This chapter introduces vertebrate animals that practice egg laying (oviparity) rather than live birthing (viviparity) and emphasizes that the distinction between these two seemingly distinct methods of procreation can often be somewhat artificial or arbitrary. Although oviparous vertebrates seldom meet the stereotypical criteria for pregnancy, oviparous females do bear their young internally (albeit encased in eggshells) before parturition, and their offspring are of course alive inside the eggs at the time they exit the dam’s body. So, technically, even oviparous species could be deemed live-bearers. Thus, depending on how one defines pregnancy, oviparous vertebrates display reproductive modes that can be interpreted either as alternatives to pregnancy or as alternative forms of pregnancy. Either way, the practice of laying internally fertilized eggs helps to contextualize the more conventional phenomenon of viviparous pregnancy, which was discussed in chapter 2.
This chapter begins with an introduction to the concept of oviparity and some of its nuances. It then describes the cast of oviparous vertebrates before concluding with a return to the broader thesis that oviparity and viviparity are reproductive phenomena along a continuum that also includes various intermediate ontogenetic conditions and transitional evolutionary states.
More than two millennia ago, in Historia Animalium, the Greek philosopher and naturalist Aristotle dichotomized many things biological. For example, he grouped species into several binary morphological categories such as winged versus wingless and two legged versus four legged, and he also noted a fundamental distinction between species that give birth and those that lay eggs. In modern parlance, the former reproductive mode is termed viviparity (chapter 2), which biologists continue to contrast with oviparity, or egg laying, a major topic of this chapter. However, before proceeding to the lineup of oviparous vertebrates, several questions about the nature of the lain egg must be addressed.
Oviparous females lay eggs (by definition), but the nature of the shed egg has a profound impact on each species’ reproductive lifestyle. One key distinction is between fertilized eggs (containing a zygote or perhaps a multicellular embryo) and unfertilized eggs (ova or oocytes). When a female lays fertilized eggs, this implies that she was fertilized internally, either following a mating event involving direct insemination by a male or perhaps after acquiring sperm by some other means such as spermatophore capture. Conversely, when a female merely lays oocytes, the usual implication is that she belongs to a species with external fertilization. Although we can say that an ova-laying female was gravid, whether she qualifies as being pregnant is a matter of definition.
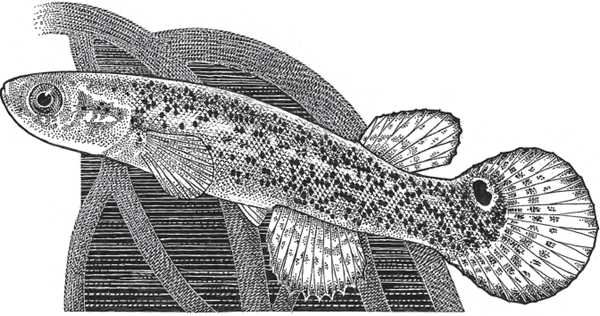
Interestingly, one sexual fish species has abandoned copulation entirely but sheds fertilized eggs nevertheless. It is the mangrove killifish (fig. 3.1), a hermaphroditic species in which each dual-sex individual simultaneously produces eggs and sperm that normally unite within the fish’s body to yield zygotes that are then laid externally. Kryptolebias marmoratus and some of its close relatives are the only vertebrate animals known to reproduce via self-fertilization (an extreme version of inbreeding). They and other hermaphroditic species (Avise 2011) also provide a rather technical exception to the standard rule that only pure females can produce ova.
Factoid: Did you know? The mangrove killifish is actually an “androdioecious” species; a few pure males also exist in populations otherwise composed solely of hermaphroditic individuals. Furthermore, these males sometimes mediate outcross events when their sperm encounter unfertilized ova that the hermaphrodites occasionally release.
FIGURE 3.1 The mangrove killifish (Kryptolebias marmoratus), the world’s only hermaphroditic vertebrate that regularly self-fertilizes.
A second key distinction relevant to the concept of “pregnancy” is whether the fertilized egg was hatched or unhatched at its time of shedding. In some species, each female carries fertilized eggs that hatch inside her body, whereas the hatching events in other species may be delayed until after parturition. Progeny are live-born in the former (viviparous) circumstance, whereas they are hatched externally in the latter (oviparous) situation, but the relative durations and degrees of embryonic development that take place within (rather than outside) the mother’s body vary greatly across species. As described later, ovoviviparous taxa display varying elements of oviparity and viviparity in the sense that the intrafemale gestation of embryos occurs partly inside and partly outside an egg.
After an egg has been fertilized within a female, it seldom remains naked for long. Instead, in amniotes (all birds, reptiles, and mammals), each embryo soon becomes surrounded by several membranes: an amnion, which protects and suspends the embryo in a fluid-filled sac; a yolk sac, which stores nutrients; an allantois, which serves as a repository for some of the embryo’s wastes; and a chorion, which collaborates with the allantois in gas exchange and embryonic respiration. Collectively, these membranes represent a major evolutionary advance over the reproductive apparatuses in nonamniotes, including all fishes and amphibians. Another ancient innovation during vertebrate evolution was the emergence of a cleidoic, or closed, egg, which, because of its waterproof casing or shell, is relatively resistant to desiccation and to some other kinds of environmental insults. The cleidoic eggs of reptiles and birds are very different from their gelatinous predecessors, which continue to confine reproduction in most fishes and amphibians to aqueous environments. Indeed, the initial evolution of the cleidoic egg, several hundred million years ago, played a key role in enabling vertebrates to colonize the terrestrial world. Interestingly, mammals still retain the four extraembryonic membranes, but most extant mammalian species have jettisoned the cleidoic egg in favor of maternal protection and internal gestation of their embryos via a placenta.
The eggshell secreted by the oviduct of any female vertebrate can serve several functions: protect the embryo from physical damage; permit the exchange of respiratory gases necessary for life; and perhaps provide an additional source of nutrition for the embryo. Sometimes these functions come into opposition, producing evolutionary trade-offs. For example, whereas a thick and highly calcified eggshell offers greater protection and may provide additional nutrients to a developing embryo, it also lowers porosity and generally reduces the efficiency of gaseous transfer when compared to an egg with a much thinner covering. Such functional trade-offs must be resolved somehow in any evolutionary transition between different modes of egg production and deployment (Stewart et al. 2010).
All major vertebrate groups, including bony fishes, cartilaginous fishes, reptiles, amphibians, and mammals, contain at least some oviparous species, as do countless invertebrate taxa. However, oviparity is ubiquitous in only one vertebrate class (Aves), so it is with birds that we begin our survey of oviparous vertebrates.
In all 10,000 species of extant birds, each embryo is encased in a hard-shelled cleidoic egg that hatches only after exiting the mother’s body. A bird’s egg is an impressive contraption, typically tapered at one end so that it cannot easily roll out of a nest, large enough to provision an embryo, yet small enough to pass through a female’s vaginal opening (cloaca), and protected by a hard casing that withstands the weight of incubating parents yet fragile enough to permit a hatchling to peck its way free. Furthermore, housed inside each cleidoic egg is everything necessary to support a developing embryo, including a nutrient-rich yolk, a layer of albumin, which supplies water and serves as a shock absorber, an allantoic sac, which helps the embryo respire and acts as a septic tank for waste products, and other specialized membranes that surround and separate all of the above. In short, the avian egg is a beautifully self-contained, out-of-body incubator.
The genesis of an egg begins deep within a hen (or, we might say, the genesis of a hen lies deep within an egg). Let us begin with the hen. In her upper reproductive tract is an ovary filled with oocytes at various stages of maturation. During the breeding season, as each ovum matures in its follicle, it swells and acquires a yolk. Then, during ovulation, the mature ova are released one at a time into the infundibulum (upper portion of the oviduct), where fertilization may take place if the hen has recently mated. Each fertilized egg (henceforth, an embryo as the zygote begins to divide) then begins its journey down the oviduct, first encountering the magnum region, where it settles for about an hour while albumin is incorporated. The embryo then moves to the isthmus section of the oviduct, where it resides for an hour as egg membranes are added. Next comes the uterine portion of the oviduct, where the embryo and its associated paraphernalia become encased in a carbonate shell, a process that takes several hours. Finally, the hen lays her egg, which later hatches an independent chick (fig. 3.2).

FIGURE 3.2 Hen, chick, and cleidoic eggshell.
FIGURE 3.3 Phylogeny for representative vertebrates showing the approximate evolutionary ages of chickens vis-à-vis cleidoic eggs (from Avise 2006).
From an evolutionary perspective, eggs are a hen’s way of making more chickens, and chickens are an egg’s way of making more eggs. But which came first? With respect to an individual’s development (ontogeny), this proverbial question has no answer because eggs and hens are just alternating phases of the avian life cycle. However, we can now declare (with tongue only partly in cheek) that eggs came long before chickens, and here is the phylogenetic reason. As summarized in figure 3.3, the domestic chicken (Gallus domesticus) is descended from red junglefowl (Gallus gallus), which people domesticated about 5,000 years ago, probably in India. Also producing cleidoic eggs are all other birds, plus the reptilian lineages from which ancestral birds separated several hundred million years ago. Phylogenetically speaking, birds are feathered reptiles, one subset of a far more ancient reptilian clade. Thus, the cleidoic egg arose approximately 300 million years ago, making it vastly older than the chicken. Clearly, the cleidoic egg came first!
In approximately 97% of all extant species of bony fishes (Osteichthyes), sexually mature individuals spawn oocytes (unfertilized eggs) that typically undergo syngamy in the external environment. As in other oviparous vertebrate and invertebrate species, spawning females are normally considered to be gravid but not necessarily pregnant. After the oocytes have been fertilized and have exited the mother’s body, their fates vary tremendously, depending on the taxon. Broadcast spawners normally abandon their young, but in many other species the sire (or occasionally the dam) tends the embryos and the fry, typically in nests or other special brooding sites. As detailed in chapters 7 and 8, it can sometimes be useful to think of such extended care-giving species as displaying “external pregnancy.”
Unlike in bony fishes, where oviparity is extremely common and taxonomically widespread, oviparity in the cartilaginous fishes (Chondrichthyes) is confined mostly to a few taxonomic families of sharks, skates, and chimaeras. An example of the latter is the monstrous Chimaera (fig. 3.4), a deepwater fish sometimes dredged from the North Sea. In this species, internal fertilization follows copulation, which is probably facilitated by a forehead clasper that each male uses to hold his mate. An impregnated female then houses the resulting embryos in half-foot-long egg capsules, which hatch after she has laid them outside her body.
Despite the relatively high frequency of viviparity in sharks and rays (chapter 2), oviparity is almost certainly the ancestral reproductive mode from which live-bearing has evolved independently several times in this clade of cartilaginous fishes (Dulvy and Reynolds 1997).
Except for the viviparous caecilians described in chapter 2, nearly all female amphibians lay their eggs externally (typically in water) before they are fertilized. However, with respect to any subsequent parental care of the zygotes or young, what happens next in these oviparous animals varies greatly from species to species (Duellman and Trueb 1986; Wake 1993). Most parents just walk, hop, or swim away, but some show more attention to their offspring. For example, some frogs make foam nests, some actively transport fertilized eggs to nearby water, and some even deliver food to their developing progeny (Weygoldt 1980). In the so-called marsupial frogs (fig. 3.5), mothers are even more attentive: they carry their developing progeny in brood pouches (special skin folds) on their backs (Jones et al. 1973). Here is how this happens: A male catches a female’s unfertilized eggs as she lays them; then he deposits and fertilizes these ova in the dam’s “backpack,” or dorsal brood pouch, where she protects her babies until they hatch and drop into the water to begin independent lives.

FIGURE 3.4 Chimaera monstrosa (Chimaeriformes), an example of an oviparous cartilaginous fish.
FIGURE 3.5 The Andean marsupial frog, Gastrotheca riobambae, a species in which mothers carry offspring in a pouched “backpack.”
As precursors to the birds (and mammals), reptiles were the first vertebrates to deploy cleidoic eggs and thereby free themselves from the need to reproduce in an aqueous environment. Thus, although many snake and lizard lineages include species that give birth to live young (chapter 2), oviparity was clearly the ancestral (and remains the most common) mode of reptilian reproduction. In squamate reptiles, the embryos of most oviparous species are lecithotrophic and thus receive their nourishment primarily from yolk (albeit supplemented with calcium from the eggshell). Viviparous squamates are mostly lecithotrophic, too, but they also show various degrees of matrotrophy (placentotrophy). From these and other observations, Stewart and Thompson (2000) argue that the pattern of embryonic nutrition in oviparous snakes and lizards is probably an exaptation (biological precondition) for the evolution of viviparity in this taxonomic assemblage.
Except for five living species of monotremes (echidnas and platypuses), all extant mammals are viviparous. The semiaquatic duck-billed platypus (fig. 3.6) is a bizarre Australian creature with many odd features such as a rubbery ducklike bill, a flattened beaverlike tail, otterlike webbed feet, a venomous spur on the male’s hind foot, and the distinctly unmammal-like habit of oviparity. During the breeding season, a female lays 1–3 small leathery eggs following a month of internal gestation, which precedes another 10 days of external incubation before her eggs finally hatch. Reproduction in Australia’s short-beaked echidna (fig. 3.7) is generally similar, but a female typically lays only 1 egg per year.
Monotremes get their name from the Greek “monos” (one) and “trema” (hole), which refers to the single cloacal opening through which a female urinates, defecates, and lays her fertilized eggs. (Marsupials have a separate opening for the genital tract, and female placental mammals have three such openings—one each for their reproductive, excretory, and digestive systems.) Although monotremes appear to differ rather fundamentally from marsupial and placental mammals by virtue of being oviparous, the mother retains the fertilized eggs internally and supplies them with nutrients for some time, so in that respect each monotreme female exhibits several elements of standard mammalian pregnancies. She also lactates and thereby offers prolonged support to her hatchlings (known as “puggles”). Extant monotremes are thought to be the descendants of an early offshoot of the mammalian evolutionary tree.
Factoid: Did you know? When European naturalists first encountered the platypus in Australia in 1798 and sent dried skins back to England, British scientists at first thought it might be the hoax of a taxidermist who had sewn together body parts of unrelated animals such as a duck and a beaver.
Factoid: Did you know? Echidnas are also called spiny anteaters because their bodies are covered in spines and they eat ants.
FIGURE 3.6 The duck-billed platypus, Ornithorhynchus anatinus, an egg-laying monotreme mammal.
Factoid: Did you know? Although female platypuses and echidnas have mammary glands that secrete nutrient-rich milk, they lack teats. Instead, their milk is released through pores in the skin and then pools in abdominal grooves where it is lapped up by their offspring.
FIGURE 3.7 The short-beaked echidna, Tachyglossus aculeatus, another egg-laying monotreme mammal.
With regard to pregnancy-like phenomena in vertebrate animals, the gestational distinction between oviparity and viviparity may be more apparent than real for at least two reasons. First, when an oviparous female lays a fertilized egg, she has actually already incubated a genetically distinct individual at least to the zygotic stage before parturition, so such offspring were borne internally for some time, even if hatched externally. Second, in many species with internal fertilization, eggs carried by a gravid female hatch inside her reproductive tract before she then gives birth to free-living offspring. Such ovoviviparous species highlight the notion that various gestational modes can be viewed as gradations along an ontogenetic continuum that ranges from the fully endogenous to the mostly exogenous incubation of young.
Following tradition, many authors have recognized ovoviviparity as a third category of gestation (Balinsky 1975) that in effect combines some of the elements of egg bearing (oviparity) and live birthing (viviparity). An ovoviviparous gestation typically begins with egg-encased embryos that sooner or later hatch inside the female’s reproductive tract before eventually being live-born.
In ovoviviparous taxa, the relative durations of intradam embryonic gestation within (rather than outside) the egg vary from early hatching in the mother and near-complete gestation as nonencased embryos, to hatching that may occur so late in a pregnancy as to barely precede parturition (fig. 3.8). Furthermore, in some ovoviviparous species such as the whale shark, in which fertilizations occur over an extended period of time, different embryos within a pregnant female can show a broad range of developmental stages (Schmidt et al. 2010). In any event, because parturition in ovoviviparous species technically involves the birth of free-living progeny, most textbooks view oviviviparity as a subcategory of viviparity rather than as a version of oviparity. This also means that, whereas ovoviviparous animals certainly can be counted among the live-bearing species that display pregnancy, the many gravid oviparous animals that lay fertilized eggs stretch the usual definition of pregnancy just a bit more.
Many additional subtleties often accompany attempts to formally define ovoviviparity and distinguish it from viviparity and oviparity. For example, Balinsky (1975) added the stipulation that a truly ovoviviparous dam must provide no supplemental nutrition to her embryos, and Mackie (1978) agonized over several considerations, including the nature of the egg membranes and the precise site of embryonic gestation within the female. Such ontological details notwithstanding, the broader point is that gestational mechanisms (and therefore pregnancy modes) are so diverse in the biological world that no single distinction or set of definitional criteria is likely to apply to all cases.
In any event, as stated in chapter 2, different degrees of ovoviviparity characterize several families of bony fishes (such as rockfish and scorpion-fish in the family Scorpionidae) and are also quite common in cartilaginous fishes. Exemplifying the latter is the frilled shark (fig. 3.9), a rare but widely distributed inhabitant of oceanic waters in mostly tropical regions. In this peculiar-looking species, litter size ranges from 2–15, with a mean of about 6. Newly ovulated eggs and early stage embryos are housed in golden-brown ellipsoid capsules that a pregnant female sheds soon after the embryos hatch inside her body after having reached a length of about 8 cm each (Ebert 2003; Tanaka et al. 1990). Thereafter, the embryos continue to gestate and slowly grow inside their mother until they are born, when they are about 50 cm long. The frilled shark appears to be aplacental (without a placenta), but the mechanism by which gestating embryos receive nutrients from their pregnant dam remains to be determined.
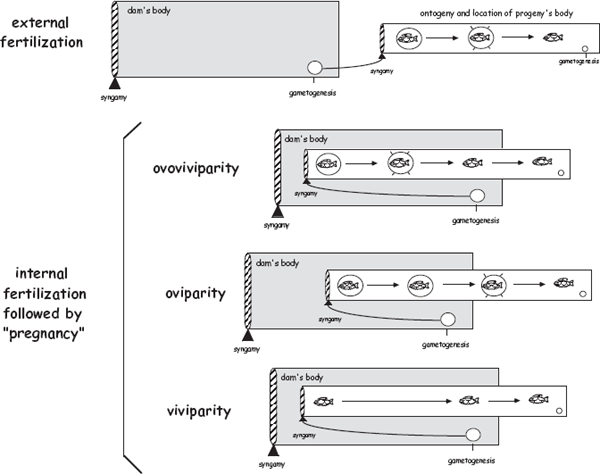
FIGURE 3.8 Diagrams of viviparity, oviparity, and ovoviviparity. Note that even simple spatiotemporal shifts in juvenile ontogeny (vis-à-vis the dam) might account for evolutionary interconversions among these three gestational modes. This can be seen from the diagram by imagining that the boxes representing generation 2 (the developmental timeline of progeny) shift either to the left or to the right with respect to generation 1 (the body of a mother) during the evolutionary process.
Factoid: Did you know? Some sharks and other live-bearing fishes produce what in effect are “trophic eggs,” which are eaten by developing embryos within the mother prior to hatching (Heemstra and Greenwood 1992). Perhaps even more remarkably, females in one species of catfish reportedly extrude unfertilized eggs to help feed hungry fry in their nests (Helfman et al. 1997, 360).
FIGURE 3.9 The frilled shark, Chlamydoselachus anguineus (Chlamydoselachidae), an ovoviviparous deep-sea shark.
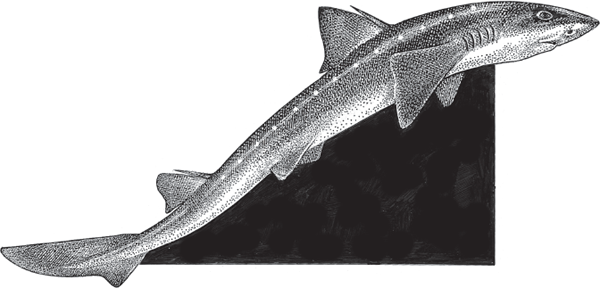
FIGURE 3.10 The Atlantic spiny dogfish, Squalus acanthias (Squalidae), an ovoviviparous shark that is mostly lecithotrophic (yolk-fed).
Factoid: Did you know? The spiny dogfish used to be one of the world’s most abundant shark species, but overfishing for pet food, fertilizers, liver oil, and dissection specimens for biology classes have led to its recent listing as critically endangered in the northeastern Atlantic Ocean.
Another example of an ovoviviparous species is the Atlantic spiny dogfish, Squalus acanthias (fig. 3.10) (Squalidae). In this cartilaginous species, several embryos are housed in an elongated egg case that remains intact for half a year before hatching internally, after which the hatchlings gestate in their mother’s uterus for another year or more, apparently without receiving much more nutritional support from the dam.
In chapter 2, we noted that although viviparity is much less common than oviparity in bony fishes, live-bearing and its associated reproductive conditions (including internal fertilization and embryonic gestation within the dam) clearly are polyphyletic, having arisen on at least several independent occasions in these lineages (see figs. 2.2 and 2.3). To address such evolutionary transitions in greater depth, Mank et al. (2005) employed a “supertree” for ray-finned fishes as a phylogenetic backdrop against which to tally the deduced numbers of evolutionary switches among alternative reproductive modes and among various methods of parental care (which also include the external care of embryos by at least one parent in the many bony fishes with external fertilization).
The results of this exercise in phylogenetic character mapping (PCM) yielded two major conclusions: (a) internal fertilization arose from external fertilization on more than a dozen occasions in these fishes, whereas evolutionary switches in the reverse direction (internal → external fertilization) were rare at best, and (b) syngamy inside the female body (internal fertilization) was the usual stepping-stone on the evolutionary pathway toward internal gestation (conventional female pregnancy) in these piscine lineages.
Dulvy and Reynolds (1997) used similar PCM approaches to address evolutionary transitions between oviparity and viviparity in a group of cartilaginous fishes that includes sharks and rays. Figure 3.11 summarizes the authors’ interpretations of their exercise in PCM, which indicated the following: (A) live-bearing evolved from egg laying on nearly a dozen occasions in these fishes, whereas changes in the opposite direction were rare, and (B) transitions among various types of maternal provisioning (along the scale from lecithotrophy to matrotrophy) were less consistent in magnitude and direction, often showing evolutionary reversals. Although some of these conclusions remain provisional (see Blackburn 2005), the findings again demonstrate the general evolutionary fluidity of interconversions between oviparity and viviparity in fishes.
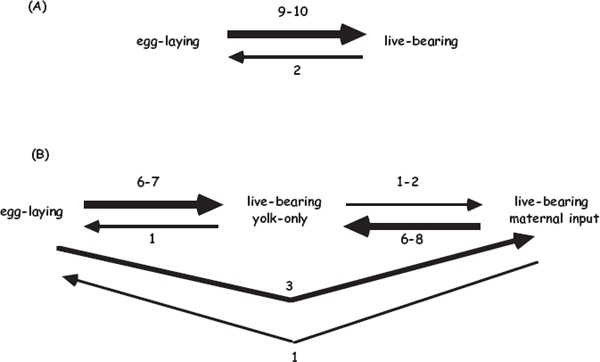
FIGURE 3.11 Phylogenetically deduced numbers of evolutionary transitions among various reproductive modes in the sharks and rays (after Dulvy and Reynolds 1997). (A) transitions between egg laying and live-bearing; (B) transitions among additional parental-care modalities. Arrow widths are proportional to the deduced numbers of evolutionary transitions along the pathways.
Traditionally, biologists often asserted that viviparity in vertebrates can evolve rather readily from oviparity but that changes in the reverse direction have been rare to nonexistent (but see de Fraipont et al. [1996] for an opposing view and see Lynch and Wagner [2010] for a contrasting empirical example involving boa snakes). The basis for the conventional viewpoint seemed to be that producing an eggshell plus all of the other structures associated with oviparity presumably requires special metabolic pathways and organ systems that could not easily be regained once lost during evolution (box 3.1). However, the evolution of viviparity in any vertebrate lineage would also seem to involve the acquisition of numerous complex adaptations (Amoroso 1968; Schindler and Hamlett 1993), such as for fetal respiration and nutrition inside the female’s body and the avoidance of maternal-fetal physiological incompatibilities. Thus, it is not altogether clear which is more complex: viviparity or oviparity. Furthermore, both egg laying and live-bearing have advantages and disadvantages that probably depend at least in part on ecological circumstances, such that neither of these reproductive strategies is likely to be universally superior to the other in all taxa or in all environmental settings. Thus, natural selection might be expected to push different species in different evolutionary directions along the oviparity-to-viviparity scale. For this and other reasons, some evolutionary biologists began to question the assumption that viviparity is always the derived condition and that oviparity is necessarily the ancestral state in all vertebrate groups that include live-bearing species.
BOX 3.1 Dollo’s Law and the Supposed Irreversibility of Evolution
In 1893, the Belgian paleontologist Louis Dollo proposed a “law of irreversibility,” stating that any complex adaptation, once lost, can never be regained in precisely the same form. In other words, if any complicated biological feature decays during evolution and then disappears (for whatever reason), presumably it can never be precisely recouped. If valid, this law implies a strong asymmetry or bias in the direction of evolutionary loss as opposed to the gain of each complex adaptation. It also implies that any organisms possessing the identical complex adaptation must have inherited that elaborate phenotype from a shared ancestor. Although Dollo’s rule makes considerable sense and is a useful generality, all evolutionary laws seem made to be broken, and Dollo’s rule is no exception (see Avise [2006] for several empirical examples).
Apart from the empirical violations, there are also theoretical reasons to be suspicious of the universality of Dollo’s thesis. For example, Dollo’s law presumes that every complex feature has an intricate if not a labyrinthine genetic and ontogenetic basis such that, if the trait were lost, it would not likely reemerge by an identical succession of mutations and selective events. However, biologists know of several instances in which the sudden appearance of a seemingly complicated phenotype (such as the presence of extra legs in a fruit fly or of four wings rather than two) is due to simple mutations in homeotic genes that switch the organism into a different development program (Raff 1996). Thus, although Dollo’s law assumes that many genes are modified during the evolution of a complex phenotype, this may not always be true. Furthermore, even if a long and complex chain of genetic causation underlies an elaborate phenotype, one altered link in that developmental chain might cause a complex adaptation to disappear, perhaps merely to reemerge later via a restoration of that crucial link.
Another related point is that some seemingly major interconversions among complex phenotypes might occur rather simply when fine ontogenetic thresholds are crossed. An example relevant to pregnancy-like phenomena involves the standard textbook distinction between oviparity and ovoviviparity. By definition, an egg-carrying female is oviparous if her fertilized eggs hatch after parturition, but technically she is viviparous if her eggs hatch even a millisecond earlier, just before birth. When a phylogenetic lineage goes back and forth across such narrow definitional lines, even tiny mechanistic steps might cause frequent recurrences of evolutionary conditions that otherwise might seem to be highly complicated and thus implausibly polyphyletic.
To address this issue empirically for reptiles, Lee and Shine (1998) mapped the distributions of live-bearing and egg laying onto a phylogenetic tree for representative extant species. Figure 3.12 depicts a representative subset of this phylogeny, from which it appears that viviparity evolved from oviparity more than a dozen independent times and that few (if any) successful evolutionary transitions occurred in the opposite direction. Thus, although oviparous reptiles might have evolved from viviparous ancestors in some instances, such transitions seem to have been rather uncommon at best. In other words, viviparity appears to have been easier for snakes and lizards to acquire than to jettison during the evolutionary process.
Interestingly, several species of snakes and lizards are polymorphic for oviparity and viviparity, meaning that some populations employ one reproductive mode and other conspecific populations employ the other (Stewart et al. 2010). Surget-Groba et al. (2001) used mtDNA sequences to study the intraspecific phylogeography (Avise 2000) of one such species, Zootoca (formerly Lacerta) vivipara (fig. 3.13) across much of its range in Europe. From these PCM analyses, these authors concluded that a single evolutionary conversion between reproductive modes had occurred (probably in the eastern portion of this species’ distribution) and that the direction of change had been from oviparity to viviparity. Results thus proved to be consistent with the general phylogenetic trends described earlier, and they also indicate that such evolutionary transitions can occur quite rapidly (well within a species’ geological lifespan, which is probably on the order of one million years or less). One hypothesis for this specific transition is that climatic deterioration during the Pleistocene Ice Ages offered fitness advantages to any gravid females who were able to retain fertilized eggs and actively seek suitable microhabitats where their advanced offspring could be born alive (rather than merely hatched from immobile eggs that had been lain outside).
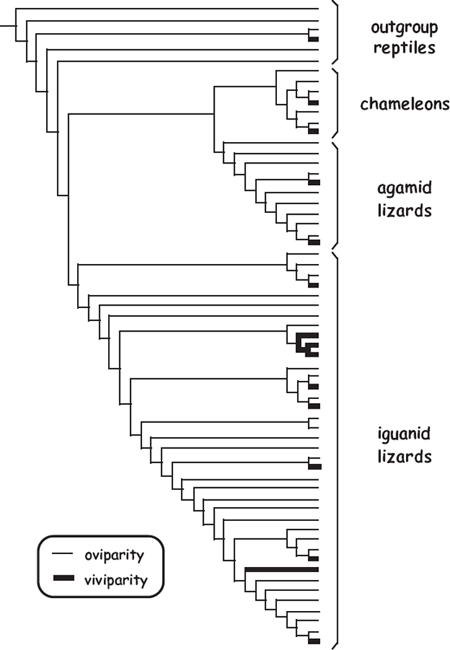
FIGURE 3.12 Phylogenetic tree for more than 60 representative species of lizards and other reptiles, showing more than a dozen independent evolutionary origins of viviparity from oviparity (after Avise 2006, following Lee and Shine 1998).
FIGURE 3.13 The common sand lizard, Zootoca (formerly Lacerta) vivipara, a species that is polymorphic for oviparity and viviparity.
1. For the many nonviviparous vertebrate species, several other reproductive modalities can be interpreted either as alternatives to pregnancy or as alternative forms of pregnancy, depending upon one’s definition of “pregnancy.” The traditional antithesis of viviparity is oviparity, in which a gravid female lays eggs rather than births free-living young. However, the varied structures of vertebrate eggs and the diverse hatching times and places in different species can complicate attempts to cleanly demarcate oviparity and viviparity.
2. With regard to the nature of the shed egg, biologically important distinctions relate to the following: (a) fertilized eggs versus unfertilized ova; (b) eggs with relatively impervious casings versus those without such shells; (c) fertilized eggs that hatch before versus after parturition; and (d) eggs that contain substantial food reserves in yolk versus those that do not.
3. All major vertebrate groups include at least some egg-laying species, but only birds are exclusively oviparous. The eggs that birds and reptiles lay are cleidoic or closed, in contrast to the gelatinous eggs of fishes and amphibians, which must be laid in water to prevent desiccation. The cleidoic egg was a key evolutionary invention that facilitated vertebrate colonization of the terrestrial realm several hundred million years ago, meaning also that the proverbial cleidoic egg came long before the chicken. Even extant mammals include a few egg-laying species: echidnas and the platypus, both of whom are in the order Monotremata.
4. Ovoviviparity is a reproductive mode with elements of both oviparity and viviparity. An ovoviviparous gestation begins with egg-encased embryos that later hatch inside their dam’s reproductive tract before eventually being live-born. The relative durations of embryonic gestation within versus outside the egg vary among taxa—from early hatching within the mother to hatching that may occur so late in a pregnancy as to barely precede parturition. Even minor changes in the relative time of hatching can precipitate shifts between egg-laying and live-bearing. Indeed, populations of some species are polymorphic for oviparity and viviparity.
5. To deduce the evolutionary histories of alternative reproductive modes, phylogenetic character mapping has been applied to several vertebrate groups, including fishes and reptiles. In addition to confirming the general fluidity of evolutionary interconversions between egg-laying and live-bearing in various piscine and reptilian taxa, such PCM analyses have indicated that viviparity is often polyphyletic and appears to evolve from oviparity more readily than vice versa.