63(12), 1163–1170.
Spellman, T., Peterchev, A., & Lisanby, S. (2009). Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology, 34(8), 2002–2010.
Takeuchi, N., & Izumi, S. (2012). Noninvasive brain stimulation for motor recovery after stroke: mechanisms and future views. Stroke Research Treatment, 584727, 1-10.
Vedeniapin, A., Cheng, L., & George, M. (2010). Feasibility of Simultaneous Cognitive Behavioral Therapy (CBT) and Left Prefrontal rTMS for Treatment Resistant Depression. Brain Stimulation, 3(4), 207–210.
Wassermann, E. M. (1998). Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop in the safety of repetitive transcranial magnetic stimulation. Electroencephalography Clinical Neurophysiology, 108, 1–16.
Westin, G., Bassi, B., Lisanby, S., & Luber, B. (2013). Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: Safety implications. Clinical Neurophysiology. In Press.
Wu, S., Shahana, N., Huddleston, D., Lewis, A., & Gilbert, D. (2012). Safety and tolerability of theta-burst transcranial magnetic stimulation in children. Developmental Medicine Child Neurology, 54(7), 636–639.