The difference in mind between man and the higher animals, great as it is, is one of degree and not of kind.
—Charles Darwin, The Descent of Man (1871)
SOON AFTER HIS RETURN from his around-the-world voyage, Darwin visited Jenny, the first orangutan displayed in the London Zoo and among the first apes ever displayed in Britain. She made a profound impression on the naturalist. He was astonished at how she interacted with her keeper, and he admired her playfulness and intelligence.
Her emotions appeared to be those of a child, and from that first encounter onward, Darwin would look at children, including his own, as a comparative primatologist.
Close encounters with apes can be as unsettling as they are fascinating. Queen Victoria, after viewing another orangutan (also named Jenny) wrote that she was “frightful, and painfully and disagreeably human.”
In the expressions on the faces of chimps, orangutans, and gorillas, their mannerisms, and their beautiful, dexterous hands, we see reflections of ourselves. These reflections have always raised provocative and, for some, discomforting questions about the gap between man and beast. What do the apes see when they glance toward their hairless, bipedal visitors? What is going on behind the long stare of a gorilla? What rolls of the ecological and genetic dice put us on the outside of those enclosures looking in, and not the other way around?
My fourteen-year-old niece, Caitie, impressed by an ape exhibit in Tampa, Florida, turned to her father and asked, “You are always telling us that we are 99 percent identical to chimpanzees. Okay, but what makes us different?”
Excellent question.
Caitie was referring to the often quoted figure of our nearly 99 percent identity at the DNA sequence level to chimpanzees. In this chapter, I will frame the beginning of an answer to her question. I have to say “beginning” for two reasons. The first is that biology has just arrived at the point where we can explore the question of specific genetic differences between ourselves and apes. Many more discoveries lie ahead than are yet in hand. The second is that certain kinds of data, such as the visualization of gene expression patterns, which has taught us so much about the evolution of animal form, will be scarce for human embryos.
The psychologist Erich Fromm once said, “Man is the only animal for whom his own existence is a problem he has to solve.” It is clear that this solution requires an integrated picture that encompasses many areas of science, including traditional fields such as paleontology and comparative neuroanatomy that have long sought to understand human history and the biological basis for our mental faculties, as well as emerging disciplines, such as comparative genomics, human medical genetics, and Evo Devo, that are just now taking the stage.
The changes in human form and function that have occurred in the 6 million years since the last common ancestor we shared with chimps are the product of the evolution of human development and genes. Understanding how the features of greatest interest evolved—such as our skeleton (bipedalism, limb lengths, hand and thumb, pelvis, and skull), life history (gestation time, prolonged juvenile state, and longevity), and, most especially, our larger brain, speech, and language—presents some of the greatest puzzles in biology, and for Evo Devo in particular.
In this chapter, I will examine the evolution of human form from several perspectives—the fossil record, comparative neurobiology, embryology, and genetics—and explore four big questions of these fields:
1. What was the actual pattern of human evolution in terms of the changes that occurred among species leading to modern humans?
2. Was human evolution in any way atypical of other mammals?
3. Where in our brain do human capabilities reside?
4. Where in our DNA are the differences that distinguish us from other apes?
The central message of this chapter is that what we have learned thus far about the evolution of form in other animals—butterflies and zebras, fruit flies and finches, spiders and snakes—fully applies to the evolution of human form. Our physical evolution was no different than that of other species. The evolution of human features—including our upright posture, large brain, opposable thumb, speech, and language—is due to developmental changes that modified existing primate or great ape structures, and that were accumulated over several million years and many speciation events. Some of the specific genetic differences between ourselves and living apes are now being revealed.
In order to understand the origins of human traits at any level, we must have an accurate picture of our history and of the characters that distinguish it. We cannot just simply take a snapshot of humans, chimps, and other living apes and then infer how the differences among these forms are made. Each of these species has an independent lineage that reaches back as much as 6 million years or more. To get a picture of the magnitude, rate, and order of changes within or between species, we rely entirely on fossil evidence. Ever since Darwin’s time, generations of paleontologists have sought to uncover the history of human origins.
The record of deep human history first began to be revealed in 1856. As workers were digging mud from a limestone cave in the Neanderthal Valley in Germany, they discovered a skull, some ribs, arm and shoulder bones, and part of a pelvis. At first, one worker took the skeleton to be that of a bear, but the brow ridge on the skull and other features convinced a local school teacher that this find might be something special—but what? It took a few years to sort out reality from various guesses.
Anatomist Hermann Schaaffhausen concluded that the bones were those of a member of an ancient race of European barbarians. A leading German pathologist pronounced that the unusual bone structures were just a consequence of rickets. Another anatomist decided that the leg bones were bent through horseback riding, and that the remains were of a Cossack soldier who had received a mortal wound in battle with Napoleon’s army and crawled into the cave to die.
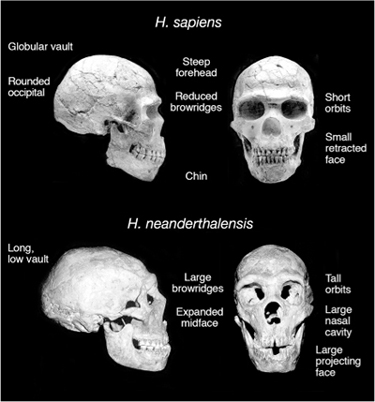
None of these explanations satisfied Thomas Huxley. Darwin’s bulldog could not see how a dying man climbed up seventy feet inside this cave, nor how or why he buried himself without equipment or clothes. No, Huxley concluded, this skeleton had odd, apelike characteristics. He was part of our genus Homo, but different. The great geologist Charles Lyell determined that bones found nearby were those of an extinct mammoth and woolly rhinoceros and, therefore, the “Neanderthal” skull was of great antiquity (figure 10.1 compares H. neanderthalensis and H. sapiens skull features).
FIG. 10.1 Comparison of H. sapiens and H. neanderthalensis skulls. Differences between the skulls are noted. PHOTOS COURTESY OF DR. DANIEL LIEBERMAN, DEPARTMENT OF ANTHROPOLOGY, HARVARD UNIVERSITY
The recognition of these bones as fossil humans could not have been more timely: the appreciation for and wider knowledge of these skeletons came on the heels of the furor over The Origin of Species in 1859. Although Darwin had very carefully avoided the topic in his opus, other than the one sentence “light will be shed on the origin of man and his history,” the evolution of humans was, of course, the topic that aroused the most passion—both then and now.
It was Huxley who took the lead in explicit discussion of human origins. Huxley’s brilliant Evidence as to Man’s Place in Nature (1863) illustrated the state of human relations, with its frontispiece depicting the skeletons of great apes and man (figure 10.2). The Athenaeum magazine derided Huxley and his supporters as degraders of man’s nobility who would make man “a hundred thousand years old.” Ironically, this was a remarkably good guess as the earliest H. sapiens fossils now known are dated at about 160,000 years old.
The story has come a long way since this first Golden Age of paleoanthropology. The fossil record has continued to expand our knowledge, with some of the most provocative discoveries coming in just the last few years. The current spectrum of fossils informs us about three of the most crucial issues in hominid evolution (the term “hominid” refers to both humans and the African apes; “hominin” refers only to humans and our ancestors back to our separation from the apes). First, what distinguishes the hominin lineage from the apes? Second, what distinguishes modern humans (Homo sapiens) from earlier hominins? And third, what was the nature of the last common ancestor of hominins and chimpanzees?
FIG. 10.2 Evolution of ape and human skeletal forms. FROM THE FRONTISPIECE OF T. H. HUXLEY’s EVIDENCE AS TO MAN’S PLACE IN NATURE (1863)
FIG. 10.3 Hominid evolutionary tree. The relationships between various apes and human fossil lineages are shown. This is a conservative tree that does not include all proposed species. The time span of fossil lineages is indicated by shaded bars. Note that the history of H. sapiens is but a small fraction of the roughly 6 million years of hominin evolution. DRAWING BY LEANNE OLDS; THANKS TO DRS. TIM WHITE AND BERNARD WOOD FOR INPUT AND ADVICE
Over the past two decades, the number of recognized hominin species, as well as the number of proposed species, has grown significantly. Depending upon many elements of interpretation, such as whether some fossils are variants of the same species, or “chronospecies”—a single line that evolves over time into a morphologically distinct form—we know of between fifteen and twenty hominin species dating back over the past 6 to 7 million years. A conservative picture of the hominid tree is shown in figure 10.3 (conservative in that several other fossils have been proposed to represent additional taxa, but there is not full agreement on their status). The oldest hominin is the most recently discovered, Sahelonthropus tchadensis, which had a chimpanzee-size brain but homininlike dental and facial features. As the hominin evolutionary tree becomes fuller and stretches back to the point where we think the chimps and human lines split, it is becoming more apparent that near the base of this tree may be a number of apelike species, from one of which the hominin line emerged.
Body fossils or crania are known for only a subset of hominin species, so we cannot always make conclusions about every aspect of anatomy that we might like. However, sufficient material is available to recognize some trends in the evolution of those hominin characters that differentiate us from other apes. The morphological or developmental characters of primary interest in human evolution are:
![]() Relative brain size
Relative brain size
![]() Relative limb length
Relative limb length
![]() Cranial size and shape
Cranial size and shape
![]() Body and thorax shape
Body and thorax shape
![]() Elongated thumb and shortened fingers
Elongated thumb and shortened fingers
![]() Small canine teeth
Small canine teeth
![]() Reduced masticatory structures
Reduced masticatory structures
![]() Long gestation period and life span
Long gestation period and life span
![]() Skull in upright position on vertebral column
Skull in upright position on vertebral column
![]() Reduced body hair
Reduced body hair
![]() Dimensions of the pelvis
Dimensions of the pelvis
![]() Presence of a chin
Presence of a chin
![]() S-shaped spine
S-shaped spine
![]() Brain topology.
Brain topology.
In addition, associated anthropological evidence, such as tools, reflects the capability and behaviors of individual species, as well as the state of the evolution of certain cognitive or motor skills. Tool use was evident as early as 2.5 million years ago with Homo habilis.
In general, more recent species are notable for their larger body size, relatively larger brains, longer legs relative to the torso, and smaller teeth, while earlier species had smaller brains and bodies, shorter legs relative to the torso, and large teeth. The important points to keep in mind are the timescale, magnitude of character change, and the number of species over which these changes occurred. Regardless of the exact branching pattern of the hominin evolutionary tree, change was occurring over an extensive time span and many species. It is crucial to realize that our own species has been around for only a tiny fraction (about 3 percent) of the total time span of hominin evolution. Most of the physical evolution of interest predated the origin of H. sapiens.
Some of the major physical traits that distinguish us are not singular changes, but involve concomitant evolution of the skeleton and musculature. For example, the evolution of bipedal locomotion required changes in the vertebral column, pelvis, feet, and limb proportions and freed up the hands to evolve greater dexterity. Chimpanzees can walk on two legs when necessary, but their gait is entirely different, and they cannot extend their knee joint to straighten their leg.
The evidence for bipedalism in early hominins is derived from features of skeletal morphology. The most stunning evidence of all, though, was discovered in 1976 around the Laetoli archeological site in Tanzania. Paleoanthropologist Andrew Hill was engaging in some typical primate behavior, tossing elephant dung at a colleague, when he stumbled upon sets of hominin footprints that trailed for about eighty feet through a volcanic ash bed (figure 10.4). These astounding prints were made by at least two individuals, one large, one small, who were walking through a fresh ash fall 3.6 million years ago. These prints were then covered until Hill’s discovery and Mary Leakey’s field team excavated and studied the site. The only known hominin species of that age in that location was Australopithecus afarensis, a small-brained, upright-walking species first made famous by the “Lucy” skeleton discovered in Ethiopia by Donald Johanson.
FIG.10.4 Ancient hominin footprints. These footprints in an ancient ash bed, inferred to be those of an adult and juvenile Australopithecus afarensis, were discovered in 1976 at Laetoli, Tanzania. PHOTOS COURTESY OF PETER JONES AND TIM WHITE, UNIVERSITY OF CALIFORNIA-BERKELEY
While bipedalism and its associated features evolved early in our lineage, our large brains did not. Australopithecines such as Au. afarensis and Au. africanus had brains about 450-500 cm3 in volume, not much larger that that of a chimpanzee (about 400 cm3). Brain and body size increased dramatically in the genus Homo in the past 2 million years (figure 10.5), but again this was not a simple, steady increase. Rather, there appears to have been a burst in absolute brain size by the early Pleistocene (1.8 million years ago) and another in the middle Pleistocene (600,000-150,000 years ago), separated by a period of about 1 million years of relative stasis.
FIG. 10.5 There is a broad trend toward an increase in body and brain size from older to more recent species. Body fossils or complete skulls are not available (NA) for all species.
Why did our brains get so much bigger during these periods? There are many theories. I’ll mention just one, the adaptation to climatic change, because I think it reflects a view that is becoming more widely accepted about the role of external forces in driving the pace of evolution. About 2.3 million years ago, there began a global shift toward a cooler and drier climate. This caused the forests of Africa to shrink and to be replaced with drier savannah. While the great apes stayed in more stable rain-forest habitats, hominins adapted to more variable habitats. After a period of relative stability, in the last 700,000 years the Earth’s climate has, on average, been colder than during any other period since the extinction of the dinosaurs 65 million years ago. Abrupt fluctuations in temperature have occurred many times, with some major shifts taking place in the course of just several years. The changing climate and its effects on food availability, water, hunting, and migration may have selected for hominins better adapted to such constantly changing conditions. Under the changing climate, brain size roughly doubled in a million years, encompassing perhaps 50,000 hominin generations. This is impressive, but far from instantaneous.
It is fun to point out that body and brain size were even greater in Neanderthals than in modern humans. We have no obvious physical indication of why we succeeded and our cousin died out about 30,000 years ago without leaving descendants. Our line and the Neanderthal lineage split off from each other well before the origin of H. sapiens, around 500,000 years ago. H. neanderthalensis did not contribute to the H. sapiens gene pool. This was demonstrated conclusively in a remarkable study, one of the really great contributions of genetics to paleoanthropology. Svante Paabo and his colleagues, then at the University of Munich, managed to sequence DNA extracted from a bone of a Neanderthal specimen, and this sequence proved that Neanderthals are a dead twig on the human evolutionary tree.
H. sapiens and Neanderthals did coexist: several sites have been uncovered that reveal the presence of both species at the same time. Both species used tools, made fire, and had other signs of culture, language, and self-awareness, but only one prevailed. Whatever intellectual advantage modern humans might have held over Neanderthals as they took over their range, this would most likely have been subtle in terms of neuroanatomy and will be difficult to ascertain. However, the bigger picture of hominin brain development and evolution, relative to that of the great apes, appears more tractable.
The marked increase in brain size in more recent hominins is only a crude measure of a potential increase in cognitive capabilities. Absolute brain size is not necessarily an indicator of greater power. What is more telling is the relative increase in brain size compared with body mass. The brain is a very expensive organ in terms of energy consumption, drawing up to 25 percent of an adult human’s energy (and 60 percent of an infant’s). The relative increase in hominin brain size in the Pleistocene is a marked deviation from typical mammalian and primate ratios. While whales and elephants have much larger brains than we do, our brains are, as a percentage of body weight, 15-20 times larger than those of these mammals. The challenge for neuroanatomists has been to identify what aspects of brain increase are most meaningful in terms of human capabilities.
The magnitude of this challenge was captured by Emerson Pugh, an IBM computer research scientist, who wrote, “If the human brain was so simple that we could understand it, we would be so simple that we couldn’t.” Understanding the brain and understanding the biological basis of behavior are two of the great frontiers yet to be conquered in biology.
The roles of certain areas of the brain in visual, motor, and cognitive functions have been well studied in mammals and primates, including humans. The top portion of our brains, the cerebral cortex, is a sheet of neural tissue that covers most of the brain. Part of this sheet, the six-layered neocortex, is a structure found only in mammals. In humans, the cortex is arbitrarily divided into several lobes whose boundaries are defined by particular grooves and bumps on the brain surface. Neurobiologists have been particularly successful in localizing functions to lobes (figure 10.6). This includes the frontal lobe, which is involved in thinking, planning, and emotion; the parietal lobe, which is involved in the sensation of pain, touch, taste, temperature, and pressure, as well as in mathematics and logic; the temporal lobe, which is primarily involved with hearing, as well as memory and the processing of emotions; the occipital lobe, which is involved in the processing of visual information; and the limbic lobe, which is involved in emotional and sexual behavior, and in memory processing.
FIG. 10.6 Physical landmarks in human and chimpanzee brains. Broca’s area and Wernicke’s area in the human planum temporale are associated with speech functions. Anatomical features associated with these structures have been reported in chimpanzees. DRAWING BY LEANNE OLDS
One of the first areas of the cortex to be functionally identified was mapped by Paul Broca, who in 1861 examined the brain of a stroke patient who could utter only a single word, “tan.” Broca found a lesion in the frontal lobe of the brain and concluded this was a speech area. His observations have since been supported by many kinds of evidence, including imaging of normal brains of individuals when speaking. Ever since Broca’s era, comparative neuroanatomists have sought to identify areas that might be central to the evolution of human talents. The major point that can be drawn from comparisons of brain anatomy is reminiscent of the stories I have told about other inventions, such as butterfly wing spots, spider spinnerets, and the insect wing—namely, that the current form of the structure owes itself to many inventions that long predated what we see now. Mammalian brains are distinct from what preceded them, early primate brains are a further elaboration upon the mammalian foundation, and ape and human brain evolution was superimposed upon the advanced state of the primate condition.
A key early invention was certainly that of the neocortex in mammals. Not only did this add processing power to the brain, but it opened the way to evolutionary specialization in particular functional subsystems. Changes in brain size among mammals are not simply a matter of enlarging or reducing all parts of the brain proportionately. Rather, brain evolution exhibits a “mosaic” pattern, with certain parts of the brain changing in concert with another, but independently of other parts. For example, the tenrec (an insectivore, a small bug-eating mammal) has a non-neocortical brain volume that is greater than that of the marmoset (a primate), but the marmoset’s neocortex is almost ten times larger (figure 10.7). In primates overall, the neocortex has been expanded such that it is, on average, about 2.3 times larger than in nonprimates of similar body weight. Within primates, shifts from a dependence on a sense of smell to a greater reliance on vision are associated with relative shifts in the sizes of cortical areas involved in each task.
FIG. 10.7 Evolution of mammalian brain areas. The tenrec, an insect-eating mammal, has a much smaller cerebral cortex than the marmoset, a primate. Relative shifts in the size of brain regions are a common feature of specializations. PHOTOS COURTESY OF CAROL DIZACK AND WALLY WALKER, THE WISCONSIN COMPARATIVE MAMMALIAN BRAIN COLLECTION, UNIVERSITY OF WISCONSIN
In addition to shifts in proportion, new centers have evolved. One area of the primate brain that appears to be novel is a center for coordination of visually guided motor activities. Reaching for, grasping, and manipulating objects are obviously important for primate lifestyles. There is a region called the ventral premotor area that is activated during visually guided movements and, very interestingly, also when monkeys observe these tasks being performed. This suggests that this primate motor area may be critical to learning through visual observation.
Because speech and language have played such an enormous part in our evolution, the origin of these capacities has been of immense interest. Broca’s area in the human brain is located in the primate premotor area and may be a specialization for speech and language. The burning issue has been whether brain areas for these activities are unique to humans. One of the gross anatomical features associated with Broca’s area is that this region is larger in the left hemisphere of the brain than in the right. We know that the left hemisphere of the brain dominates speech production, so this asymmetry in Broca’s area has been proposed to reflect the specialization of the left hemisphere. The left hemisphere also controls right-handedness, and hand gestures are also part of our communication process. A second language area, called Wernicke’s area, is in the temporal lobe (figure 10.6). A site within this area, the planum temporale, is implicated in spoken and gestural communication and in musical talent, both of which are also left-hemisphere dominant. In most humans, there is also a left-right hemisphere anatomical asymmetry in this area where a particular fissure extends farther back in the left hemisphere than the right.
Evidence for these anatomical asymmetries has been reported in great apes. This would suggest that anatomical areas that have become specialized in humans were also defined in the common ancestor of humans and great apes. There is also some evidence that communication in captive apes is left-hemisphere dominated, so that would support the inference that the anatomical architecture for communication long predated hominins. However, more recent studies on a larger number of specimens are not supportive.
There is now clear evidence in humans that these anatomical asymmetries are not required templates for speech production or handedness. In 1 out of about 10,000 humans, the normal left-right asymmetry of the distribution of internal organs is reversed (this is called “situs inversus”), but these individuals are generally functionally normal. Recent imaging studies of the brains of situs inversus individuals reveal that the left-right asymmetries in the frontal lobe and planum temporale are also reversed. However, these individuals still have left-hemisphere dominance in speech production and are generally right-handed. These observations show that these two long recognized anatomical asymmetries in the human brain are not necessarily required for the development of speech and language function.
Gross and detailed comparisons of human and ape brains have been undertaken to search for other areas that might account for our functional aptitudes. There is a long-standing idea that areas of the brain involved in planning, organizational behavior, personality, and other “higher” cognitive processes might differ. These properties map to areas of the frontal cortex, which is larger in humans than in chimps, but not disproportionately so. Could it be then that what makes us different is more subtle? Probably. The stuff of our evolution is more likely to be found in the “microanatomy” of our brain, including the interconnections between cortical regions, the architecture of local wiring circuits, or the arrangement of neurons in the cortex. For example, the dimensions of vertical columns of neurons in the planum temporale do differ between chimps and humans. Evolutionary tinkering in the number, arrangement, and connectivity of neurons formed during development in specialized areas of our ancestors’ brains was the most likely path to the origins of our capabilities. Neurobiologists are in hot pursuit of potential fine-scale differences among apes and human brains using a host of high-resolution technologies.
The physical differences between the forms of modern humans, earlier hominins, and the great apes are the products of evolutionary changes in development. In order to understand the nature of those changes, detailed study has been made of the rates of growth and maturation of humans and chimps, and some deductions have also been made from fossil material.
One of the long appreciated, fundamental differences in chimpanzee and human development is the relative rate of skull growth and maturation. Human babies have less mature skulls in terms of their shape than do young chimpanzees, even though human skulls and brains are much larger. In humans, the maturation of the skull is slowed dramatically compared with the chimp, which allows for its greater initial size. Chimpanzee and human skulls eventually grow to the same size, but attain very different face sizes and brain case volumes. The relative shift in skull maturation rates indicates that the timing of similar developmental processes has been shifted.
Evolutionary shifts in the timing of other developmental processes have also been revealed from the study of hominin fossils. Paleontologists can tell from enamel patterns on fossil teeth that tooth formation times were shorter in Australopithecines and early Homo species than in modern humans. The stages of dental development are reliable indicators of stages of juvenile development and the relative age of sexual maturation. The fossil record reveals that these aspects of modern human biology appeared later than other changes such as those of brain size and body proportions. In contrast, all of the skeletal changes associated with our bipedal posture are due to structural changes in bones and musculature and were attained earlier and independently of the slower maturation of the skull. Overall then, the picture of hominin evolution is a mosaic pattern, with different traits appearing at different times and evolving at different rates in hominin history.
The importance of this mosaic pattern to an Evo Devo perspective on human evolution is that it tells us that the development of different structures was evolving in a patchy, nonlinear way over a long course of time. The fossil record dispels any notion of a sudden, instantaneous change in human form. Rather, our history involved quantitative shifts—in brain size, body proportion, skull size, gestation time, juvenile development, and more—assimilated over tens of thousands of generations. Furthermore, the rates of change in human characters were not at all exceptional with respect to what was also transpiring in other mammals during the time period of human evolution. For instance, fossil horses show similar rates of change in body size and other characters.
The body of evidence tells us that the evolution of human form was not special or atypical of other animals. We should expect then that what we know about the evolution of animal form, in general, applies as well to humans. Indeed, our extremely close genetic relationship to chimpanzees, as well as the genetic similarity of primates to other mammals, underscores a now familiar theme. The sets of genes for making these animals and humans are very similar; the differences in form between them, both great and small, must lie in how they are used—or, as we will see in one case, not used.
The ultimate cause of developmental and physical change in the evolution of humans is genetic. Somewhere in our DNA reside the differences between ourselves and apes, and earlier hominins. The critical questions then are:
![]() How many significant differences are there?
How many significant differences are there?
![]() Where are they?
Where are they?
![]() How have they contributed to differences in form?
How have they contributed to differences in form?
The good news is that we now have complete genome sequences for a human, a chimp, and a mouse.
The bad news comes from doing a little arithmetic.
The DNA sequence of a human contains about 3 billion base pairs. In chimp DNA, about 98.8 percent of these bases are identical. That is a difference of just 1.2 percent, the smallest fraction of DNA sequence differences between ourselves and any other animal on the planet. However, that 1.2 percent difference translates to 36 million different base pairs. Because humans and chimps diverged from a common ancestor about 6 million years ago, we can assume that one-half of these differences are chimp-specific (occurred in the chimp line) and one-half are human-specific (occurred in our lineage). That still leaves 18 million changes in our line since our last common ancestor. (I am simplifying the numbers here a bit for discussion purposes. I am not counting the deletion or insertion of bases, or the gain or loss of larger DNA elements.)
Do all of these changes matter? Or, are some just noise? How can we decide which of these 18 million differences contributed to evolution?
We do know that not all mutations in a gene are meaningful. Because the genetic code is redundant, some bases can change without altering a protein. These “silent” substitutions accumulate as a function of time because there is little or no selective pressure to eliminate them. In addition, because only about 5 percent of our DNA is involved in coding or regulatory functions, mutations occurring elsewhere in the vast expanse of human DNA sequence are of no or little consequence. Furthermore, an additional fact to consider is that any two unrelated humans will differ, on average, at about 3 million bases. While that seems to be a large number in absolute terms, it is only 0.1 percent of all DNA bases; and, despite these differences, we clearly belong to one species. This tells us that millions of differences may be of no consequence whatsoever. So, in fact, nobody knows how many changes shaped human form. My guess would be somewhere on the order of a few thousand. The challenge now is to find those differences that do matter.
Before I analyze chimp-human differences further, I think that the paradox, and its general solution, becomes clearer when we compare the human genome with that of another mammal, the mouse. Mice are rodents, and the rodent and primate lines separated a long time ago, probably on the order of about 75 million years ago. Mice are small-brained; they possess a neocortex but it is much smaller relative to that of primates, and, of course, minuscule in comparison to ours. Yet, comparison of mouse and human genomes reveals that greater than 99 percent of all genes in the human have a mouse counterpart, and vice versa. In fact, 96 percent of all genes in the human are found in the exact same relative order in human chromosomes as in the mouse chromosomes. This is a remarkable degree of similarity. These figures tell us that in the course of 75 million years of mammalian evolution, and at least 55 million years of primate evolution, our genome and that of a rodent contain essentially the same genes in mostly the same organization. Differences in gene number and organization have not played much, if any, role in the origin of humans or primates.
If not the number or organization of genes, what else could explain the enormous differences between mice and humans? The sequences of proteins encoded by mouse and human genes do differ, by about 30 percent on average. But, based on what we have seen so far, are the differences in protein sequences likely to account for most changes in form?
Generally speaking, I don’t think so. I make this argument based more on what we know from other species rather than on direct experimental data on humans, but I think this conclusion is inescapable based on several lines of evidence. First, most proteins in the body do not affect form—they carry out other roles in physiology. There may be some interesting differences in proteins involved in physiology, such as the sense of smell, immunity, or reproduction, but these do not affect the way mice or humans appear. Second, the tool kit proteins are a small fraction of all the proteins in the body, and we have seen that because each protein usually has many jobs in development, they are even less likely to change in meaningful ways (because mutations would usually affect all functions, not just one). Rather, as we have seen in previous chapters, changes in genetic switches account for many differences in animal form. Because human evolution is largely a matter of the evolution of the size, shape, and fine-scale anatomy of structures, and of timing in development, it is only logical that switch evolution would be important in the evolution of humans as well. Everything in our bodies is a variation on a mammalian or primate template. Thus, I believe that the weight of genetic evidence is telling us that the evolution of primates, great apes, and humans is due to changes more in the control of genes than in the proteins the genes encode.
I am not the first to reach this conclusion. In a classic study three decades ago, Mary Claire King and Allan Wilson showed that the sequences of chimp and human proteins were nearly identical and drew the conclusion that evolutionary differences were due to changes in gene regulation. A host of eminent biologists in the 1960s and 1970s—including Linus Pauling, Emile Zuckerkandl, Eric Davidson, Roy Britten, and François Jacob—also deduced the same. However, at the time, we knew nothing at all about the logic and function of genetic switches in animals, nor of even a single gene that controlled development. The weight of evidence from Evo Devo and comparative genomics tells us that these earlier deductions were on the right track.
However, despite their importance, it is much more difficult to study human gene switches than those of other species (because we can’t study their function in living human embryos). This makes the identification of evolutionary changes in human switches very challenging. While various kinds of efforts are under way, it has been easier thus far to spot differences in protein-coding sequences that may be responsible for or associated with aspects of human evolution. I will focus on two genes that have been implicated in human evolution. Their stories illustrate the kind of detective work that is needed to implicate particular genes with the evolution of human features. These examples should be taken as illustrative of how such associations are made, as the first stars to be seen through new genetic telescopes. They are not necessarily, or even likely to be, the most important or exclusive genetic causes of the evolution of these traits.
Among the traits that distinguish us from other apes, or earlier hominins such as the Australopithecines, is the reduced size of our jaw muscles. Living primates, such as the macaque or gorilla, have large, powerful jaw muscles for breaking down food. One muscle that elevates the mandible, the temporalis, is attached over most of the temporal region of the skulls of living primates, but is much reduced in proportion in humans (figure 10.8).
FIG. 10.8 Evolution of jaw musculature in primates. Macaques and gorillas have a large temporal region to which the temporalis muscle attaches. This large region is necessary to generate sufficient force for the large jaw and chewing pressure of these animals. In humans, the temporalis is much reduced—this feature is correlated with at least one mutation in a muscle fiber protein. COURTESY OF DR. HANSELL STEDMAN; FROM NATURE 428 (2004): 415; REPRINTED BY PERMISSION
One genetic clue to the origin of the shift in jaw muscle size has been uncovered by Hansell Stedman and colleagues at the University of Pennsylvania. They noticed that the human gene encoding a particular protein called myosin heavy chain 16 (MYH16 for short) had a mutation in it that disrupted most of the protein sequence. Myosin heavy chains play a crucial role as parts of the fibers within muscles that generate force by contraction. When these proteins are absent or altered, the fibers and muscles are usually reduced in size.
MYH16 is a specialized myosin found in only a subset of muscles. In the macaque, MYH16 is made in the temporalis muscle and a second muscle nearby, but not in others. The human MYH16 gene is expressed in the human temporalis muscle, but the mutation in the gene has inactivated the protein’s function. The muscle fibers in the human temporalis are only about one-eighth the size of those of the macaque. This genetic and anatomical evidence suggests that the inactivation of the MYH16 protein is associated somehow with the reduction of the temporalis muscle sometime in hominid evolution.
When might this genetic change have occurred? It definitely occurred after the split of the human and chimp lineages because chimps (as well as other apes and monkeys) have an intact MYH16 gene that encodes a full-size MYH16 protein. Based upon the number of changes in the human gene relative to other species, the Pennsylvania group has estimated that the inactivating mutation occurred somewhere between 2.1 and 2.7 million years ago. This is tantalizingly close to the period of the origin of the genus Homo.
The significance of the evolutionary reduction in jaw musculature extends beyond how hominins chewed their food. Muscle anatomy has a large influence on bone growth, and experimental studies have shown that jaw muscle growth has a significant impact on the size and shape of the craniofacial skeleton. Reduction in the jaw musculature, and the force imposed on the mandible, would reduce the stress on bones in the skull. This could have allowed the braincase to become thinner and larger. Thus, the expansion in brain size that took hold in early Homo may have been enabled, to some degree, by changes in jaw musculature and related skull features. Furthermore, the reduction in jaw musculature may have facilitated the eventual evolution of finer control of the mandible, as is required for speech.
All of these connections and associations are intriguing, but we must be cautious not to attribute all of this anatomical change to a single mutation. While the inactivation of the previously functional MYH16 gene is certainly a noteworthy occurrence, we cannot say whether this inactivating mutation was the initial genetic change toward reduction of the temporalis, or one of many sequential or parallel changes, or the last change that occurred once the role of the MYH16 protein in the temporalis became dispensable. For reasons I will explain shortly, there is no reason to assert that it was the single, critical evolutionary trigger. This will always be difficult to ascertain for any gene implicated in human evolution, such as, for example, the recent discovery of a gene implicated in the evolution of human speech.
One advantage that searchers for human genes of potential evolutionary interest have is that there is a lot of us, about 6 billion now, and when some function isn’t quite right, humans show up at medical clinics. This allows even very rare mutations to be discovered that might arise only once in 1 billion individuals. One striking example of such a very rare and informative mutation was detected in a small family in which members of three generations exhibited a severe speech and language impairment. What is most interesting about the affected individuals is that their impairment is not due to some muscular problem with the production of speech; rather, they have some deficits in the neural circuitry that affects language processes. State-of-the-art imaging techniques have revealed that affected individuals have some detectable abnormalities in several brain areas. Furthermore, magnetic resonance imaging of affected individuals during the performance of silent (thought) and spoken tasks reveals underactivity in Broca’s area and a few other language-related areas. The patients appear to have a deficit in a neural network involved in the learning and/or planning and execution of speech sequences.
The gene that is mutated in this family has been identified and is called FOXP2. The FOXP2 protein is a transcription factor, a tool kit protein that binds to DNA and regulates the expression of other genes. This mutation changes one amino acid in FOXP2, and this one change appears to knock out the FOXP2 protein function. Because these patients also carry one copy of the normal FOXP2 gene, they still have some FOXP2 function. The speech and language impairment is due to a reduction in the total amount of functional FOXP2 protein, not a complete loss. Perhaps the first question that springs to mind about FOXP2 is whether it is a novel, uniquely human gene.
I hope that all I have said for the past several chapters has prepared you to guess the answer to that question. No, FOXP2 is not at all unique to humans. The gene has been identified in a bunch of primates, rodents, and a bird. This distribution is typical of human tool kit genes, in that most, if not all, have counterparts in other species. In fact, the human FOXP2 protein differs at only 4 out of 716 positions from that of the mouse, at 3 positions from the FOXP2 of the orangutan, and at just 2 positions from those of the gorilla and chimpanzee. This is a smaller amount of change in sequence than most proteins show, indicating that there has been a lot of pressure to conserve the FOXP2 protein sequence throughout mammalian evolution.
Did the evolution of the FOXP2 gene play a role in the origin of speech and language? This is a trickier question to answer; the changes in FOXP2 are more subtle than the inactivating mutation in MYH16. Another way to test whether a gene might have played a recent role in evolution is to look for signs of what is called a “selective sweep.” The action of natural selection can leave a trail of evidence in the form of the pattern of DNA sequence variation that arises after a favorable mutation is selected for. Variation in a length of DNA sequence accumulates as a function of time unless or until selection acts to favor a particular variant. Selection for a variant causes a “sweep” that reduces overall variation. From the pattern of reduced variation at a gene relative to its neighbors, geneticists can tell if a gene has experienced a selective sweep. The signal of a selective sweep at the human FOXP2 locus is one of the strongest at any human gene. This is a good indication that for some period of the past 200,000 years, during the evolution of our species, mutations in the FOXP2 gene were favored and spread throughout H. sapiens.
What changes in FOXP2 might have contributed to the evolution of speech? There are just two coding differences between the human and chimp proteins. While it is possible that these could be responsible, there are hundreds more differences in noncoding DNA around FOXP2, in switches and regions that affect the place and amount of FOXP2 expression. It is very difficult to pinpoint the changes that might have been meaningful to human evolution with current technology. My money is on the noncoding regions because tinkering with switches of the FOXP2 gene would allow fine tuning of FOXP2 expression in the formation of neural networks. It is known that FOXP2 is expressed in many sites in the developing human brain. It is also expressed in the counterparts of these regions in the mouse, so FOXP2 would appear to have a widespread role in mammalian brain development. It is not clear yet precisely what FOXP2 does in development, but it is likely that it affects how subregions of the brain form and connect with other parts. Because, as I have said again and again, it is difficult to change a tool kit protein in such a way that only one or a subset of functions is affected, I suspect that evolution in the switches controlling the FOXP2 gene has enabled the evolution of fine-scale differences in individual brain regions.
The discoveries of FOXP2 and MYH16 have generated a great deal of excitement in scientific and medical circles, as well as in the general press. But are they the whole story of the development and evolution of jaw musculature and craniofacial form, or of speech and language? Not at all. They are just the beginning. In order to put the discovery and role of FOXP2 and MYH16 in context, we have to shed a longstanding tendency in many circles, including scientists as well as the general press, to imagine evolution occurring in one leap through the occurrence of a single dramatic mutation. Such ideas have been forwarded for the origin of speech and language and other complex human traits and are often coupled to the idea that the evolution of some characteristic was “rapid.” But we have seen that brain size, skeletal anatomy, dental development, skull shape, and other features evolved over many tens of thousands of generations or more. There is no need to invoke single dramatic mutations as causes of great leaps in form and function or as explanation for the origins of human traits. Nor is there any scientific foundation for doing so.
The term “genetic architecture” has been coined to refer to the number of genes and the relative effect of individual genes contributing to the evolution of a particular trait. Decades of work on quantitative characters, such as body size, or the number of some particular structure has shown that variation within species, or differences between species, are often due to many genetic differences that are individually responsible for relatively small effects. They suggest that evolutionary shifts in characters occur in small increments, via changes in potentially many genes. The genetic architecture of human trait evolution should be no different and, in fact, studies of human variation suggest that many genes contribute to differences in height, body size, and other quantitative characters. We do not know whether the inactivation of MYH16 was an early step in the evolution of the temporalis muscle, or a very late step after the MYH16 function became irrelevant. It is very likely that changes in other genes contributed to the reduction in jaw musculature over an extended time period. Likewise, FOXP2 is certain to be just one part of the story of the evolution of speech. We should expect that selection for evolutionary changes at other genes—or, more specifically, in their genetic switches—also contributed to the evolution of this human talent. We know about FOXP2 because of a lucky strike, a one-in-a-billion mutation that happened to be clinically detectable when present in just one copy of the gene. We know about MYH16 because we can easily tell that this gene was inactivated. There are many more genes to discover and study in stitching together the history of human evolution, some of which will have much more subtle effects and histories than these two genes.
Because there are more stories like that of FOXP2 and MYH16 to come, we should resist the natural tendency to treat new discoveries—of a fossil, a brain landmark, or of a particular gene—as “the” solution to the puzzle of human evolution. Rather, most discoveries are individual pieces of a more complex mosaic. Paleoanthropology now recognizes a complex pattern of hominin evolution, with more species than earlier views, as well as numerous dead twigs rather than a single straight line leading from a distant ancestor to modern humans. In fact, as more fossils are discovered that cluster near the fork between the human and chimp lineages, any claim to finding “the” ancestor should be viewed with suspicion. Similarly, comparative neurobiology must now search for more subtle explanations of human capabilities, as the most obvious anatomical landmarks in the brain appear to have deeper origins than first thought and do not strictly account for human behaviors. Similarly, it is very unlikely that the evolution of any traits that define us—bipedalism, skeletal form, craniofacial form, brain size, or speech—was the result of selection on just a few major genes. FOXP2 and MYH16 are the first pieces in the puzzle to be identified, but we have no reason to believe they are the biggest or most important ones. Rather, the more likely picture is that hominin evolution was forged by selection for variants of many genes, responsible for small increments of differences in size, shape, and tissue composition, over sustained intervals of many thousands of generations.
I mention this caveat to oversimplifying new discoveries not to diminish the excitement they warrant, but because of the larger issues at stake in deciphering the material basis of human evolution. Evolutionary biology has faced resistance since birth and it has been difficult enough for basic concepts drawn from rock-solid data on finches, moths, or fruit flies to gain acceptance. Some claims about human evolution are certain to require revision as more data become available, as has occurred continually in paleoanthropology for the past century. Opponents of evolutionary sciences always seek to exploit even appropriately cautious statements by scientists as evidence of doubt or uncertainty and grounds for not teaching evolutionary principles. Simplification may indeed be necessary for news articles, but it can distort the more complex and subtle realities of evolutionary patterns and mechanisms.
The discoveries of Evo Devo are illuminating the evolutionary process and particular evolutionary events in powerful new ways. Evo Devo has expanded the foundations of evolutionary biology, changed the way we think, and provided a new opportunity to change the way evolutionary biology is reported, taught, and discussed. In my concluding chapter, I will discuss the place of Evo Devo in an overall evolutionary synthesis and the role it must play in the teaching of evolutionary biology, and in the vanguard of the perennial social controversy over evolution.
The geometric beauty and diversity of seashells. JAMIE CARROLL