Figure 2-6 Action of calcium on the actin-myosin complex.
© Jones & Bartlett Learning.
DescriptionMembrane Channels and Action Potential Phases
Eventually, the cell becomes so positive that a new set of channels opens. The point at which the channels open is called the threshold potential, and the channels are the fast sodium channels. Think of this as a one-way valve at the end of a tube. When the positive charges inside the cell reach a certain point, the valve opens. Because it is a one-way valve, ions can only enter the cell, and what is the most common ion outside the cell? Sodium! This influx of sodium makes the cell even more positive, and the cycle continues. The rapid increase in sodium ions causes the cell to “spike,” or fire. This is called phase 0 (Figure 2-5). The impulse is transmitted down the cell, which begins to influence the cell next to it, and so on, until they are all stimulated. At this point, the cell is no longer polarized, or negatively charged; it is now depolarized, and just as positive as the outside solution.
The next phase, when the cell is at its peak positive charge, is phase 1. At this point, some negatively charged chloride ions enter the cell and cause the influx of sodium to slow down. This initial slowdown slams shut the one-way valve on the rapid sodium channels. Two more types of channels now open, the slow sodium channels and the calcium channels, and a slow “plateau” phase begins—phase 2. The slow sodium channels are responsible for a slow influx of sodium ions, but not to the degree of the fast sodium channels. The calcium channels open and begin to allow calcium to enter the cell. Calcium is a double positive ion; it has two positive charges instead of one. The influx of calcium and the slow influx of sodium help maintain the cell in the depolarized state.
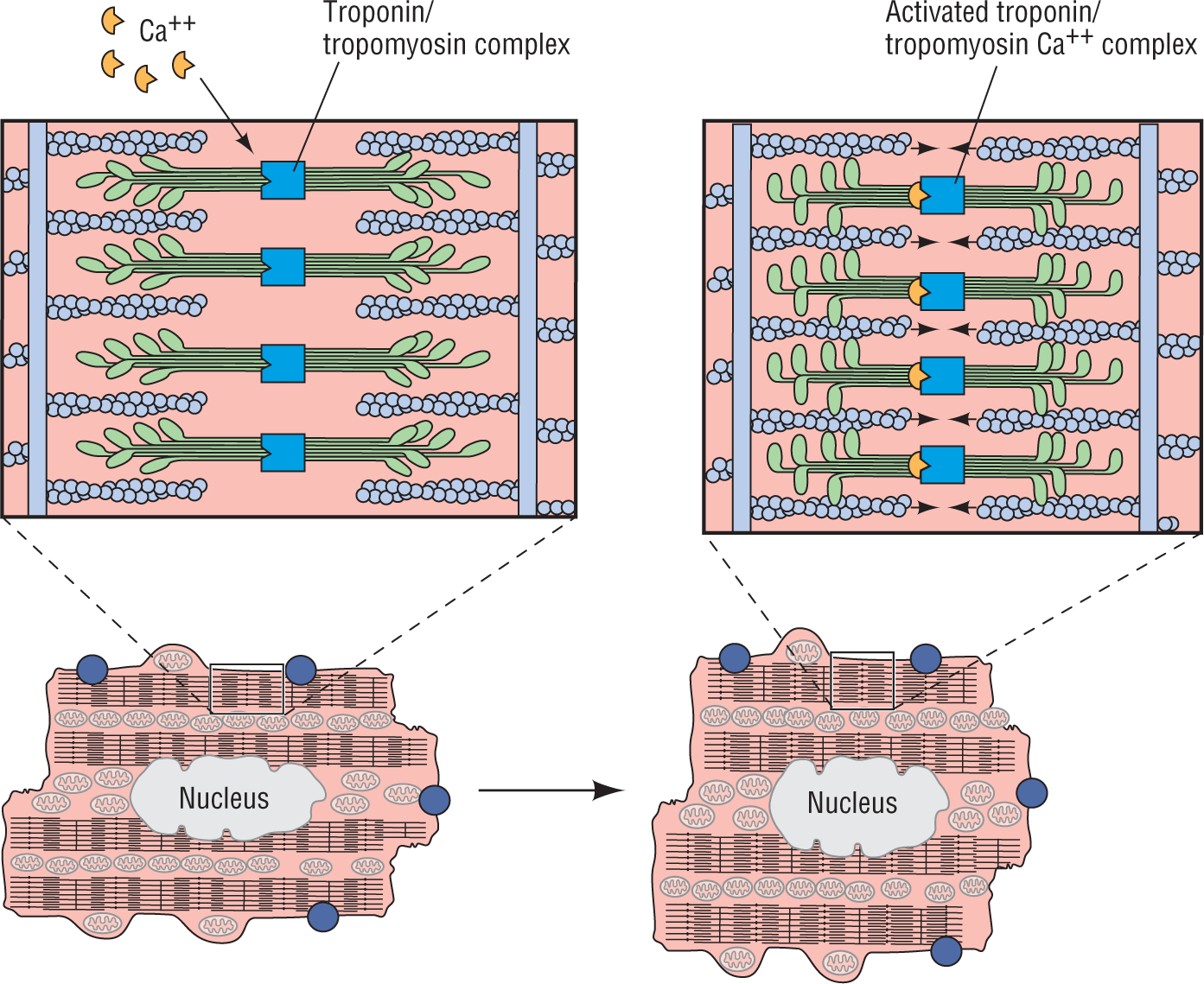
This is where the fun starts: calcium is needed for the cell to contract. Calcium acts like a key, activating a clamp composed of the proteins troponin and tropomyosin. The clamp brings together the two ratcheting proteins, actin and myosin, and allows them to move along each other and cause the cell to contract (Figure 2-6). Without calcium, the right key configuration is not present to unlock and free the clamping proteins, and the actin and myosin do not come close enough together to engage their “teeth” with each other. The more calcium, the faster the clamping action, and the longer the contraction is maintained.

Figure 2-6 Action of calcium on the actin-myosin complex.
© Jones & Bartlett Learning.
DescriptionNext is phase 3. In this phase, some potassium channels open and allow potassium to escape the inside of the cell. During this phase of rapid repolarization, the exit of positive ions imparts a relatively negative charge to the inside of the cell (repolarizes it).
After the cell reaches resting potential, the whole process begins again. The sodium-potassium ATPase pump begins to move sodium out and potassium in, the cell leaks, and it slowly creeps back up to the threshold potential to fire again. One critical point to understand about phase 4 is that different myocytes reach the threshold potential at different rates. Which ones reach it first? The ones that maintain the pacemaking function of the heart, the sinoatrial (SA) nodal cells. In sequence, the next ones are the atrial cells, the atrioventricular (AV) nodal cells, the bundle cells, the Purkinje cells, and finally the ventricular myocytes. Isn’t it interesting that the independent rate for each of these systems is slower than the ones before? This is the body’s protective mechanism, rather than having just one set of cells responsible for the pacing function. If all of the cells in the SA node die, then the next fastest phase 4 belongs to the atrial myocytes; they will fire before the other cells and will set the pace. This continues down the line, as needed.
NOTE: Moving On
As a closing thought, imagine that there are millions of action potentials occurring throughout the heart. Each individual cell is polarizing and depolarizing about 70 to 100 times each minute, and there are quite a few million myocytes in the heart. This translates to millions or billions of action potentials occurring each minute. Miraculously, they will all act in unison, thanks to the electrical conduction system we reviewed in Chapter 1, Anatomy and Basic Physiology. The sum of these collective electrical discharges will create one large electrical current—the electric axis of the heart. In the next few chapters, we will see how the ECG machine measures these electrical potentials and changes them into the patterns that we will learn to recognize on an ECG tracing. We will see how the normal heart gives off some characteristic waves and complexes and how these complexes are altered in pathologic states.
Laying down the foundation for electrocardiography may appear tedious. It is important, however, if we really want to understand and interpret the ECG or rhythm strip correctly. Remember, it isn’t enough just to read the rhythm strip; you must understand what causes the tracings, and the pathology it represents, in order to translate that information into a diagnosis. In turn, that diagnosis will be used to guide therapy—therapy that could save your patient’s life.