If the soft-bodied components had never been found, the Burgess Shale would be an entirely unremarkable Middle Cambrian fauna of about thirty-three genera. It contains a rich assemblage of sponges (Rigby, 1986) and algae, seven species of brachiopods, nineteen species of ordinary trilobites with hard parts, four of echinoderms, and a mollusk and coelenterate or two (Whittington, 1985b, pp. 133–39, presents a complete list). Among the soft-bodied organisms, bringing the total biota to about 120 genera, some are legitimate members of major groups. Whittington lists five certain and two probable species of priapulid worms, six species of polychaetes, and three soft-bodied trilobites (Tegopelte and two species of Naraoia).
My five-act drama, just concluded, emphasizes a different theme, taught to me by the soft-bodied components alone. The Burgess Shale includes a range of disparity in anatomical design never again equaled, and not matched today by all the creatures in all the world’s oceans. The history of multicellular life has been dominated by decimation of a large initial stock, quickly generated in the Cambrian explosion. The story of the last 500 million years has featured restriction followed by proliferation within a few stereotyped designs, not general expansion of range and increase in complexity as our favored iconography, the cone of increasing diversity, implies. Moreover, the new iconography of rapid establishment and later decimation dominates all scales, and seems to have the generality of a fractal pattern. The Burgess revisions of Whittington and colleagues have specified three ascending levels.
1. Major groups of a phylum. No group of invertebrate fossils has received more study, or stands higher in general popularity, than trilobites. The mineralized skeletons of conventional fossils show extraordinary diversity, but all conform to a basic design. One would hardly have anticipated, after all this study, that the total anatomical range of the group could have been far broader in its early days. Yet soft-bodied Naraoia is undoubtedly a trilobite in its distinctive series of head appendages (one pair of antennae and three post-oral biramous pairs), and its conventional body appendages of the “right” form and number of segments. Yet the exoskeleton of Naraoia, with its two valves, stands far outside the anatomical range of the group as seen in conventional fossils.
2. Phyla. We can completely grasp the extent of a surprise only when we also know the full range of conventional possibilities—for we need a baseline of calibration. I find the story of Burgess arthropods particularly satisfying because the baseline has “no vacancy,” and all additional disparity truly supplements a full range of membership in major groups. The orphaned arthropods of the Burgess are spectacular, but the representatives of conventional groups are just as important for documenting the first phrase of the primary theme—“all we could expect and then a great deal more.” The recent discovery of Sanctacaris brings the conventional roster to completion. All four great groups of arthropods have representatives in the Burgess Shale:
Trilobita—nineteen ordinary species plus three soft-bodied
Crustacea—Canadaspis and perhaps Perspicaris
Uniramia—Aysheaia, if correctly identified as an onychophoran
Chelicerata—Sanctacaris
But the Burgess Shale contains an even greater range of anatomical experiments, equally distinct in design and functionally able, but not leading to subsequent diversity. A few of these orphans may show relationships among themselves—Actaeus and Leanchoilia, perhaps, on the basis of their distinctive frontal appendages—but most are unique, with defining features shared by no other species.
The monographic work of Whittington and colleagues has identified thirteen unique designs (table 3.3), all discussed in the preceding chronology. But how many more have yet to be described? Whittington lists twenty-two species (and inadvertently omits Marrella) in his category “not placed in any phylum or class of Arthropoda” (1985b, p. 138). Therefore, by best estimate, the Burgess Shale contains at least twenty unique designs of arthropods, in addition to the documented representatives of all four great groups within the phylum.*
3. Multicellular animal life as a whole. The weird wonders of the Burgess Shale excite our greatest fascination, though the arthropod story is every bit as satisfying intellectually, especially for its completion of the baseline and consequently firm estimate for the relative frequency of oddballs. Still, whereas Marrella and Leanchoilia may be beautiful and surprising, Opabinia, Wiwaxia, and Anomalocaris are awesome—deeply disturbing and thrilling at the same time.


The Burgess revision has identified eight anatomical designs that do not fit into any known animal phylum: in order of publication, Opabinia, Nectocaris, Odontogriphus, Dinomischus, Amiskwia, Hallucigenia, Wiwaxia, and Anomalocaris. But this list is nowhere near complete—surely less exhaustive than the account of documented oddballs among arthropods. The best estimates indicate that only about half the weird wonders of the Burgess Shale have been described. Two recent sources have provided lists of all potential creatures in this category of ultimate strangeness. Whittington counts seventeen species of “miscellaneous animals” (1985b, p. 139), and I would add Eldonia to his total. Briggs and Conway Morris count nineteen “Problematica from the Middle Cambrian Burgess Shale of British Columbia” (1986). Finding no basis for genealogical or anatomical arrangement among the weird wonders, they simply list their nineteen creatures in alphabetical order.
What may the future bring us in further surprises from the Burgess Shale? Consider Banffia, namesake of the more famous national park adjoining Yoho and the Burgess Shale. Walcott’s “worm”—with an annulated front portion separated from a saclike posterior—is almost surely a weird wonder. Or Portalia, an elongate animal with bifurcating tentacles arrayed along the body axis. Or Pollingeria, a scalelike object with a meandering tubelike structure on top. Walcott interpreted Pollingeria as a covering plate from a larger organism, akin to the sclerites of Wiwaxia, and explained the meandering tube as a commensal worm, but Briggs and Conway Morris think that the object could be an entire organism. The general form of the Burgess story may now be well in hand, but Walcott’s quarry has not yet yielded all its particular treasures.
This book, long enough already, cannot become an abstract treatise on the rules of evolutionary inference. But I do need to provide a few explicit comments on how paleontologists move from descriptions of anatomy to proposals about genealogical relationships—so that my numerous statements on this subject receive some underpinning and do not stand as undefended pronouncements ex cathedra.
Louis Agassiz, the great zoologist who founded the institution that now houses both me and the Raymond collection of Burgess Shale fossils, picked a superficially peculiar name that we retain with pride—the Museum of Comparative Zoology. (Anticipating the hagiographical urges of his contemporaries, he even explicitly requested that his chosen title be retained in perpetuity, and that the museum not be renamed for him upon his demise.) Experiment and manipulation may form the stereotype of science, Agassiz argued, but disciplines that treat the inordinately complex, unrepeatable products of history must proceed differently. Natural history must operate by analyzing similarities and differences within its forest of unique and distinctive products—in other words, by comparison.
Evolutionary and genealogical inferences rest upon the study and meaning of similarities and differences, and the basic task is neither simple nor obvious. If we could just compile a long list of features, count the likenesses and unlikenesses, gin up a number to express an overall level of resemblances, and then equate evolutionary relationship with measured similarity, we could almost switch to automatic pilot and entrust our basic job to a computer.
The world, as usual, is not so simple—and thank goodness, for the horizon would probably be a disappointing place anyway. Similarities come in many forms: some are guides to genealogical inferences; others are pitfalls and dangers. As a basic distinction, we must rigidly separate similarities due to simple inheritance of features present in common ancestors, from similarities arising by separate evolution for the same function. The first kind of similarity, called homology, is the proper guide to descent. I have the same number of neck vertebrae as a giraffe, a mole, and a bat, not (obviously) because we all use our heads in the same way, but because seven is the ancestral number in mammals, and has been retained by descent in nearly all modern groups (sloths and their relatives excepted). The second kind of similarity, called analogy, is the most treacherous obstacle to the search for genealogy. The wings of birds, bats, and pterosaurs share some basic aerodynamic features, but each evolved independently; for no common ancestor of any pair had wings. Distinguishing homology from analogy is the basic activity of genealogical inference. We use a simple rule: rigidly exclude analogies and base genealogies on homology alone. Bats are mammals, not birds.
Using this cardinal rule, we can go a certain distance with the Burgess Shale. The tail flukes of Odaraia bear an uncanny resemblance to functionally similar structures of some fishes and marine mammals. But Odaraia is clearly an arthropod, not a vertebrate. Anomalocaris may have used its overlapping lateral flaps to swim by undulation, much as certain fishes with continuous lateral fins or flattened body edges do—but this functional similarity, evolved from different anatomical foundations, indicates nothing about genealogical relationship. Anomalocaris remains a weird wonder, no closer to a vertebrate than to any other known creature.
But the basic distinction between homology and analogy will not carry us far enough. We must make a second division, among homologous structures themselves. Rats and people share both hair and a vertebral column. Both are homologies, structures inherited from common ancestors. If we are searching for a criterion that will properly unite rats and people into the genealogical group of mammals, we can use hair, but the shared vertebral column will not help us at all. Why the difference? Hair works because it is a shared-and-derived character, confined to mammals among the vertebrates. A vertebral column is no help because it is a shared-but-primitive character, present in the common ancestor of all terrestrial vertebrates—not just mammals—and most fish.
This distinction between properly restricted (shared and derived) and overly broad homologies (shared but primitive) lies at the core of our greatest contemporary difficulties with Burgess organisms.* For example, many Burgess arthropods have a bivalved carapace; many others share the basic “merostomoid” form, a broad head shield followed by numerous short and wide body segments capped by a tail spike. These two features are, presumably, genuine arthropod homologies—each bivalved lineage doesn’t start from scratch and develop the same complex structure, slowly and separately. But neither the presence of a bivalved carapace nor “merostomoid” body form can identify a genealogically coherent group of Burgess arthropods because both are shared-but-primitive characters.
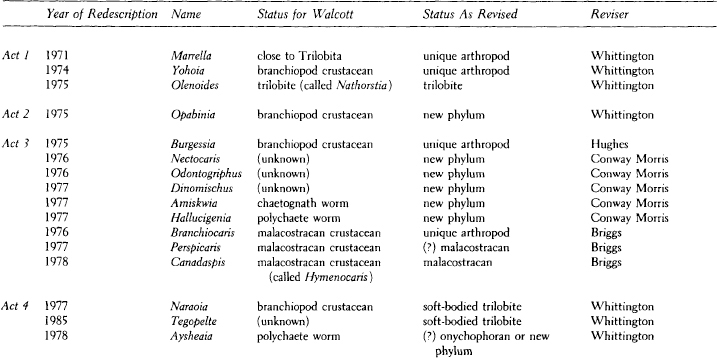
Figure 3.71 should clarify the reason for rejecting shared-but-primitive traits as a guide to genealogy. This evolutionary tree represents a lineage that has diversified into three great groups—I, II, and III—by the time marked by the dashed line. A star indicates the presence of a homologous trait—call it five digits on the front limb—inherited from the distant common ancestor (A). In many branches, this trait has been lost or modified beyond recognition. Every loss is marked by a double-headed arrow. Note that at the selected time, four species (1–4) still retain the shared-but-primitive trait. If we united these four as a genealogical group, we would be making the worst possible error—missing the three true groups entirely, while taking members from each to construct a false assemblage: species 1 might be the ancestor of horses; species 2 and 3, early rodents; and species 4, an ancestor of primates, including humans. The fallacy of basing groups on shared-but-primitive traits should be apparent.*
3.71. A hypothetical evolutionary tree illustrating why shared-but-primitive traits must be rejected as guides in identifying genealogical groups. Lineages and branching points marked with a star possess the shared-but-primitive trait. Double-headed arrows mark the loss of this trait.
But the Burgess problem is probably even worse. In my five-act chronology, I often spoke of a grabbag of available arthropod characters. Suppose that such shared-but-primitive features as the bivalved carapace, unlike the starred trait of figure 3.71, do not indicate continuous lineages. Suppose that in this early age of unparalleled experimentation and genetic lability, such traits could arise, again and again, in any new arthropod lineage—not by slow and separate evolution for common function (for the traits would then represent classic analogies), but as latent potentials in the genetic system of all early arthropods, separately recruitable for overt expression in each lineage. Then traits like merostomoid body form and bivalved carapaces would pop up again and again all over the arthropod evolutionary tree.
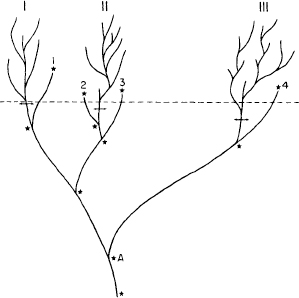
I suspect that such a strange phenomenon did prevail in Burgess times, and that we have had so little success in reconstructing Burgess genealogies because each species arose by a process not too different from constructing a meal from a gigantic old-style Chinese menu (before the Szechuan, yuppie, and other gastronomical revolutions)—one from column A, two from B, with many columns and long lists in each column. Our ability to recognize coherent groups among later arthropods arises for two reasons: First, lineages lost this original genetic potential for recruitment of each major part from many latent possibilities; and second, the removal of most lineages by extinction left only a few survivors, with big gaps between (figure 3.72). The radiation of these few surviving lineages (into a great diversity of species with restricted disparity of total form) produced the distinct groups that we know today as phyla and classes.
3.72. A hypothetical evolutionary tree reflecting a view of life’s history suggested by the reinterpretation of the Burgess fauna. The removal of most groups by extinction leaves large morphological gaps among the survivors. The dashed line represents the time of the Burgess Shale, with disparity at a maximum.
I think that Derek Briggs had a model like this in mind when he wrote of the difficulty in classifying Burgess arthropods: “Each species has unique characteristics, while those shared tend to be generalized and common to many arthropods. Relationships between these contemporaneous species are, therefore, far from obvious, and possible ancestral forms are unknown.” (1981b, p. 38).*
I also think that the model of the grabbag might be extended to all Burgess animals taken together, not only to the arthropods separately. What are we to make of the feeding appendages on Anomalocaris? They do seem to be fashioned on an arthropod plan, but the rest of the body suggests no affinity with this great phylum. Perhaps they are only analogous to arthropod limbs, separately evolved and truly devoid of any genetic continuity with the jointed structures of arthropods. But perhaps the Burgess grabbag extended across phyla. Perhaps jointed structures with a common genetic underpinning were not yet restricted to the Arthropoda. Their limited presence elsewhere would not imply close genealogical relationship with arthropods, but only a broad range of latent and recruitable structures that did not yet respect the later, unbridgeable boundaries of modern phyla. The jaws of Wiwaxia (recalling the molluscan radula) and the feeding organ of Odontogriphus (recalling the lophophore of several phyla) come to mind as other possible features from the mega-grabbag.
The model of the grabbag is a taxonomist’s nightmare and an evolutionist’s delight. Imagine an organism built of a hundred basic features, with twenty possible forms per feature. The grabbag contains a hundred compartments, with twenty different tokens in each. To make a new Burgess creature, the Great Token-Stringer takes one token at random from each compartment and strings them all together. Voilà, the creature works—and you have nearly as many successful experiments as a musical scale can build catchy tunes. † The world has not operated this way since Burgess times. Today, the Great Token-Stringer uses a variety of separate bags—labeled “vertebrate body plan,” “angiosperm body plan,” “molluscan body plan,” and so forth. The tokens in each compartment are far less numerous, and few if any from bag 1 can also be found in bag 2. The Great Token-Stringer now makes a much more orderly set of new creatures, but the playfulness and surprise of his early work have disappeared. He is no longer the enfant terrible of a brave new multicellular world, fashioning Anomalocaris with a hint of arthropod, Wiwaxia with a whiff of mollusk, Nectocaris with an amalgam of arthropod and vertebrate.
The story is old, and canonical. The youthful firebrand has become the apostle of good sense and stable design. Yet the former spark is not entirely extinct. Something truly new slips by now and then within the boundaries of strict inheritance. Perhaps his natural vanity finally got the better of him. Perhaps he couldn’t bear the thought of running such an exquisite play for so long, and having no chronicler to admire the work. So he let the token for more brain tumble from compartment 1 of the primate bag—and assembled a species that could paint the caves of Lascaux, frame the glass of Chartres, and finally decipher the story of the Burgess Shale.
The chief fascination of the Burgess Shale lies in a paradox of human comprehension. The most stunning and newsworthy parts of the story involve the greatest oddities and strangest creatures. Anomalocaris, two feet long, and crunching a trilobite in its circular “jellyfish” jaw, rightly wins the headlines. But the human mind needs anchors in familiarity. The Burgess teaches us a general lesson, and reverses our usual view of life, because so much about this fauna has the clear ring of conventionality. Its creatures eat and move in ordinary ways; the entire community strikes a working ecologist as comprehensible in modern terms; key elements of the fauna also appear in other locations, and we learn that the Burgess represents the normal world of Cambrian times, not a bizarre marine grotto in British Columbia.
I emphasized throughout my five-act chronology that the discovery of conventional creatures, true crustaceans and chelicerates, was every bit as important as the reconstruction of weird wonders in forging a complete interpretation for the Burgess Shale. If we now take a larger look, and consider the entire fauna as a totality, as a functioning ecological community, the same theme holds with even more force. The anatomical oddness of the Burgess gains its meaning against a backdrop of global spread and conventional ecology for the fauna as a whole.
In 1985, Briggs and Whittington published a fascinating article summarizing their conclusions on the modes of life and ecology of Burgess arthropods; (the focus of almost all their previous monographic work had been anatomical and genealogical). Taking all the arthropods together, they inferred a range of behaviors and feeding styles comparable with modern faunas. They divided the Burgess genera into six major ecological categories.
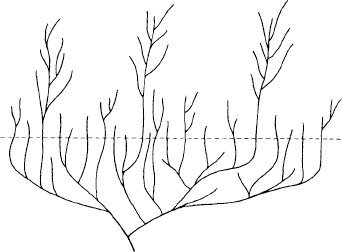
1. Predatory and scavenging benthos. (Benthic creatures live on the sea floor and do little or no swimming.) This large group includes the trilobites and several of the “merostomoid” genera—Sidneyia, Emeraldella, Molaria, and Habelia (figure 3.73D and F–K). All have biramous body appendages bearing strong walking branches with a spiny inner border on the first segment, facing the central food groove. The alimentary canal (where identified) curves down and backward at the mouth—indicating that food was passed from the rear forward, as in most benthic arthropods. The strong spines imply that relatively large food items were caught or scavenged, and passed forward to the mouth.
2. Deposit-feeding benthos. (Deposit feeders extract small particles from sediment, often by processing large quantities of mud; they do not select or actively pursue large food items.) Several genera fall into this category, primarily on the evidence of weak or absent spines on the inner borders of the food groove—Canadaspis, Burgessia, Waptia, and Marrella, for example (figure 3.74E and H–J). Most of these genera could probably either walk across the bottom sediment or swim weakly in the water column just above.
3. Scavenging, and perhaps predatory, nektobenthos. (Nektobenthonic creatures both swim and walk on the sea floor.) The genera in this category—Branchiocaris and Yohoia (figure 3.74D and F)—were not primarily benthic because they did not possess biramous appendages with strong walking branches. Yohoia has three biramous appendages on the head, but probably uniramous limbs with gill branches, used for respiration and swimming, alone on the body; Branchiocaris has biramous body appendages, but with short, weak walking branches. The absence of strong inner branches on the body appendages also suggests that these genera did not eat by passing food forward from the rear. But both genera possess large head appendages with claws at the tip, and probably brought discrete food items from the front end of the body directly to the mouth.
4. Deposit-feeding and scavenging nektobenthos. Like the genera of the preceding category, the members of this group have body appendages with weak or absent inner branches, implying little walking and food processing from the rear; stronger outer branches for swimming; and head appendages that could have gathered food directly. But these genera—Leanchoilia, Actaeus, Perspicaris, and Plenocaris (figure 3.74A–C and G)—do not have strong claws on the tips of their frontal appendages, and probably did not capture large food items; hence they are regarded as probable deposit feeders.
3.73. Burgess arthropods, all drawn to the same scale to show their relative sizes (Briggs and Whittington, 1985). (A) Odaraia. (B) Sarotrocercus. (C) Aysheaia. (D) Habelia. (E) Alalcomenaeus. (F) Emeraldella. (G) Molaria. (H) Naraoia. (I) Sidneyia. (J) The trilobite Olenoides. (K) The large soft-bodied trilobite Tegopelte.
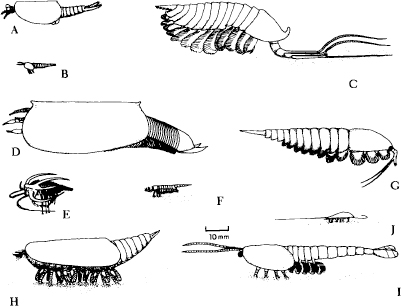
3.74. From Briggs and Whittington, 1985. Additional Burgess arthropods, all drawn to the same scale. (A) Perspicaris. (B) Plenocaris.(C) Leanchoilia.(D) Branchiocaris.(E) Marrella.(F) Yohoia.(G) Actaeus.(H) Canadaspis.(I) Waptia.(J) Burgessia.
5. Nektonic suspension feeders. This small category—consisting of Odaraia and Sarotrocercus (figure 3.73A–B)—includes the true swimmers among Burgess arthropods. These genera either had no walking branches (Sarotrocercus) or possessed short inner branches that could not extend beyond the carapace (Odaraia). They had the biggest eyes among Burgess arthropods, and both probably sought small prey for filter feeding.
6. Others. Every classification has a residual category for unusual members. Aysheaia (figure 3.73C) may have been a parasite, living among and feeding on sponges. Alalcomenaeus (figure 3.73E) bears strong spines all along the inner edges of its walking legs, not only on the first segment, adjoining the food groove. Briggs and Whittington conjecture that Alalcomenaeus may have used these spines either to grasp on to algae, or to tear carcasses in scavenging.
Briggs and Whittington include two excellent summary figures in their paper (figures 3.73 and 3.74). Each genus is shown in its probable habitat, and all are drawn to the same scale—so that the substantial differences in size among genera may be appreciated.
Each of the six categories crosses genealogical lines. The ensemble fills a set of ordinary roles for modern marine arthropods. The great anatomical disparity among Burgess arthropods is therefore not a simple adaptive response to a wider range of environments available at this early time. Somehow, the same basic scope of opportunity originally elicited a far greater range of anatomical experimentation. Same ecological world; very different kind of evolutionary response: this situation defines the enigma of the Burgess.
In 1986, a year after his monograph on Wiwaxia, Simon Conway Morris published a “blockbuster” of another type—a comprehensive ecological analysis of the entire Burgess community. He began with some interesting facts and figures. About 73,300 specimens on 33,520 slabs have been collected from the Burgess Shale. Ninety percent of this material resides in Washington, in Walcott’s collection; 87.9 percent of these specimens are animals, and nearly all the rest are algae. Fourteen percent of the animals have shelly skeletons; the remainder are soft-bodied.
The fauna contains 119 genera in 140 species; 37 percent of these genera are arthropods. Conway Morris identified two main elements in the fauna: (1) An overwhelmingly predominant assemblage of benthic and near-bottom species that were transported into a stagnant basin by the mudslide. Conway Morris inferred, from abundant algae needing light for photosynthesis, that this assemblage originally lived in shallow water, probably less than three hundred feet in depth. He called this element the Marrella-Ottoia assemblage, to honor both the most common substrate walker (the arthropod Marrella) and the most common burrower (the priapulid worm Ottoia). (2) A much rarer group of permanently swimming creatures that lived in the water column above the stagnant basin, and settled amidst the animals transported by the mudslide. Conway Morris called this element the Amiskwia-Odontogriphus assemblage, to honor two of his pelagic weird wonders.
He found that the Burgess genera, despite their odd and disparate anatomies, fall into conventional categories when classified by feeding style and habitat. He recognized four major groups: (1) Deposit-feeding collectors (mostly arthropods)—60 percent of the total number of individuals; 25–30 percent of the genera. (This category includes Marrella and Canadaspis, the two most common Burgess animals, hence the high representation for individuals). (2) Deposit-feeding swallowers (mostly ordinary mollusks with hard parts)—1 percent of individuals; 5 percent of genera. (3) Suspension feeders (mostly sponges, taking food directly from the water column)—30 percent of individuals; 45 percent of genera. (4) Carnivores and scavengers (mostly arthropods)—10 percent of individuals; 20 percent of genera.
Traditional wisdom, with its progressionist bias and its iconography of the cone of increasing diversity, has viewed Cambrian communities as more generalized and less complex than their successors. Cambrian faunas have been characterized as ecologically unspecialized, with species occupying broad niches. Trophic structure has been judged as simple, with detritus and suspension feeders dominating, and predators either rare or entirely absent. Communities have been reconstructed with broad environmental tolerances, large geographic distributions, and diffuse boundaries.
Conway Morris did not entirely overturn these received ideas of a relatively simple world. He did, for example, find comparatively little complexity in the attacking and maneuvering capacities of Burgess predators: “It seems plausible that the degree of sophistication in styles of predation (search and attack) and deterrence in comparison with younger Paleozoic faunas was substantially less” (1986, p. 455).
Still, his primary message made the ecology of the Burgess Shale more conventional, and more like the worlds of later geological periods. Over and over again, when the full range of this community could be judged by its soft-bodied elements, Conway Morris found more richness and more complexity than earlier views had allowed. Detritus and suspension feeders did dominate, but their niches did not overlap broadly, with all species simply sopping up everything edible in sight. Rather, most organisms were specialized for feeding on particular types and sizes of food in a definitely limited environment. Suspension feeders did not absorb all particles at all levels in the water column; the various species were, as in later faunas, “tiered” in assemblages of complex interaction. (In tiering, various forms specialize, confining themselves to low, medium, or high level of the water column, as communities diversify.) Most surprising of all, predators played a major role in the Burgess community. This top level of the ecological pyramid was fully occupied and functioning. No longer could the disparity of early form be attributed to reduced pressures of an easy world, devoid of Darwinian competition in the struggle for existence, and therefore open to any contraption or jury-rigged experiment. The Burgess fauna, Conway Morris argued, “shows unequivocally that the fundamental trophic structure of marine metazoan life was established early in its evolution” (1986, p. 458).
Conway Morris had reached the same conclusion for the entire Burgess ecology that Briggs and Whittington had established for arthropod life styles. The “ecological theater” of the Burgess Shale had been rather ordinary: “It may transpire,” Conway Morris wrote, “that the community structure of the Phyllopod Bed was not fundamentally different from that of many younger Paleozoic soft-bodied faunas” (1986, p. 451). Why then was the “evolutionary play” of these early times so different?
Nothing breeds scientific activity quite so effectively as success. The excitement generated by recent work on the Burgess Shale has inspired an outburst of interest in soft-bodied faunas and the history of early multicellular life. The Burgess Shale is a small quarry in British Columbia, deposited in Middle Cambrian times, after the celebrated explosion of the Lower Cambrian. As long as its fauna remained geographically confined, and temporally limited to a mere moment after the main event, the Burgess Shale could not tell a story for all of life. The most exciting development of the past decade, continuing and accelerating as I write this book, lies in the discovery of Burgess genera all over the world, and in earlier rocks.
The first and most obvious extension occurred close to home. If a mudslide down an unstable slope formed the Burgess, many other slides must have occurred in adjacent regions at about the same time; some must have been preserved. As previously discussed, Des Collins of the Royal Ontario Museum has pioneered the effort to find these Burgess equivalents, and he has been brilliantly successful; during the 1981 and 1982 field seasons, Collins found more than a dozen Burgess equivalents in areas within twenty miles or less of the original site. Briggs and Conway Morris joined the field party in 1981, and Briggs returned in 1982. (See Collins, 1985; Collins, Briggs, and Conway Morris, 1983; and Briggs and Collins, 1988.)
These additional localities are not mere carbon copies of the Burgess. They contain the same basic organisms, but often in very different proportions. One new site, for example, entirely lacks Marrella—the most common species by far in Walcott’s original quarry. The champion here is Alalcomenaeus, one of the rarest creatures, with only two known examples, in the phyllopod bed. Collins also found a few new species. Sanctacaris, as already noted, is especially important as the world’s first known chelicerate arthropod. Another specimen, a weird wonder, has yet to be described; it is “a spiny animal with hairy legs, of unknown affinities” (Collins, 1985).
Above all, Collins has supplied the most precious themes of diversity and comparison to supplement Walcott’s canonical find. His additional localities include five assemblages sufficiently distinct in mix and numbers of species to be called different assemblages. Significantly, these additional sites include four new stratigraphic levels—all close in time to the phyllopod bed, to be sure, but still teaching the crucial lesson that the Burgess fauna represents a stable entity, not an unrepeatable moment during an early evolutionary riot of change.
A few basically soft-bodied Burgess species have lightly skeletonized body parts that can fossilize in ordinary circumstances—notably the sclerites of Wiwaxia and the feeding appendages of Anomalocaris. These have long been known from distant localities of other times. But a few bits do not make an assemblage. The Burgess fauna, as a more coherent entity, has now been recognized away from British Columbia, in soft-bodied assemblages in Idaho and Utah (Conway Morris and Robison, 1982, on Peytoia; Briggs and Robison, 1984, on Anomalocaris; and Conway Morris and Robison, 1986). These contain some forty genera of arthropods, sponges, priapulids, annelids, medusoids, algae, and unknowns. Most have not yet been formally described, but about 75 percent of the genera also occur in the Burgess Shale. Many species once known only for a moment in time, at a dot in space, now have a broad geographic range and an appreciable, stable duration. Writing about the most common Burgess priapulid, Conway Morris and Robison mark the “notable geographic and stratigraphic extensions of a previously unique occurrence.… Ottoia prolifica has a range through much of the middle Cambrian (?15 million years) during which time it shows minimal morphological changes” (1986, p. 1).
More exciting still has been the recognition of many Burgess elements in older sediments. The Burgess Shale is Middle Cambrian; the famous explosion that originated modern life occurred just before, during the Lower Cambrian. We would dearly like to know whether Burgess disparity was achieved right away, in the heart of the explosion itself.
Even before the most recent discoveries, a few positive hints were already in hand, notably some Burgess-like elements in the Lower Cambrian soft-bodied Kinzers fauna of Pennsylvania, and a suspected weird wonder from Australia, described as an annelid worm in 1979. Then, in 1987, Conway Morris, Peel, Higgins, Soper, and Davis published a preliminary description of an entire Burgess-like fauna from the mid-to-late Lower Cambrian of north Greenland. The fauna, like the Burgess itself, is dominated by nontrilobite arthropods. The most abundant creature, about a half inch in length, has a semicircular bivalved carapace; the largest, at about six inches, resembles the Burgess soft-bodied trilobite Tegopelte. Existing collections are poor, and the area is, as we say in the trade, “difficult of access.” But Simon will be visiting next year, and we can expect some new intellectual adventures. In the meantime, he and his colleagues have made the crucial observation, confirming that the Burgess phenomenon occurred during the Cambrian explosion itself: “The extension of stratigraphic ranges of at least some Burgess Shale–like taxa back into the early Cambrian also suggests that they were an integral part of the initial diversification of metazoans” (1987, p. 182).
Last year, my colleague Phil Signor, knowing of my Burgess interests, sent me a spare reprint from a colleague in China (Zhang and Hou, 1985). I could not read the title, but the Latin name of the subject stood out—Naraoia. Chinese publications are notorious for poor photography, but the accompanying plate shows an unmistakable two-valved, soft-bodied trilobite. A key Burgess element had been found half a world away. Far more important, Zhang and Hou date this fossil to the early part of the Lower Cambrian.
One creature is tantalizing; but we need whole faunas for sound conclusions. I am delighted to report—for it promises to be the most exciting find since Walcott’s original discovery itself—that Hou and colleagues have since published six more papers on their new fauna. If the djinn of my previous fable (see page 62) had returned five years ago and offered me a Burgess-style fauna at any other place and time, I could not have made a better choice. The Chinese fauna is half a world away from British Columbia—thus establishing the global nature of the Burgess phenomenon. Even more crucially, the new finds seem well dated to a time deep in the Lower Cambrian. Recall the general anatomy of the Cambrian explosion: an initial period, called Tommotian, of skeletonized bits and pieces without trilobites—the “small shelly fauna”; then the main phase of the Cambrian explosion, called Atdabanian, marked by the first appearance of trilobites and other conventional Cambrian creatures. The Chinese fauna comes from the second trilobite zone of the Atdabanian—right in the heart, and near the very beginning, of the main burst of the Cambrian explosion!
Hou and colleagues describe a rich and well-preserved assemblage, including priapulid and annelid worms, several bivalved arthropods, and three new genera with “merostomoid” body form (Hou, 1987a, 1987b, and 1987c; Sun and Hou, 1987a and 1987b; Hou and Sun, 1988).
The Burgess phenomenon, then, goes right back to the beginning of the Cambrian explosion. In a preliminary report, based on admittedly uncertain dating, Dzik and Lendzion (1988) describe a creature like Anomalocaris and a soft-bodied trilobite from Eastern European strata below the first appearance of ordinary trilobites. We can no longer doubt that Walcott found products of the Cambrian explosion itself in his slightly later strata of British Columbia. Burgess disparity is astounding enough for a time just 30 to 40 million years after the beginning of the Cambrian. But we cannot even view the Burgess range as accumulating steadily during this relatively short period. The main burst occurred well down in the Lower Cambrian—and probably produced the full Burgess range, if the Chinese fauna proves to be as rich as preliminary accounts suggest. The Burgess Shale represents the slightly later period of stabilization for the products of the Cambrian explosion. But what caused the subsequent decimation, and the consequent pattern of modern life, marked by great gaps between islands of extensive diversity within restricted anatomical designs?
The Burgess revision poses two great problems about the history of life. These are symmetrically disposed about the Burgess fauna itself, one before and one after: First, how, especially in the light of our usual views about evolution as a stately phenomenon, could such disparity arise so quickly? And second, if modern life is a product of Burgess decimation, what aspects of anatomy, what attributes of function, what environmental changes, set the pattern of who would win and who would lose? In short, first the origin, second the differential survival and propagation.
In many ways, the first is a juicier problem for evolutionary theory. How in heaven’s name could such disparity have arisen in the first place, whatever the later fortunes of its exemplars? But the second problem is the subject of this book, for the decimation of the Burgess fauna raises the fundamental question that I wish to address about the nature of history. My key experiment in replaying the tape of life begins with the Burgess fauna intact and asks whether an independent act of decimation from the same starting point would yield anything like the same groups and the same history that our planet has witnessed since the Burgess maximum in organic disparity. Hence, I shall shamelessly bypass the first problem—but not without presenting a brief summary of possible explanations, if only because one aspect of the potential solution does bear crucially on the second problem of differential fate.
Three major kinds of evolutionary explanation are available for the explosion that led to Burgess disparity. The first is conventional, and has been assumed—largely faute de mieux—in almost all published discussions. The last two have points in common and represent recent trends in evolutionary thinking. I have little doubt that a full explanation would involve aspects of all three attitudes.
1. The first filling of the ecological barrel. In conventional Darwinian theory, the organism proposes and the environment disposes. Organisms provide raw material in the form of genetic variation expressed in morphological differences. Within a population at any one time, these differences are small and—more important for the basic theory—undirected.* Evolutionary change (as opposed to mere variation) is produced by forces of natural selection arising from the external environment (both physical conditions and interactions with other organisms). Since organisms supply only raw material, and since this raw material has been judged as nearly always sufficient for all changes occurring at characteristically stately Darwinian rates, environment becomes the motor for regulating the speed and extent of evolutionary alteration. Therefore, according to conventional theory, the maximal rates of the Cambrian explosion must indicate something odd about environments at that time.
When we then inquire about the environmental oddity that could have engendered the Cambrian explosion, an obvious answer leaps at us. The Cambrian explosion was the first filling of the ecological barrel for multicellular life. This was a time of unparalleled opportunity. Nearly anything could find a place. Life was radiating into empty space and could proliferate at logarithmic rates, like a bacterial cell alone on an agar plate. In the bustle and ferment of this unique period, experimentation reigned in a world virtually free of competition for the one and only time.
In Darwinian theory, competition is the great regulator. Darwin conceived the world in metaphor as a log with ten thousand wedges, representing species, tightly hammered in along its length. A new species can enter this crowded world only by insinuating itself into a crack and popping another wedge out. Thus, diversity is self-regulating. As the Cambrian explosion proceeded, it drove itself to completion by filling the log with wedges. All later change would occur by a slower process of competition and displacement.
This Darwinian perspective also addresses the obvious objection to the model of the empty barrel as the cause of the Cambrian explosion: Life has suffered some astounding mass extinctions since the Cambrian—the Permian debacle may have wiped out 95 percent or more of all marine species—yet the Burgess phenomenon of explosive disparity never occurred again. Life did rediversify quickly after the Permian extinction, but no new phyla arose; the recolonizers of a depleted earth all remained within the strictures of previous anatomical designs. Yet the early Cambrian and post-Permian worlds were crucially different. Five percent may not be a high rate of survivorship, but no mode of life, no basic ecology, was entirely wiped out by the Permian debacle. The log remained populated, even if the wedges had become broader or more widely spaced. To shift metaphors, all the big spheres remained in the barrel, and only the pebbles in the interstices needed a complete recharging. The Cambrian barrel, on the other hand, was flat empty; the log was unscathed, with nary a woodsman’s blow nor a lover’s knife scratch (see Erwin, Valentine, and Sepkoski, 1987, for an interesting, quantitative development of this general argument).
This conventional view has been assumed in essentially all the Burgess literature—not as an active argument explicitly supported by Burgess evidence, but as the dues that we all properly pay to traditional explanations when we make a side comment on a subject that has not engaged our primary attention. “Less severe competition” has been the watchword of interpretation. Whittington has written, for example: Conway Morris has also supported this traditional view. He wrote to me, in response to my defense of unconventional alternatives to follow: “I think that ecological conditions may have been sufficient to account for the observed morphological diversity.… Thus, perhaps the Cambrian explosion can be regarded as one huge example of ‘ecological release’” (letter of December 18, 1985).
Presumably there was abundant food and space in the varied marine environments which were being occupied initially by these new animals, and competition was less severe than in succeeding periods. In these circumstances diverse combinations of characters may have been possible, as new ways of sensing the surroundings, of obtaining food, of moving about, of forming hard parts, and of behavior (e.g. predation and scavenging) were being evolved. Thus may have arisen strange animals, the remains of some of which we see in the Burgess Shale, and which do not fit into our classifications (1981b, p. 82).
This argument is simply too sensible to dismiss. I haven’t the slightest doubt that the “empty ecological barrel” was a major contributor to Burgess disparity, and that such an explosion could never have occurred in a well-filled world. But I don’t for a minute believe that external ecology will explain the entire phenomenon. My main defense for this gut feeling relies upon scale. The Cambrian explosion was too big, too different, and too exclusive. I just can’t accept that if organisms always have the potential for diversification of this kind—while only the odd ecology of the Lower Cambrian ever permitted its realization—never, not even once, has a new phylum arisen since Burgess times. Yes, the world has not been so empty again, but some local situations have made a decent approach. What about new land risen from the sea? What about island continents when first invaded by new groups? These are not large barrels, but they are at least fair-to-middling bowls. I have to believe that organisms as well as environments were different in Cambrian times, that the explosion and later quiescence owes as much to a change in organic potential as to an altered ecological status.
Ideas about organisms playing such active roles in channeling their own directions of evolutionary change (not merely supplying raw material for the motor of natural selection) have recently grown in popularity, as the strict forms of conventional Darwinism yield their exclusive sway, while retaining their large and proper influence. Evolution is a dialectic of inside and outside, not ecology pushing malleable structure to a set of adaptive positions in a well-oiled world. Two major theories, described in the next two sections, grant a more active role to organic structure.
2. A directional history for genetic systems. In the traditional Darwinian view, morphologies have histories that constrain their future, but genetic material does not “age.” Differences in rates and patterns of change are responses of an unchanging material substrate (genes and their actions) to variations in environment that reset the pressures of natural selection.
But perhaps genetic systems do “age” in the sense of becoming “less forgiving of major restructuring” (to cite a phrase from J. W. Valentine, who has thought long and deeply about this problem). Perhaps modern organisms could not spawn a rapid array of fundamentally new designs, no matter what the ecological opportunity.
I have no profound suggestions about the potential nature of this genetic “aging,” but simply ask that we consider such an alternative. Our exploding knowledge of development and the mechanics of genetic action should provide, within a decade, the facts and ideas to flesh out this conception. Valentine mentions some possibilities. Were Cambrian genomes simpler and more flexible? Has the evolution of multiple copies for many genes, copies that then diverge into a range of related functions, tied up genomes into webs of interaction not easily broken? Did early genes have fewer interactions with others? Did ancient organisms develop with more direct translation of gene to product, permitting such creatures to interchange and alter their parts separately? Most important, do increased complexity and stereotypy of development from egg to adult put a brake upon potential changes of great magnitude? We cannot, for now, go much beyond such crude and preliminary suggestions.
But I can present a good argument against the usual reason for dismissing such ideas in favor of conventional control by external environment. When evolutionists observe that several unrelated lineages react in the same way at the same time, they usually assume that some force external to the genetics of organisms has provoked the common response (for the genetic systems are too unlike, and a similar push from outside seems the only plausible common cause). We have always viewed the creatures that made the Cambrian explosion as unrelated in just this profound way. After all, they include representatives of nearly all modern phyla, and what could be more different, one from the other, than a trilobite, a snail, a brachiopod, and an echinoderm? These morphological designs were as distinct in the Cambrian as they are today, so we assume that the genetic systems were equally unlike—and that the common evolutionary vigor of all groups must therefore record the external push of ecological opportunity.
But this argument assumes the old view of a long, invisible Precambrian history for creatures that evolved skeletons during the Cambrian explosion. The discovery of the Precambrian Ediacara fauna, with the strong possibility that this first multicellular assemblage may not be ancestral to modern groups (see pages 312–13), suggests that all Cambrian animals, despite their disparity of form, may have diverged not long before from a late Precambrian common ancestor. If so—if they had been separate for only a short time—all Cambrian animals may have carried a very similar genetic mechanism by virtue of their strictly limited time of separate life. No ties bind so strongly as the links of inheritance. In other words, the similar response of Cambrian organisms may reflect the homology of a genetic system still largely held in common, and still highly flexible, not only the analogy of response to a common external push. Of course, life needed the external push of ecological opportunity, but its ability to respond may have marked a shared genetic heritage, now dissipated.
3. Early diversification and later locking as a property of systems. My friend Stu Kauffman of the University of Pennsylvania has developed a model to demonstrate that the Burgess pattern of rapid, maximal disparity followed by later decimation is a general property of systems, explicable without a special hypothesis about early relaxed competition or a directional history for genetic material.
Consider the following metaphor. The earthly stage of life is a complex landscape with thousands of peaks, each a different height. The higher the peak, the greater the success—measured as selective value, morphological complexity, or however you choose—of the organisms on it. Sprinkle a few beginning organisms at random onto the peaks of this landscape and allow them to multiply and to change position. Changes can be large or small, but the small shifts do not concern us here, for they only permit organisms to mount higher on their particular peak and do not produce new body plans. The opportunity for new body plans arises with the rarer large jumps. We define large jumps as those that take an organism so far away from its former home that the new landscape is entirely uncorrelated with the old. Long jumps are enormously risky, but yield great reward for rare success. If you land on a peak higher than your previous home, you thrive and diversify, if you land on a lower peak or in a valley, you’re gone.
Now we ask, How often does a large jump yield a successful outcome (a new body plan)? Kauffman proves that the probability of success is quite high at first, but drops precipitously and soon reaches an effective zero—just like the history of life. This pattern matches our intuitions. The first few species are placed on the landscape at random. This means that, on average, half the peaks are higher, half lower, than the initial homes. Therefore, the first long jump has a roughly 50 percent chance of success. But now the triumphant species stands on a higher peak—and the percentage of still loftier peaks has decreased. After a few successful jumps, not many higher peaks remain unoccupied, and the probability of being able to move at all drops precipitously. In fact, if long jumps occur fairly often, all the high peaks will be occupied pretty early in the game, and no one has anyplace to go. So the victors dig in and evolve developmental systems so tied to their peaks that they couldn’t change even if the opportunity arose later. Thereafter, all they can do is hang tough on their peak or die. It’s a difficult world, and many meet the latter fate, not because ecology is a Darwinian log packed tight with wedges, but because even random extinctions leave spaces now inaccessible to everyone.
Kauffman could even quantify the precipitous decline of possibilities for successful jumps. The waiting time to the next higher peak doubles after each successful jump. (Stu told me that a mountain of athletic data shows that when a record is fractured, the average time to the next break doubles.) If your first success needed only two tries on average, your tenth will require more than a thousand. Soon you have effectively no chance of ever getting anywhere better, for geological time may be long, but it is not infinite.
We need no more than the descriptive pattern of Burgess disparity and later decimation to impose a major reform upon our traditional view of life. For the new iconography (see figure 3.72) not only alters but thoroughly inverts the conventional cone of increasing diversity. Instead of a narrow beginning and a constantly expanding upward range, multicellular life reaches its maximal scope at the start, while later decimation leaves only a few surviving designs.
But the inverted iconography, however notable, does not have revolutionary impact by itself because it does not exclude the possibility of a fallback to conventionality. Remember what is at stake! Our most precious hope for the history of life, a hope that we would relinquish with greatest reluctance, involves the concepts of progress and predictability. Since the human mind arose so late, and therefore threatens to demand interpretation as an accidental afterthought in a quirky evolutionary play, we are incited to dig in our heels all the harder and to postulate that all previous life followed a sensible order implying the eventual rise of consciousness. The greatest threat lies in a history of numerous possibilities, each sensible in itself after the fact, but each utterly unpredictable at the outset—and with only one (or a very few) roads leading to anything like our exalted state.
Burgess disparity and later decimation is a worst-case nightmare for this hope of inevitable order. If life started with a handful of simple models and then moved upward, any replay from the initial handful would follow the same basic course, however different the details. But if life started with all its models present, and constructed a later history from just a few survivors, then we face a disturbing possibility. Suppose that only a few will prevail, but all have an equal chance. The history of any surviving set is sensible, but each leads to a world thoroughly different from any other. If the human mind is a product of only one such set, then we may not be randomly evolved in the sense of coin flipping, but our origin is the product of massive historical contingency, and we would probably never arise again even if life’s tape could be replayed a thousand times.
But we can wake up from this nightmare—with a simple and obvious conventional argument. Granted, massive extinction occurred and only a few original designs survived. But we need not assume that the extinction was a crap shoot. Suppose that survivors prevailed for cause. The early Cambrian was an era of experimentation. Let a bunch of engineers tinker, and most results don’t work worth a damn: the Burgess losers were destined for extinction by faulty anatomical construction. The winners were best adapted and assured of survival by their Darwinian edge. What does it matter if the early Cambrian threw up a hundred possibilities, or a thousand? If only half a dozen worked well enough to prevail in a tough world, then these six would form the rootstocks for all later life no matter how many times we replayed the tape.
This idea of survival for cause based on anatomical deftness or complexity—“superior competitive ability” in the jargon—has been the favored explanation, virtually unchallenged, for the reduction of Burgess disparity, and indeed for all episodes of extinction in the history of life. This traditional interpretation is tightly linked with the conventional view for the origin of Burgess disparity as a filling of the empty ecological barrel. An empty barrel is a forgiving place. It contains so much space that even a clap-trap disaster of anatomical design can hunker down in a cranny and hang on without facing competition from the big boys of superior anatomy. But the party is soon over. The barrel fills, and everyone is thrown into the maelstrom of Darwinian competition. In this “war of all against all,” the inefficient survivors from gentler times soon make their permanent exit. Only the powerful gladiators win. Thumbs up for good anatomy!
You will read this interpretation in textbooks, in articles of science magazines, even in the Yoho National Park Highline, the official newsletter for the home of the Burgess Shale (1987 edition). Under the headline “Yoho’s Fossils Have World Significance,” we are told: “The first animals moved into the environment devoid of competition. Later, more efficient life forms held sway only to be supplanted again and again as changing conditions and evolution took its course.” And when, in 1988, Parks Canada put out the first tourist brochure for its nation’s most famous fossils (“Animals of the Burgess Shale”), they wrote that all creatures outside the bounds of modern phyla (the weird wonders of my text) “appear to have been evolutionary dead ends, destined to be replaced by better-adapted or more efficient organisms.”
Whittington and colleagues did not, until recently, challenge this comforting view. It makes too much sense. For example, in the summary comments of his monograph on Wiwaxia, Conway Morris explicitly linked the two traditional scenarios—barrel filling as a cause of disparity followed by stringent competition as the source of later extinction:
It may be that diversification is simply a reflection of the availability of an almost empty ecospace with low levels of competition permitting the evolution of a wide variety of bodyplans, only some of which survived in the increasingly competitive environments through geological time (1985, p. 570).
Briggs made the same point for a French popular audience:
Perhaps this [disparity] is the result of an absence of competition before all the ecological niches of Cambrian seas were filled. Most of these arthropods rapidly became extinct, no doubt because the least well adapted animals were replaced by others that were better adapted (1985, p. 348).*
Whittington also made the almost automatic equation between survival and adaptive superiority:
The subsequent eliminations among such a plethora of metazoans, and the radiations of the forms that were best adapted, may have resulted in the emergence of what we recognize in retrospect as phyla (1980, p. 146).
Conway Morris and Whittington put the matter most directly in an article for Scientific American—probably the best-read source on the Burgess Shale:
Many Cambrian animals seem to be pioneering experiments by various metazoan groups, destined to be supplanted in due course by organisms that are better adapted. The trend after the Cambrian radiation appears to be the success and the enrichment in the numbers of species of a relatively few groups at the expense of the extinction of many other groups (1979, p. 133).
Words have subtle power. Phrases that we intend as descriptions betray our notions of cause and ultimate meaning. I suspect that Simon and Harry thought they were only delineating a pattern in this passage, but consider the weight of such phrases as “destined to be supplanted” and “at the expense of.” Yes, most died and some proliferated. Our earth has always worked on the old principle that many are called and few chosen. But the mere pattern of life and death offers no evidence that survivors directly vanquished the losers. The sources of victory are as varied and mysterious as the four phenomena proclaimed so wonderful that we know them not (Proverbs 30:19)—the way of an eagle in the air, the way of a serpent upon a rock, the way of a ship in the midst of the sea, and the way of a man with a maid.
Arguments that propose adaptive superiority as the basis for survival risk the classic error of circular reasoning. Survival is the phenomenon to be explained, not the proof, ipso facto, that those who survived were “better adapted” than those who died. This issue has been kicking around the courtyards of Darwinian theory for more than a century. It even has a name—the “tautology argument.” Critics claim that our motto “survival of the fittest” is a meaningless tautology because fitness is defined by survival, and the definition of natural selection reduces to an empty “survival of those who survive.”
Creationists have even been known to trot out this argument as a supposed disproof of evolution (Bethell, 1976; see my response in Gould, 1977)—as if more than a century of data could come crashing down through a schoolboy error in syllogistic logic. In fact, the supposed problem has an easy resolution, one that Darwin himself recognized and presented. Fitness—in this context, superior adaptation—cannot be defined after the fact by survival, but must be predictable before the challenge by an analysis of form, physiology, or behavior. As Darwin argued, the deer that should run faster and longer (as indicated by an analysis of bones, joints, and muscles) ought to survive better in a world of dangerous predators. Better survival is a prediction to be tested, not a definition of adaptation.
This requirement applies in exactly the same way to the Burgess fauna. If we wish to assert that Burgess extinctions preserved the best designs and eliminated predictable losers, then we cannot use mere survival as evidence for superiority. We must, in principle, be able to identify winners by recognizing their anatomical excellence, or their competitive edge. Ideally, we should be able to “visit” the Burgess fauna in its heyday, while all its elements flourished, and pick out the species destined for success by some definable, structural advantage.
But if we face the Burgess fauna honestly, we must admit that we have no evidence whatsoever—not a shred—that losers in the great decimation were systematically inferior in adaptive design to those that survived. Anyone can invent a plausible story after the fact. For example, Anomalocaris, though the largest of Cambrian predators, did not come up a winner. So I could argue that its unique nutcracker jaw, incapable of closing entirely, and probably working by constriction rather than tearing apart of prey, really wasn’t as adaptive as a more conventional jaw made of two pieces clamping together. Perhaps. But I must honestly face the counterfactual situation. Suppose that Anomalocaris had lived and flourished. Would I not then have been tempted to say, without any additional evidence, that Anomalocaris had survived because its unique jaw worked so well? If so, then I have no reason to identify Anomalocaris as destined for failure. I only know that this creature died—and so, eventually, do we all.
As the monographic revisions of Burgess genera continued, and as Harry, Derek, and Simon became more adept at reconstructing such unconventional creatures as functioning organisms, their respect grew for the anatomical integrity and efficient feeding and locomotion of the Burgess oddballs. They talked less and less about “primitive” designs, and labored more and more to identify the functional specializations of Burgess animals—see Briggs (1981a) on the tail of Odaraia, Conway Morris (1985) on the protective spines of Wiwaxia, Whittington and Briggs (1985) on the inferred mode of swimming for Anomalocaris. They wrote less about predictable, ill-adapted losers, and began to acknowledge that we do not know why Sanctacaris is cousin to a major living group, while Opabinia is a memory frozen into stone. The later articles talk more and more about good fortune. Briggs tacked a proviso onto his claim, quoted earlier, about survival due to superior adaptation: “… and also, without doubt, because certain species were luckier than others” (1985, p. 348).
All three scientists also begin to emphasize—as a positive note of interest, not an admission of defeat in the struggle to rank Burgess organisms by adaptive worth—the theme that a contemporary observer could not have selected the organisms destined for success. Whittington wrote of Aysheaia as a potential cousin to insects, the greatest of all multicellular success stories:
Looking forward from the Burgess Shale, it would have been difficult to predict which [the survivors] would have been. Aysheaia, slow-moving around sponge colonies, hardly would have looked to be the ancestors of those formidable conquerors of the land, myriapods and insects (1980, p. 145).
Conway Morris wrote that “a hypothetical observer in the Cambrian would presumably have had no means of predicting which of the early metazoans were destined for phylogenetic success as established body plans and which were doomed to extinction” (1985, p. 572). He then commented explicitly on the dangers of circular reasoning. Suppose that the jaw of Wiwaxia is homologous with the molluscan radula and that the two groups, as closest cousins, represent alternative Burgess possibilities. Since wiwaxiids died and mollusks lived to diversify, one might be tempted to argue that the wiwaxiid molting cycle was less efficient than the continuous accretionary growth of mollusks. But Conway Morris acknowledged that if wiwaxiids had lived and mollusks died, we could have ginned up just as good an argument about the benefits of molting:
Nevertheless, molting as a mode of growth is widely used in a number of phyla including arthropods and nematodes, these latter two groups being arguably the most successful of all metazoan phyla. In conclusion, if the clock was turned back so metazoan diversification was allowed to rerun across the Precambrian–Cambrian boundary, it seems possible that the successful body plans emerging from this initial burst of evolution may have included wiwaxiids rather than mollusks (1985, p. 572).
Thus, all three architects of the Burgess revision began with the conventional view that winners conquered by dint of superior adaptation, but eventually concluded that we have no evidence at all to link success with predictably better design. On the contrary, all three developed a strong intuition that Burgess observers would not have been able to pick the winners. The Burgess decimation may have been a true lottery, not the predictable outcome of a war between the United States and Grenada or a world series pitting the 1927 New York Yankees against the Hoboken Has-Beens.
We can now fully appreciate the force of so much patient work in documenting the Burgess arthropods. Whittington and colleagues reconstructed some twenty-five basic body plans. Four led to enormously successful groups, including the dominant animals of our world today; all the others died without issue. Yet, except for the trilobites, each surviving group had only one or two representatives in the Burgess. These animals were not marked for success in any known way. They were not more abundant, more efficient, or more flexible than the others. How could a Burgess observer ever have singled out Sanctacaris, an animal known from only half a dozen specimens? How, as Whittington argued, could the Burgess handicapper ever have given the nod to Aysheaia, a rare and odd creature crawling about on sponges? Why not bet on the sleek and common Marrella, with sweeping spines on its head shield? Why not on Odaraia, with its subtle and efficient tail flukes? Why not on Leanchoilia, with its complex frontal appendage? Why not on sturdy Sidneyia, with nothing fancy but everything in order? If we could wind the tape of life back to the Burgess, why should we not have a different set of winners on a replay? Perhaps, this time, all surviving lineages would be locked into a developmental pattern of biramous limbs, well suited for life in the water but not for successful invasion of the land. Perhaps, therefore, this alternative world would have no cockroaches, no mosquitoes, and no black flies—but also no bees and, ultimately, no pretty flowers.
Extend this theme beyond arthropods to the weird wonders of the Burgess. Why not Opabinia and Wiwaxia? Why not a world of grazing marine herbivores bearing sclerites, not snail shells? Why not Anomalocaris, and a world of marine predators with grasping limbs up front and a jaw like a nutcracker? Why not a Steven Spielberg film with a crusty seaman sucked into the cylindrical mouth of a sea monster, and slowly crushed to death by multiple layers of teeth lining a circular mouth and extending well down into the gullet?
We do not know for sure that the Burgess decimation was a lottery. But we have no evidence that the winners enjoyed adaptive superiority, or that a contemporary handicapper could have designated the survivors. All that we have learned from the finest and most detailed anatomical monographs in twentieth-century paleontology portrays the Burgess losers as adequately specialized and eminently capable.
The idea of decimation as a lottery converts the new iconography of the Burgess Shale into a radical view about the pathways of life and the nature of history. I dedicate this book to exploring the consequences of this view. May our poor and improbable species find joy in its new-found fragility and good fortune! Wouldn’t anyone with the slightest sense of adventure, or the most weakly flickering respect for intellect, gladly exchange the old cosmic comfort for a look at something so weird and wonderful—yet so real—as Opabinia?