This chapter introduces “live-bearing” vertebrates as ambassadors of pregnancy and as exemplars of diverse evolutionary topics that the pregnancy syndrome motivates. In other words, it describes vertebrate creatures in which progeny are borne internally by their mothers before being delivered as free-living beings to the outside world. This overview should prepare readers for later chapters that focus on other manifestations of pregnancy-like phenomena in both vertebrates and invertebrates.
Viviparity (from “vivi,” meaning “alive,” and “parity,” meaning “borne”) refers to the gestation and subsequent delivery of offspring from within the body of a biological parent. Viviparous species, including Homo sapiens and most but not all other mammals, offer quintessential examples of the pregnancy phenomenon. However, female mammals are not the only creatures that become pregnant. Except for birds (class Aves), all major vertebrate groups, including fishes, reptiles, and amphibians, contain at least some live-bearing species. Pregnancies in these taxa, like those in many viviparous invertebrates, otherwise vary in many features, such as the precise site of internal fertilization, the location and duration of embryonic incubation, the size of a brood, the number of genetically involved parents (as when a brood has multiple sires), the mode and magnitude of nutrient passage from adult to offspring, and even the gender of the gestating parent.
Viviparity in any animal species has some obvious evolutionary advantages, including the amelioration of direct predation on eggs and a buffering of embryos against environmental extremes (Packard et al. 1977; Bull and Shine 1979). However, viviparity also has some clear evolutionary downsides, including (a) a typical reduction in fecundity of the pregnant parent (compared to species without pregnancy); (b) survival costs to the adult gestator due to locomotory and other challenges (Miles et al. 2000; Ghalambor et al. 2004; Plaut 2002); (c) the fact that the death of a pregnant parent necessarily results in the loss of an entire brood; and (d) inevitable evolutionary conflicts of interest that arise between a gestating embryo and the parent that houses it (chapter 6). Thus, it is an oversimplification at best to view internal gestation as an evolutionary panacea for rearing offspring because many fitness costs and benefits must be considered (Trexler and DeAngelis 2003).
Among vertebrate animals (creatures with backbones), viviparity made its first evolutionary appearance in fishes, but the phenomenon has arisen time and again not only in a wide variety of distantly related piscine lineages (Gross and Sargent 1985; Mank et al. 2005) but also in many other vertebrate groups (Hogarth 1976; Blackburn 1992). Utilizing phylogenetic considerations, Blackburn (2005) tallied more than 140 independent evolutionary origins for viviparity among the backboned animals alone and concluded (p. 296) that “The evolution of viviparity may represent the most striking example of convergent evolution found among vertebrates.”
Each convergent or parallel evolutionary transition from oviparity (egg laying) to viviparity typically necessitates profound shifts in ontogeny by both parents and offspring. For example, a common chain of events during this evolutionary transformation might entail the following series of biological innovations (Amoroso 1960; Wourms 1981): (a) alterations of both male and female reproductive tracts (as well as mating behaviors) in ways that promote internal as opposed to external fertilization; (b) a decrease in the proclivity of females to shed eggs (or perhaps an increase in their tendency to shed fewer eggs) (Rosen 1962); and (c) reductions in a reliance on the egg yolk for nourishing embryos and a concomitant elaboration of specialized structures (such as a placenta) and physiological mechanisms for a more direct transfer of nutrients from parent to fetus. Furthermore, all of these changes, especially the emergence of an intimate maternal-fetal relationship, pose selective challenges for biotic systems ranging from the morphological to the trophic and also for biological functions related to osmoregulation, excretion, respiration, circulation, endrocrinology, and immunology (Medawar 1953; Matthews 1955). In short, each evolutionary transition from oviparity to viviparity (or vice versa) can be a major biological undertaking.
As characterized by Turner (1947) and Wourms (1981), ontogeny in viviparous fishes and many other vertebrates includes four signature events often relevant to pregnancy: ovulation (O), the release of a mature oocyte (or sometimes an embryo) from its follicle or pit in the ovary; fertilization (F) or conception, the genetic union of egg and sperm; hatching (H), the escape of an embryo from any egg membranes or shells that envelop it; and parturition (P), the final exit of an embryo from its pregnant parent’s body. By convention, P is called birth or delivery in viviparous species, and it is termed extrusion or oviposition in oviparous (egg-laying) species. Across species, the temporal sequence of these four events includes both fixed and variable elements. For example, hatching cannot precede fertilization, and parturition cannot precede ovulation. Otherwise, however, various evolutionary shuffles have occurred in the orders of these four ontogenetic mileposts, and, indeed, many of the shifts help to define different reproductive modalities. For example, because viviparity normally entails internal fertilization, a common sequence of events in live-bearing fish is O-F-H-P, whereas the normal order in an egg-laying species with external fertilization is O-P-F-H. However, some temporal inversions in ontogeny are subtler or even counterintuitive. For example, fertilization in viviparous bony fishes typically occurs in ovarian follicles prior to ovulation, such that the overall sequence of events is F-O-H-P. Apart from evolutionary changes in the orders of these ontogenetic events, there are also several instances in which distinctions between the events themselves can become blurred. For example, hatching and parturition essentially coincide in some shark species in which each embryo retains its egg capsule throughout the gestational phase of its development within its mother. In addition, in live-bearing rockfishes of the genus Sebastes (fig. 2.1), embryos typically hatch as relatively undeveloped larvae, but whether they do so just before or just after parturition is not always clear from the evidence available for species in the wild.

FIGURE 2.1 The tiger rockfish, Sebastes nigrocinctus, representing a taxonomic family (Scorpionidae) that includes ovoviviparous species (chapter 3) in which each pregnant female retains egg-encased embryos until close to the time of both hatching and parturition.
Another important consideration for all forms of pregnancy involves the mechanism(s) by which embryos receive nourishment from their gestating parent. The delivery routes and the magnitudes of nutrient transfer vary greatly among taxa. Most mammals and many viviparous fishes have a special device known as a placenta, which serves as a maternal-fetal conduit that delivers parental resources to embryos and removes fetal wastes. Placentation is an extreme and direct form of matrotrophy, or “mother feeding” of embryos. However, embryos in some other live-bearing fishes secure their nourishment by other means, such as lecithotrophy (exclusive reliance on egg yolk), or perhaps expressions of matrotrophy that entail limited or indirect nutritional support from the dam. For example, depending on the species, matrotrophy sometimes involves a placental analogue (Blackburn 1999b) or “pseudoplacenta” (Turner 1940b), or it may entail the direct absorption of maternal secretions or other assets from the mother’s uterine or ovarian environment. Although lecithotrophy versus matrotrophy is a standard distinction in the reproductive literature, the line between these two trophic modalities often blurs, such as when embryonic gestation in a live-bearing species begins with yolk feeding but later proceeds to maternal feeding after the progeny have hatched internally (chapter 3). The line can also blur in a structural sense, such as in some fish species in which derivatives of the yolk sac participate in the direct absorption or transfer of nutrients from the mother’s reproductive tract (Turner 1947).
Factoid: Did you know? Placenta-like connections between a pregnant parent and its progeny are common in cartilaginous fishes (Chondrichthyes) but, with a few notable exceptions, are relatively rare in most groups of bony fishes (Osteichthyes) (Blumer 1979; Wourms 1981).
With respect to the magnitude of maternal provisioning, different live-bearing vertebrates collectively show a full gradation from little or no supplementation beyond the yolk per se to extensive investments that enable the dramatic growth of embryos in utero. A standard way that scientists gauge the magnitude of maternal support during a pregnancy is to monitor weight changes or developmental progress in offspring between conception and parturition. In matrotrophic species, embryos typically gain much weight depending on the exact amount of maternal investment. In lecithotrophic species, by contrast, a substantial net loss of total organic weight (up to 50%) typically occurs during gestation as embryos gradually use up their finite yolk supplies.
Although placental mammals may represent the epitome of pregnancy, they are hardly alone in exhibiting intraparent gestation of their young. To introduce the taxonomic scope of viviparous pregnancy and some of the many species associated with it, the following sections present the vertebrate animals in which a pregnant female gives birth to live offspring following a substantial period of embryonic gestation within the dam. Most of the viviparous vertebrates described here are obligate live-bearers. In addition, a few fish species that normally display external fertilization and oviparity show occasional or “facultative viviparity” when a female is sometimes fertilized internally and then retains at least some eggs until the embryos hatch within her body. Indeed, internal fertilization and facultative viviparity are two of the initial transitional stages that Rosen (1962) proposed as evolutionary antecedents to constitutive viviparity.
Among the approximately 18,000 extant species of bony fishes (superclass Osteichthyes), only slightly more than 500 (ca. 3%) display female pregnancy (Wourms 1981). These live-bearing species are distributed throughout more than 120 osteichthyan genera, representing at least 14 taxonomic families (table 2.1). (One additional family [Syngnathidae; Syngnathiformes] also has viviparous species, but the gestating gender in that special case is the male [chapter 7].) Clearly, female-pregnant viviparity in bony fishes is polyphyletic, having evolved independently on more than a dozen separate occasions in different osteichthyan lineages (Gross and Sargent 1985; Blackburn 2005; Mank et al. 2005; Mank and Avise 2006a, 2006b). Some appreciation of the diversity (and polyphyletic origins) of viviparity in extant bony fishes can be gained by inspecting figure 2.2, which shows how modes of parental care and fertilization relevant to live-bearing are taxonomically arranged across a phylogeny for these animals.
Evolutionary pathways to female pregnancy in bony fishes have also been deduced from these exercises in “phylogenetic character mapping,” or PCM (Mank et al. 2005). As summarized in figure 2.3, the standard evolutionary route proved to be as follows: external fertilization → internal fertilization → internal gestation (with few if any evolutionary transitions occurring in the opposite directions along this two-step pathway).
TABLE 2.1 Taxonomic distribution of live-bearing in extant fishes
Shown are 14 taxonomic families of bony fishes (class Osteichthyes) plus 11 orders containing more than 40 taxonomic families of cartilaginous fishes (class Chondrichthyes), which all include at least some live-bearing, female-pregnant species. For the many primary references that underlie these reports, see tables 1 and 2 in Blackburn 2005.
| Osteichthyes |
Chondrichthyes1 |
1. Latimeridae (coelacanths) |
1. Hexanchiformes (2 families) |
2. Zoarcidae (eelpouts) |
2. Squaliformes (6 families) |
3. Parabrotulidae (false brotulas) |
3. Pristiophoriformes (1 family) |
4. Bythitidae (brotulas) |
4. Orectolobiformes (7 families) |
5. Aphyonidae (deep-sea livebearers) |
5. Lamniformes (7 families) |
6. Hemirhamphidae (half-beaks) |
6. Carcharhiniformes (8 families) |
7. Goodeidae (splitfins) |
7. Squatiniformes (1 family) |
8. Anablepidae (four eyes) |
8. Echinorhiniformes (1 family) |
9. Poeciliidae (aquarium live-bearers) |
9. Pristiformes (1 family) |
10. Scorpaenidae (scorpionfish and rockfish) |
10. Torpediniformes (2 families)2 |
11. Comephoridae (oilfish) |
11. Myliobatiformes (10 families)2 |
12. Embiotocidae (surfperches) |
|
13. Clinidae (blennies) |
|
14. Labrisomidae (weed blennies) |
|
1Taxonomy from Nelson 2006.
2All members of this order (the rays) show aplacental viviparity (as do many sharks).
FIGURE 2.2 Ordinal-level phylogeny of Actinopterygii (a major subset of bony fishes) showing the phylogenetic distributions of alternative modes of fertilization and parental care (after Mank et al. 2005).
FIGURE 2.3 Evolutionary pathways to parental care in bony fishes as deduced from PCM analyses (after Mank et al. 2005). Arrow sizes reflect the relative numbers of transitions (numerals show minimum and maximum estimates) along each pathway. The left part of this figure shows the usual route to female pregnancy. The other forms of parental care in fish are discussed in chapter 7.
The following interesting details describe the better-known or most noteworthy families of extant osteichthyan fishes that include at least some species in which females become pregnant.
1. Latimeridae (coelacanths). As fossil evidence indicates, these prehistoric-looking fish, which were up to 6 feet long and had leglike fins, arose about 350 million years ago and were thought to have gone extinct about 65 million years ago, coincident with the asteroid impact that precipitated the demise of the dinosaurs. Then, in 1938, to everyone’s amazement, a living coelacanth (Latimeria chalumnae) was trawled from deep waters off the eastern coast of South Africa. This specimen (fig. 2.4) and dozens more subsequently found in the Indian Ocean revealed many details about coelacanth biology that fossils could not readily provide. For example, researchers learned that female coelacanths are live-bearers that retain the developing young in their oviduct before delivering live-born young after a gestation that lasts somewhat more than a year (Smith et al. 1975). In the oviduct, the embryos seem not to be intimately connected to the dam but rather nestle unattached in cramped compartments, where they absorb oviductal secretions as food, perhaps supplemented by occasional oophagy (box 2.1).
BOX 2.1 Oophagy, Adelphophagy, and Histrotrophe
Apart from the more standard forms of lecithotrophy and matrotrophy, embryos in viviparous species also sometimes feed themselves by eating their siblings in utero. The term oophagy is the practice of embryos ingesting their parent’s eggs during gestation. Many sharks produce “trophic eggs” apparently for this purpose. Adelphophagy (or embryonic cannibalism) is functionally similar but applies when gestating embryos eat one another rather than merely ingesting their mother’s oocytes. Either or both of these phenomena are documented in various sharks (Compagno 1977; Stribling et al. 1980; Sims 2009) and certain other viviparous fish species (Wourms 1981; Veith 1980) and particular invertebrate animals (chapter 4) that either brood their offspring internally or encase them in egg capsules (Kamel et al. 2010). Adelphophagy is a form of cannibalism sensu stricto (Elgar and Crispi 1992) and can also be considered intradam siblicide or an extreme expression of sibling rivalry.
From an evolutionary perspective, oophagy and adelphophagy are not quite as macabre as they might at first appear. Oophagy can be interpreted as merely a modest exaggeration of the standard tendency of embryos in many viviparous fish and other species to ingest material known as histrotrophe: cellular debris and the decomposed products of moribund eggs and embryos that are the routine by-products of pregnancy in many live-bearing species, especially those with large broods. Oophagy often makes good sense for both the parent and its offspring because both parties are likely to profit in terms of personal genetic fitness when these extra resources in the mother’s reproductive tract are put to good use rather than squandered. In turn, adelphophagy can be interpreted as a modest extension of oophagy to include the ingestion of live embryos. It, too, makes good genetic sense in some circumstances, such as when a pregnancy includes more offspring than could possibly survive to term, given the limited intrauterine space and other finite resources available inside a gestating parent.

FIGURE 2.4 The coelacanth, Latimeria chalumnae.
Factoid: Did you know? Young coelacanths are called “pups,” and there are typically 5–25 pups per litter. Some coelacanths may live to be more than 100 years old.
2. Aphyonidae (deep-sea fish in the order Ophidiiformes). These eel-like fishes (fig. 2.5) occur circumtropically in the bathypelagic realm, typically living at depths of 2,000–6,000 meters. Perhaps the most noteworthy features of their viviparous lifestyle relate to the fact that each male bundles his sperm into small sacs (spermatophores), which he delivers to a mate for prolonged yet viable storage inside her reproductive tract. This means that a male can mate with an immature female long before she has produced mature ova, yet his sperm may nevertheless fertilize eggs that she will produce after reaching sexual maturity much later in life. For these species, this special suite of reproductive behaviors (male sperm packing and female sperm storage) may be of crucial adaptive benefit to both sexes because conspecific mates might be hard to find in an environment characterized by perpetual darkness and perhaps low population densities.
3. Goodeidae (splitfins). In these small freshwater species (fig. 2.6) from Mexico and the southwestern United States, egg-encased embryos hatch inside their mother’s body and then soon develop special placenta-like structures known as trophotaeniae (Turner 1937; Wourms and Lombardi 1979), which in this case are evolutionarily modified derivatives of the digestive system. The primary role of these trophotaeniae, which can be either rosette shaped or ribbonlike, appears to be to soak up nutritional resources from the ovarian environment and deliver them to the embryos housed therein until parturition. Furthermore, with respect to precise modes of ovarian gestation, there appears to have been an evolutionary trend in goodeid species toward increased specialization in embryonic feeding: from strict yolk feeding (lecithotrophy) on large-yolked eggs in some of the primitive goodeid lineages to extensive maternal feeding (matrotrophy) via elaborate trophotaeniae in many of the more advanced taxa (Turner 1933, 1940a; Lombardi and Wourms 1979).

FIGURE 2.5 Sciadonus cryptophthalmus, a viviparous deep-sea fish in the family Aphyonidae.
Trophotaeniae also occur in several other live-bearing fish families, including some members of the Parabrotulidae, Zoarcidae, Clinidae, and Embiotocidae. After considering the structural diversity of piscine analogues of mammalian placentas, Wourms and Lombardi (1979) proposed that various groups of viviparous fishes convergently evolved these embryo-feeding adaptations by the following sequence of events: (a) origin of the trophotaeniae from a tubular embryonic gut; (b) precocious enlargement of the midgut or hindgut; (c) hypertrophy of the intestinal villi (fingerlike projections of the gut epithelium); (d) exteriorization of the gut lining and its villi; and (e) a further elaboration of trophotaenial rosettes and ribbons. The authors speculated that these evolutionary transitions took place via temporal shifts in the regulation of genes that govern the differentiation and ontogeny of intestinal cells.

FIGURE 2.6 The redtail splitfin, Xenotoca eiseni, a live-bearing goodeid species with placenta-like structures.
4. Poeciliidae (live-bearers). This large Neotropical family includes mosquito fish (fig. 2.7) and many popular aquarium fishes (Jacobs 1971) such as guppies, mollies, platyfish, and swordtails. Although some of these viviparous species (such as the Amazon molly, pictured earlier in fig. 1.7) consist solely of females who reproduce by parthenogenesis or related clonal mechanisms (Avise 2008), most poeciliids are fully sexual. Nearly all of the more than 200 species in this family are viviparous, typically with gestational periods of about 1 month per brood (Breder and Rosen 1966; Meffe and Snelson 1989). Sperm storage by females is quite common in this group, as is the associated phenomenon of superfetation (box 2.2).
BOX 2.2 Superfetation
Superfetation, or superembryonation, is the simultaneous occurrence of two or more broods of embryos of different ontogenetic stages during a pregnancy. This condition is common in poeciliid fishes (Scrimshaw 1944a; Thibault and Schultz 1978), among whom 6 or more such cohorts may cohabit a female’s reproductive tract (Scrimshaw 1944b). Superfetation occurs at least sporadically in several other viviparous fish assemblages as well (Turner 1947; Reznick et al. 2007), such as Zenarchopteridae (halfbeaks) and Clinidae (blennies), for whom as many as 12 broods per pregnancy have been reported (Veith 1979).
Superfetation normally requires either (a) sperm storage by females, in which case all cohorts carried by a pregnant female might have the same father, but different batches of eggs have been fertilized on various occasions and thus have matured at dissimilar times or (b) multiple mating by the dam, in which case some cohorts of offspring might have different sires. These requirements are not mutually exclusive, and females in many species of superfetatious poeciliids have both long-term sperm storage (lasting several months) (Vallowe 1953) and proclivities for mating with multiple males (Zane et al. 1999). For such taxa, genetic paternity analyses in conjunction with assessments of the ontogenetic stages of embryos can reveal how many sires contributed to each superfetatious pregnancy.
Superfetation probably arises in evolution when the periods required for oocyte maturation and/or embryonic development in a species shorten, thereby in effect permitting successive broods to be squeezed into the temporal window of a pregnancy (Turner 1947). In these situations new groups of oocytes can mature and be fertilized before other broods at more advanced stages of development have completed their gestation and undergo parturition. Such temporal adjustments in ontogeny are likely to be promoted by natural selection when they increase the reproductive outputs of pregnant dams and thereby, in effect, also happen to motivate transitions from semelparity (the occurrence of a single brood during an individual’s lifetime) to iteroparity (repeated reproductive cycles within a lifetime) (Thibault and Schultz 1978). Superfetation seems to be best developed in species with refined mechanisms for nutrient transfer between a mother and her broods (Pollux et al. 2009), probably because matrotrophy improves the effectiveness of superfetation (Reznick and Miles 1989; Wourms 1981).
Male poeciliids have a modified anal fin known as a gonopodium, which serves as an intromittent organ (analogous to a penis) that each male wields with dexterity to inseminate females with special “sperm balls” called spermozeugmata. Each spherical spermozeugmatum is a collection of radially oriented sperm cells with their heads located peripherally and tails coiled into the interior. Brood size in a typical poeciliid pregnancy is 20–30 offspring.
As was also true for goodeid species, the maternal-fetal association in poeciliids ranges from strict lecithotrophy to refined matrotrophy (Reznick et al. 2002). Embryos in lecithotrophic species typically show a weight loss during gestation as they gradually use up their finite yolks. Among the matrotrophic poeciliids, embryos in some species maintain a more or less constant gestational weight (indicating that they receive at least modest nutritional support from the mother), whereas in other species they show dramatic weight gains of perhaps 50-fold or more by the time of birth (thus indicating more extensive maternal support during gestation). These two categories of mother-fed species are called unspecialized and specialized matrotrophs, respectively. Perhaps not surprisingly, the magnitude of nutritional support that a poeciliid embryo receives from its dam is related to the nature of the physical connection between mother and fetus during each pregnancy. Some poeciliid species show little or no development of a placenta, whereas others show highly developed placenta-like structures that enable extensive maternal-fetal interchange (Panhuis et al. 2011), and still others show intermediate degrees of placentation that have helped researchers decipher the evolutionary transitions from aplacental to placental conditions (Pollux et al. 2009).
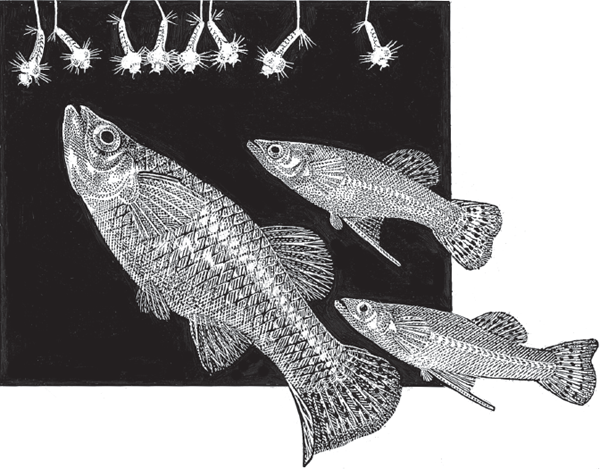
FIGURE 2.7 Mosquito fish (Gambusia affinis), a member of the live-bearing family Poeciliidae. Mosquito fish get their name from the fact that these animals eat aquatic mosquito larvae. Note that male mosquito fish (the two smaller specimens in this drawing) have a gonopodium (an anal fin with elongated rays), which acts as an intromittent organ during mating events with the larger females.
To explore the evolutionary history of maternal provisioning and placental development in poeciliid lineages, Reznick et al. (2002) used a molecular phylogeny as a backdrop. From this exercise in phylogenetic character mapping, the authors deduced that placental structures evolved on at least three separate occasions within the genus Poeciliopsis alone, plus several times elsewhere in the family (fig. 2.8). Thus, placenta-like structures and the associated phenomenon of matrotrophic feeding clearly have arisen by convergent evolution on multiple occasions in these fishes, a conclusion also reached by Pires et al. (2010). Taxa with well-developed placentas are often deeply embedded in clades that otherwise consist of species that mostly lack placentas and show little or no direct maternal provisioning of embryos.
5. Embiotocidae (surfperches). More than 20 of these small live-bearing fish species (see fig. 2.9) inhabit mostly the shallow marine waters of the northern Pacific basin. Perhaps the most interesting feature of their advanced viviparity is that the reproductive life cycles of the two sexes are about 6 months out of phase (Wiebe 1968). In males, spermatogenesis begins in early spring and eventuates in mature sperm by June or July, at which time copulations take place following elaborate courtship displays. During a mating event, each male uses a system of appendages and tubular structures in his much-modified anal fin to transfer spermozeugmatic packets, each containing about 600 spermatozoa, to a female’s reproductive tract. Afterward, his mate stores these gametes in special pockets of her ovarian epithelium. Oocyte maturation, followed by fertilization within the ovarian follicle, then takes place from October to December. Later, the nearly yolk-free eggs are released into the ovarian cavity, where the embryos gestate for about 6 months (Wiebe 1968), relying on nutrients supplied by copious secretions from their mother’s ovigerous folds (Wourms 1981). Finally, the progeny are born in an almost adult condition (Baltz 1984).
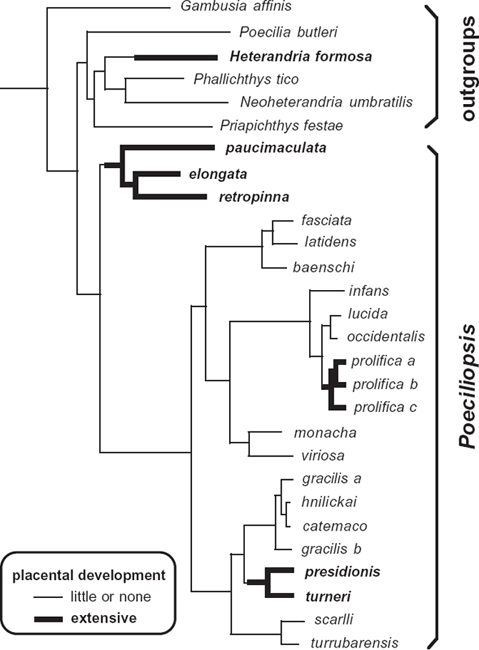
FIGURE 2.8 Molecular phylogeny for poeciliid taxa, demonstrating independent evolutionary origins of placentas in several of these piscine lineages (from Avise 2006, following Reznick et al. 2002).
FIGURE 2.9 The shiner perch, Cymatogaster aggregata, a live-bearing species representing the family Embiotocidae.
Factoid: Did you know? In another embiotocid species (Micrometrus minimus), newborn males are sexually active and inseminate newborn females, who then must grow up to become pregnant (Schultz 1993).
Multiple mating and long-term sperm storage by embiotocid females undoubtedly contribute to the genetically documented fact that broods gestated by a pregnant female often include embryos from multiple sires (Reisser et al. 2009; Liu and Avise 2011).
6. Bythitidae (viviparous brotulas). These shallow-water inhabitants of tropical and subtropical reefs illustrate the wide range of brood sizes (i.e., fecundities) that sometimes characterize even closely related viviparous fishes. For example, whereas only about a dozen embryos comprise a pregnancy in members of the bathytid genera Lucifuga and Ogilbia, several hundred embryos are borne by pregnant dams in Dinamatichthys iluocoeteoides; moreover, in Grammonus (Oligopus) longhursti (fig. 2.10), a pregnant mother gives birth to as many as 15,000 progeny. Presumably, species that fall closer to the oviparous end of the ovoviviparity spectrum (see chapter 3) can have higher fecundities than strictly viviparous species, all else being equal, because offspring from “oviparous pregnancies” tend to be born at less advanced stages of development.
FIGURE 2.10 Grammonus longhursti, a live-bearing brotula fish (family Bythitidae) with extremely large broods.
Unlike the bony fishes, among whom viviparity is relatively uncommon and taxonomically localized, about 60% of 970 extant cartilaginous species (mostly sharks and rays) give birth to live young. These female-pregnant live-bearers reside in approximately 130 genera in the superclass Chondrichthyes and in nearly 50 condrichthyan families (examples in table 2.1), representing nearly a dozen taxonomic orders (Wourms 1977, 1981). Typically, females produce small numbers of heavily yolked eggs enclosed in distinctive egg cases (fig. 2.11) that hatch either internally (in viviparous species) or externally (in oviparous species).
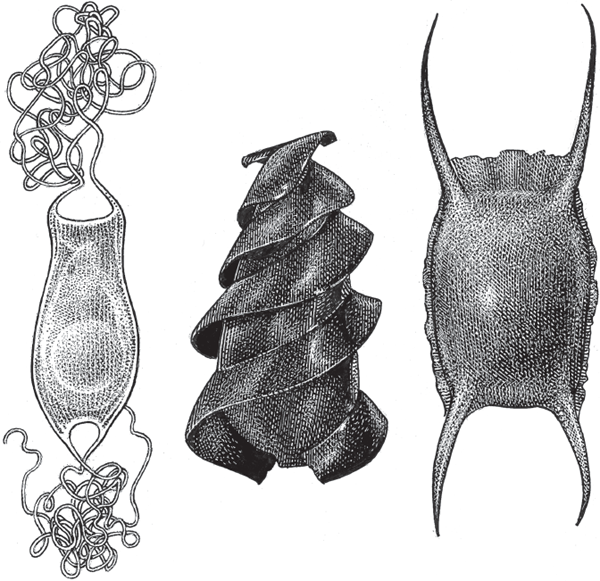
FIGURE 2.11 Examples of the beauty and varied designs of egg cases from sharks and rays. In some cartilaginous fish species, embryos hatch from such egg cases inside the mother and are born live, but in other species the embryos hatch after the egg cases have been deposited into the environment. From left to right: cat shark (Apristurus sp.), horn shark (Heterodontus francisci), and undulate ray (Raja undulata).
Placentation seems to be confined to the ground sharks (Carcharhiniformes), where it probably evolved just once or twice (Dulvy and Reynolds 1997). Thus, overall, sharks display a wide array of reproductive modes, including oviparity, aplacental viviparity, and placental viviparity (Byrne and Avise 2012).
Factoid: Did you know? The world’s largest fish species—the whale shark (Rhincodon typus)—is a live-bearer. In 1996, fishermen caught a pregnant specimen carrying more than 300 embryos. They dubbed her “megamamma” (Joung et al. 1996).
In general, there seems to be no great mystery as to why viviparity is the dominant reproductive mode in Chondrichthyes but is much less common in Osteichthyes. This disparity probably relates to the fact that viviparity normally requires internal fertilization, which is ubiquitous in extant cartilaginous fishes but rather uncommon in bony fishes (the vast majority of which have external fertilization). In general, internal fertilization must be a rather difficult evolutionary hurdle for fishes to clear due to “the extraordinary complexity of the structural modifications that make internal fertilization possible in an aquatic environment” (Rosen 1962). The achievement of successful insemination by any fish requires, for example, that males evolve an effective intromittent organ (typically a modified anal fin), which in turn necessitates a dramatic evolutionary restructuring of associated musculature and reproductive ducts (Rosen and Gordon 1953; Zauner et al. 2003), not to mention major behavioral adjustments by both sexes. For whatever reason, the ancestors of chondrichthyan fishes apparently cleared such logistical hurdles early in their evolutionary history and thereby paved a path that promoted multiple origins of viviparity among their descendants (Dulvy and Reynolds 1997; Blackburn 2005). In osteichthyan fishes, by contrast, internal fertilization (and eventual viviparity) evolved independently in the ancestors of only a modest number of taxonomic families (Gross and Shine 1981; Wourms 1981; Gross and Sargent 1985; Mank et al. 2005). Thus, before invoking any “Panglossian” adaptationist rationale (Gould and Lewontin 1979) for the prevalence of viviparity in Chondrichthyes vis-à-vis Osteichthyes, we should remember that much of the explanation might simply relate to different phylogenetic legacies of internal versus external fertilization in these two major piscine groups.
Factoid: Did you know? With a total gestational period of about 3.5 years, the frilled shark (see fig. 3.9) is highlighted in the Guinness World Records as displaying the longest pregnancy of any animal species.
Factoid: Did you know? In a pregnant manta ray (Manta alfredi), an embryo is not connected to its mother by a placenta, but nonetheless it respires and obtains oxygen by actively pumping uterine fluid into its mouth (Tomita et al. 2012).
Of course, internal fertilization is only one part of what is required to accomplish an evolutionary transition from oviparity to refined viviparity. Also normally entailed must be the following: the retention of embryos in the female reproductive tract to an advanced developmental stage, a reduction in the egg casing, the evolutionary emergence of endocrine adaptations and many other physiological mechanisms necessary to support internal gestation, and so on (Amoroso 1968; Wourms et al. 1988; Guillette 1989).
In any event, another key difference between viviparous chondrichthyans and most live-bearing osteichthyans is the site of pregnancy. In all live-bearing cartilaginous fishes, embryos gestate in an enlarged posterior region of the oviduct known as the uterus, whereas in most viviparous bony fishes (except for the coelacanth), embryos typically gestate either in follicles (pits) or in the central cavity of the ovary (or both). Thus, pregnancies are intrauterine in Chondrichthyes (plus the coelacanth) but are intraovarian in most Osteichthyes. Among all vertebrates, intraovarian gestation by some bony fishes appears to be a unique evolutionary phenomenon.
With regard to both the magnitude and the mechanism of embryonic nutrition, live-bearing chondrichthyans show a great diversity of sustenance modes. These range from strict lecithotrophy, as in the Atlantic spiny dogfish, to lecithotrophy plus uterine feeding, as in the electric ray (fig. 2.12), to placental viviparity, as in hammerhead sharks (fig. 2.13). Embryos in some shark species even engage in embryonic cannibalism or adelphophagy (box 2.1).
Another interesting mode of embryonic feeding is displayed by many species of rays (fig. 2.14), as in the family Myliobatidae. In these species, villous extensions (trophonemata) protruding from the mother’s uterine wall secrete “uterine milk,” which her embryos absorb as food.
Factoid: Did you know? In the tiger shark, a pregnant female produces a new embryo about every 2 weeks, so offspring within the womb are at different stages of development, and elder siblings who already have sharp teeth sometimes bite and even kill their younger siblings.
FIGURE 2.12 The electric ray, Torpedo ocellata (Torpedinidae), a cartilaginous fish in which gestating embryos initially are yolk-fed but later receive uterine milk from the mother.
FIGURE 2.13 The bonnethead, Sphyrna tiburo (Sphyrnidae), a matrotrophic shark species in which embryos initially are yolk-fed but later receive maternal nutrients via a placenta.
FIGURE 2.14 The bat ray, Myliobatus californica, a live-bearing species of cartilaginous fish in which gestating embryos receive much of their nutrition by absorbing uterine milk from their mother.
In addition to the many extant fish species that give birth to live young, a few serendipitously preserved fossils of pregnant individuals have confirmed that viviparity is an ancient phenomenon in backboned animals (Beltan 1977; Lund 1980), first appearing in fishes of the Devonian Period (Long et al. 2008). Of special note was the recent discovery in northwestern Australia of a 375-million-year-old expectant mother, dubbed Materpiscis attenboroughi (Long 2011). She belongs to a long-extinct group of fishes (the Placoderms) whose evolutionary roots trace at least as far back as those of some of the earliest chondrichthyan fishes. This spectacular piscine specimen and a few others like her provide direct and unequivocal evidence that copulation, internal fertilization, and pregnancy had already arisen in some vertebrate lineages a long time ago.
Among the world’s approximately 6,000 extant species of frogs and toads (order Anura), plus 600 species of salamanders and newts (Urodela), only a small handful (less than 1%) can be considered strictly viviparous in the sense of giving birth to live offspring that were conceived and then “oviductally gestated” within their mothers (Taylor and Guttman 1977; Greven 1998; Wells 2007). Indeed, only one extant species of salamander (fig. 2.15) and just a few frog and toad species exhibit this refined gestational mode (Wake 1993). All of these viviparous species have internal fertilization, but the mechanics differ (Kühnel et al. 2010): in the salamander, a female becomes pregnant after she picks up a spermatophore from the substrate, whereas an apposition of male and female cloacas (mating) precedes pregnancy in most viviparous frogs and toads.
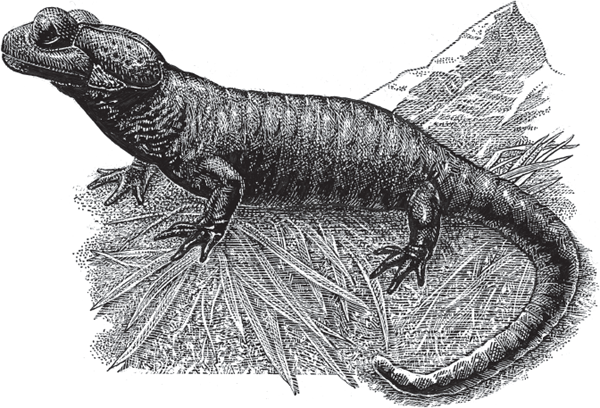
FIGURE 2.15 The alpine salamander, Salamandra atra (Salamandridae) of the Central and Eastern Alps is the only salamander species known to be an obligate live-bearer; a pregnancy may last 2–5 years.
Given the rarity of viviparity in most amphibians, it comes as quite a surprise that a majority of the approximately 190 extant species in a third amphibian order, Gymnophiona (the “caecilians”), routinely give birth to live young (Wake 1977a, 1977b, 1993). In these animals, which superficially look like worms (fig. 2.16), internal fertilization is achieved when a male everts the rear portion of his cloaca to form a phallodeum that he insets into the vent of a female. The embryos gestate internally (nourished by yolk and/or by a mother’s oviductal secretions but fed also by a pseudoplacenta in a few species). By the time of birth, the young typically have metamorphosed. Both the size and the number of ova tend to be reduced in the viviparous (as compared to oviparous) caecilians, as is also generally true for the other live-bearing amphibians. Based on a molecular phylogenetic analysis, viviparity apparently evolved independently at least four times in the Gymnophiona (Gower et al. 2008).

FIGURE 2.16 A viviparous caecilian, Gegeneophis seshachari, the only known live-bearing amphibian from Asia.
Factoid: Did you know? A variation on dermatophagy is displayed by some catfish and cichlid fish species in which newly hatched fry feed on special epidermal secretions by the parent (Herald 1961; Fryer and Iles 1972).
A remarkable category of larval feeding known as dermatophagy is displayed by some caecilians (Kupfer et al. 2006). In this peculiar culinary mode, a newly hatched progeny in an egg-laying species uses specialized dentition to peel and eat the outer layer of its mother’s modified and highly nutritious skin. In some other viviparous caecilian species, a gestating fetus actually uses its teeth to feed on the lining of its mother’s oviduct (Wake 1976). Researchers have speculated that external dermatophagous feeding by the larvae of oviparous species was an ancestral preadaptation for internal oviductal feeding by the fetuses in descendant caecilian lineages that show viviparity (Kupfer et al. 2006; Wilkinson et al. 2008).
Most reptile species, including all turtles and crocodilians, lay eggs. However, in about 20% of extant reptilian species (in the order Squamata, which includes snakes and lizards), females give birth to live young. One example of an advanced viviparous species is the common garter snake, Thamnophis sirtalis (fig. 2.17), in which gestating embryos receive substantial nutritional support and waste-disposal services via placenta-like connections to their mother. Other terrestrial snakes that give birth to live young include various pipesnakes in the families Cylindrophiidae and Uropeltidae and some dwarf boas in the family Tropidophiidae.
FIGURE 2.17 A live-bearing terrestrial snake, the common garter snake, Thamnophis sirtalis.
Factoid: Did you know? Although most viviparous lizards are primarily lecithotrophic, one small lizard species from central Africa was recently discovered to nourish its embryos via a rather human-like placenta (Blackburn and Flemming 2012).
Factoid: Did you know? In some viviparous lizards with temperature-dependent sex determination, pregnant females routinely bask on rocks, and the thermal outcomes influence the gender of the offspring to which they later give birth (Pen et al. 2010).
Also included among the viviparous squamates are sea snakes (fig. 2.18), the world’s most successful (species-rich) group of marine reptiles. All 60 extant species of true sea snakes (in the Hydrophis lineage) spend their entire lives in the ocean, have internal fertilization, and give birth to their young while at sea (Lukoschek and Avise 2011a, 2011b). Brood size averages 6–20 offspring per pregnancy. Depending on the species, viviparous reptiles nourish their embryos by several means, including yolk material either alone or in conjunction with chorionic or allantoic membranes that sometimes function like placentas (Wake 1993).
Altogether, more than 50 reptilian groups contain at least some viviparous (including ovoviviparous) species. Among squamate lineages alone, dozens of independent evolutionary changes from oviparity to viviparity have taken place (Tinkle and Gibbons 1977; Blackburn 1999a; Lee and Shine 1998). To pick just one example, although most of the world’s 150-plus species of chameleons (Chamelionidae) lay eggs, the derived live-bearing state clearly has evolved from an ancestral oviparous condition in a few chameleon sublines (fig. 2.19).
Factoid: Did you know? Viviparity is also known in several groups of aquatic reptiles of the Mesozoic Era. For example, scientists recently discovered the fossilized remains of a pregnant plesiosaur who carried her fetus nearly 80 million years ago (O’Keefe and Chiappe 2011).
FIGURE 2.18 An example of a live-bearing marine reptile, the elegant sea snake, Hydrophis elegans.
FIGURE 2.19 The Madagascar chameleon, Chamaeleo oustaleti. Although this and most other chameleons on the island of Madagascar are oviparous, a few South African members of this reptilian clade (such as the dwarf chameleon, Bradypodion pumium) have evolved viviparity.
With just a few exceptions (the egg-laying monotremes described in chapter 3), all extant mammals are viviparous. Typically, these modern live-bearing species are categorized as members of either of two related “sister taxa”—the placentals (infraclass Eutheria; see fig. 2.20) and the marsupials (infraclass Metatheria). However, this taxonomic distinction can be somewhat misleading because all living Metatheria and Eutheria have a placenta that intimately connects mother and child during each intrauterine (i.e., intrawomb) pregnancy. Both groups also suckle their postpartum young. Another source of potential confusion is that although nearly all extant mammals are viviparous, many extinct mammalian species were not, and, indeed, “Most mammalian lineages in the history of the world were probably egg-layers” (Tudge 2000, 437), from which live-bearing might well have evolved more than once independently.
If both marsupials and “placental” mammals utilize a placenta, what, then (if anything), reproductively distinguishes the eutherians and the metatherians? Actually, it is mostly just a matter of differing emphases. In comparison to the pregnancies in most marsupials, eutherian pregnancies tend to be longer and more refined such that many (but certainly not all) eutherian species deliver their young in quite an advanced state of development. Consider, for example, a foal, which is able to feed from the mare and frolic soon after parturition.
FIGURE 2.20 A representative menagerie of viviparous Eutherian mammals.
FIGURE 2.21 The western gray kangaroo, Macropus fuliginosus (Macropodidae), and musky rat kangaroo, Hypsiprymnodon moschatus (Hypsiprymnodontidae), two representative metatherian mammals or marsupials.
Marsupials (fig. 2.21), by contrast, normally give birth to less mature babies, even in species whose pregnancies can be quite long due to embryonic diapause (chapter 6). Also, many marsupial mothers compensate to some extent for giving “premature” births by carrying and suckling their newborns in special belly pouches.
1. Viviparity is the bringing forth of offspring that are first gestated internally by a parent. With few exceptions, the sex of the gestating parent is female. Except for birds (class Aves), all major vertebrate groups contain at least some viviparous (live-bearing) species. Pregnancies in these taxa vary in many features, such as the precise site of internal fertilization, the location and duration of embryonic incubation, brood size, the number of parents genetically involved (as when a brood has multiple sires), and the mode and magnitude of nutrient passage from adult to offspring.
2. In backboned animals, viviparity made its first evolutionary appearance in fishes nearly 400 million years ago, but the condition is highly polyphyletic, having arisen on more than 100 separate occasions in vertebrate lineages alone. Each evolutionary transition from oviparity (egg laying) to viviparity typically entails morphological and behavioral shifts toward internal fertilization, plus suitable adjustments between mother and embryo in biological functions related to nutrition, excretion, respiration, circulation, and endocrinology. Nearly all mammals and many viviparous fishes and reptiles have either placentas or placental analogues that serve as maternal-fetal conduits that help to carry out many of these physiological functions.
3. Viviparity is rather uncommon (3% of extant species) in bony fish (Osteichthyes) but predominates (70% of extant species) in cartilaginous fishes (Chondrichthyes). This disparity probably relates to the fact that viviparity requires internal fertilization, which is ubiquitous in extant cartilaginous fishes, whereas the vast majority of bony fishes fertilize ova externally. The site of pregnancy also differs between most viviparous chondrichthyans and osteichthyans; it is uterine in all live-bearing cartilaginous fishes but uniquely intraovarian in most viviparous bony fishes. With regard to embryonic sustenance within the dam, extant live-bearing fishes collectively show a wide diversity of nutritional modes ranging from strict lecithotrophy (yolk feeding only) to placental matrotrophy (advanced feeding by the mother) to embryonic cannibalism.
4. In the class Amphibia, viviparity is extremely rare in the taxonomic orders Anura (frogs and toads) and Urodela (salamanders and newts). However, live-bearing predominates in a third amphibian order—Gymnophiona (caecilians)—where the phenomenon is polyphyletic and entails several different mechanisms by which embryos receive nourishment while gestating within their pregnant mother.
5. Although most species in the class Reptilia lay eggs, more than 50 different reptilian groups contain at least a few live-bearing species that give birth to live young, thus indicating that viviparity in reptiles is highly polyphyletic. Nevertheless, all of these viviparous taxa reside in various lineages in the order Squamata (snakes and lizards), where females in about 20% of extant species give birth to free-living offspring following pregnancies of varied durations and different modes of embryonic gestation within the dam.
6. Extant live-bearing mammals are traditionally classified as either marsupials or placentals. However, pregnancies in both of these groups entail the intrawomb gestation of an embryo that receives nutrition via a placenta while in utero and suckles milk from its mother after birth. Thus, although marsupial females normally give birth to babies that are less mature than their placental counterparts (and then may carry their offspring in an external pouch), the different expressions of viviparity in these two mammalian groups are mostly matters of degree. Furthermore, when extinct mammalian lineages are taken into consideration, perhaps a majority of mammalian species that have inhabited the planet might well have been egg layers rather than live-bearers.