Although many invertebrate organisms manifest one or another version of viviparity, it is customary to refer to these species as internally producing their offspring rather than as giving birth by standard means of pregnancy. This chapter discusses the diverse expressions of internal (and external) brooding by invertebrate animals. Furthermore, among the external brooders are invertebrate species that retain embryos at one place or another on their bodies, plus other species that encase their embryos in special off-body capsules. Figure 4.1 introduces some of the rationales for including all of these categories of endogenous and exogenous brooding in a book on pregnancy. Pregnancy-like phenomena in nonvertebrates are interesting in their own right, and they also serve as a backdrop for later discussions (in part II) on evolutionary ramifications of pregnancy.
Many of the issues surrounding reproductive modes and pregnancy-like phenomena that arose for vertebrate animals (chapters 2 and 3) often resurface for invertebrates. For example, fertilization may be internal or external, depending on the species, brooding may be absent or present and of variable duration, the sire or the dam (or both) may be the brooding parent, and parental care of offspring can range from nil to extensive. Also, like their vertebrate counterparts, invertebrate embryos require nourishment, which can come from a variety of sources. In marine broadcast spawners with external fertilization, larvae may be either planktotrophic (feeding on plankton) or lecithotrophic (reliant on more substantial yolk reserves in the egg), and the same is true for postpartum larvae in marine invertebrates that have internal fertilization and brood their zygotes or embryos either internally or externally (Kamel et al. 2010). Understandably, planktotrophic species usually have higher fecundities (more eggs) than lecithotrophic species because the larger eggs of the latter require more space and more maternal resources (Marshall and Keough 1973; Vance 1973). The “fend-for yourself” feeding lifestyle of planktotrophic larvae places this nutritional tactic at the opposite end of the spectrum from refined matrotrophy, in which embryos receive substantial direct support from their mothers. Overall, the diversity of gestational and feeding modes for brooded invertebrate larvae parallels the varied categories of embryonic gestation and nutrition during vertebrate pregnancies.
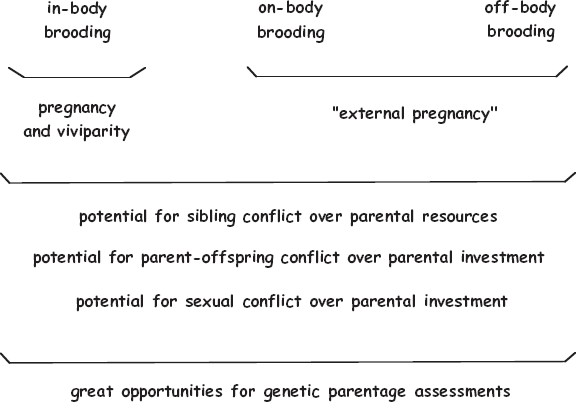
FIGURE 4.1 Three categories of embryonic brooding (in-body, on-body, and off-body) displayed by invertebrate animals. Also shown are how these alternative brooding modes might relate to several biological phenomena typically associated with pregnancy.
For invertebrate animals that brood their fertilized eggs and/or embryos (i.e., that display “pregnancy”), brooding can occur in many locations inside or outside the parent’s body: in body, on body, or off body. Furthermore, depending on the taxon, the brooding parent may be a female, a hermaphrodite (Avise 2011) or sometimes even a male (Tallamy 2001). To illustrate the taxonomic and biological breadth of brooding arrangements in invertebrate animals, the following sections introduce several phyla, each containing a potpourri of species that sometimes house embryos in specialized or otherwise interesting ways. I begin this survey with annelid worms because these small animals can serve to introduce many evolutionary topics related to different modes of larval brooding and larval feeding.
Consider first a rather obscure group of marine worms in the genus Streblospio (fig. 4.2). Within and among these tiny species, lecithotrophy and planktotrophy co-occur as life-history alternatives, with various populations showing either or both of these two life-history strategies (Schulze et al. 2000). For example, many females along the Atlantic Coast display “pouch planktotrophy,” in which hundreds of small eggs are stored initially in a dam’s dorsal brood pouch, from which plankton-feeding larvae later emerge. In the Gulf of Mexico, most females also release large numbers of planktotrophic larvae, but in this case the small eggs have been stored in their gills (“gill planktotrophy”). Finally, some females in both the Pacific and Atlantic oceans store large eggs in a dorsal pouch, from which small numbers of yolk-feeding larvae emerge (“pouch lecithotrophy”). Any such intraspecific polymorphism in larval lifestyle is known as poecilogony, a phenomenon that is of special interest to biologists because evolutionary switches between alternative feeding strategies in effect have been caught in the act in such species.

FIGURE 4.2 Benedict’s polychaete worm, Streblospio benedicti, a poecilogonous species that displays several different forms of larval development.
FIGURE 4.3 Molecular phylogeny for populations of polychaete worms in the genus Streblospio (after Schulze et al. 2000). Also shown adjacent to the tree are the occurrences of alternative modes of larval development in this taxonomic assemblage.
Using a molecular phylogeny for geographic populations of Streblospio, Schulze et al. (2000) plotted the occurrences (and thereby deduced the recent evolutionary histories) of contrasting modes of larval developmental (fig. 4.3). This exercise in phylogenetic character mapping yielded two main conclusions. First, as judged by the shallow evolutionary depths within the phylogenetic tree, conversions between larval life histories must occur quite rapidly in these polychaetes. Thus, such life-history features are evolutionarily plastic in at least some invertebrate animals. Second, because experimental studies indicated that switches between these larval modes in Streblospio are not induced by changes in environmental conditions (such as temperature, photoperiod, or food regimen), these alternative developmental trajectories seem to be genetically hardwired. Unfortunately, another key evolutionary question remained unanswered. Because lecithotrophy and planktotrophy (and also pouch brooding versus gill brooding) were closely interspersed within the Streblospio phylogeny, Schulze et al. (2000) were unable to determine from their PCM analyses whether evolutionary switches between the various modes of larval development had been more common in one direction than in another.
The genus Streblospio is not alone among polychaete worms in displaying poecilogonous larval development. Another example involves the marine species Boccardia proboscidea, which produces both planktotrophic and benthic (bottom-dwelling) larvae (Gibson 1997). Indeed, in this case both larval types sometimes emerge from a single-egg capsule of an individual female. Furthermore, some of the offspring within a brood occasionally give a boost to their own development by ingesting “nurse eggs” that their mother also produced and deposited in her egg capsules.
The flatworm Gyrodactylus elegans shows a truly bizarre form of pregnancy. In this hermaphroditic species, an egg that has been fertilized internally begins to grow and divide within the mother’s uterus. The small assemblage of mitotic cells soon divides unequally, generating a second embryo that starts to develop inside the first, but not until the first daughter embryo is released from the parent. A third embryo then begins to develop within the second, a fourth within the third, and so on, eventually yielding as many as 2,500 genetically identical progeny in four weeks (Baer and Euzet 1961, as cited in Craig et al. 1997). This Russian-doll configuration of clonemates may be nearly unique to these fish-parasitic flatworms, but it illustrates the lengths to which evolution can go in generating offspring via pregnancy.
In most corals and other cnidarians, both sperm and ova are broadcast into the ocean, such that fertilization is external, and the resulting larvae develop in the plankton without benefit of direct parental care (Veron 2000). However, in a substantial minority of species, ova are retained either by the female or by a hermaphrodite, such that fertilization is internal, and the planula larvae begin life within the body of a parent. In a survey of reproductive modes for nearly 200 coral species from several of the world’s major reef areas (the Caribbean, tropical Pacific, and Red Sea), Richmond and Hunter (1990) tallied the number of species that qualify as broadcast spawners without brooding (168 species), hermaphroditic brooders (11 species), and gonochoristic (separate-sex) brooders (7 species). Even closely related coral species sometimes show different modes of larval development. For example, in the genus Acropora most species, including cervicornis (fig. 4.4), are broadcast spawners, but several others, such as palifera and striata, produce their progeny internally (Ayre and Miller 2006).
Most coral species are hermaphroditic (Harrison and Wallace 1991), and in some species the dual-sex colonies occasionally self-fertilize, an incestuous behavior that nonetheless may be advantageous when mates are rare or sperm are in limited supply. Hermaphroditic species that retain (rather than broadcast) their ova may be predisposed to brood their young, so perhaps it is not too surprising that brooding and selfing co-occur in several coral species (Richmond and Hunter 1990; Sherman 2008). Another route to “pregnancy” in corals occurs in several taxa in which the brooded larvae are produced asexually (Stoddart 1983; Ward 1992).
A relatively small number of sea anemones, such as Epiactis prolifera (fig. 4.5) also brood their larvae. In this species, each individual begins life as a female but later becomes a hermaphrodite (a functionally dual-sex specimen). Eventually, the hermaphrodite will brood selfed or outcrossed progeny externally on the body’s central column (Bucklin et al. 1984).
Factoid: Did you know? Like many other invertebrates (and plants), most corals can propagate not only sexually but also asexually via “cuttings.” Such clonal reproduction often occurs when a branch of coral breaks off from the parent colony and falls to the substrate, where it initiates a new colony.
FIGURE 4.4 The Caribbean staghorn coral, Acropora cervicornis. Although this and most other staghorn corals are broadcast spawners, a few species in the genus brood their larvae.
Factoid: Did you know? In Epiactis prolifera, eggs are fertilized in the adult’s digestive cavity, after which the motile larvae swim out of the parent’s mouth and install themselves on the adult’s outer body, where they incubate and grow into autonomous little anemones.
FIGURE 4.5 The brooding sea anemone, Epiactis prolifera, a cnidarian species with a highly peculiar mode of fertilization and external brooding (see the factoid).
Bryozoans (phyla Entoprocta and Endoprocta) are tiny solitary or colonial marine creatures that superficially resemble corals and consist of specialized functional units known as zooids. They are of special interest in the context of pregnancy because many species possess placenta-like analogues via which larvae receive maternal nutrients during their development either within or outside the parent’s body cavity (Dyrynda and Ryland 1982). Depending on the particular Bryozoan group, placental contact between mother and larva can sometimes be intimate, even to the point of fusion and substantial nutrient transfer between fetal and parental tissues. Comparative analyses and phylogenetic character mapping have demonstrated that various categories of placentation have arisen multiple times in Bryozoa and thus are polyphyletic (Ostrovsky et al. 2009).
Factoid: Did you know? Bryozoans are often referred to as moss animals, sea mosses, or sea mats because their branched or matted colonies can resemble seaweeds when they spread plantlike across rocks or other marine substrates.
Although brooding is relatively unusual in this group of marine animals, all major echinoderm taxa include at least some species with larval brooding (Hart et al. 1997). For example, in some sea stars (class Asteroidea) and brittle stars (Ophiuroidea), a female extrudes yolk-rich eggs and then forms a brooding chamber by bending her arms ventrally around her central disk. In Leptasterias hexactis (fig. 4.6), brooding therein proceeds for about 40 days postfertilization before the juveniles develop tube feet and walk away.
Factoid: Did you know? In some hermaphroditic sea stars that brood their young internally, juveniles routinely eat some of their siblings while still housed jointly in their pregnant parent’s gonadal tissue (Byrne 1996).
FIGURE 4.6 The sea star, Leptasterias hexactis, an echinoderm species that broods its eggs and larvae.
Factoid: Did you know? In the brooding sea cucumber, Leptosynapta clarki, juveniles remain in their pregnant mother’s ovary for about 6 months before exiting by rupturing her body wall (Sewell 1994).
Other echinoderms show different forms of brooding. Although most sea cucumbers (Holothuroidea) are broadcast spawners with planktonic larvae, more than 40 holothurian species brood their young in various locations such as under their sole, in tentacles, in brood pouches, or, internally, in either the ovary or the body cavity (Smiley et al. 1991). In the sea cucumber, Synaptula hydriformis, fertilized eggs hatch, and larvae develop inside a perivisceral coelom within the parent’s body before being live-born. This species is of interest for two additional reasons: it is a self-fertilizing hermaphrodite, and it is matrotrophic, meaning that the gestating young receive at least some parental nutrition in excess of that supplied by the yolk (Frick 1998). Brooding of one form or another also occurs in various other echinoderms, including some sea urchins and sand dollars (Echinoidea), where the phenomenon has apparently evolved independently on at least a dozen occasions (Emlet 1990).
All major groups of mollusks, including snails (class Gastropoda), bivalves (Bivalvia), chitons (Polyplacophora), and squids and their allies (Cephalopoda), encompass at least some species that brood their larvae. Fertilization in such species is typically internal, either following mating or by some direct means of sperm uptake from the environment.
In gastropods, brooding either in the mantle cavity or within an enlarged oviduct is quite common, as well as polyphyletic (Köhler et al. 2004). Several examples from the marine realm involve Littorina periwinkles in the family Littorinidae (Johannesson 1988; Reid 1990), some Planaxis snails (Planaxidae), and some slipper limpets (Calyptraeidae), including Crepidula fornicata (fig. 4.7). Individuals in the last species are sequential hermaphrodites (Avise 2011) that live in stacks in which the bottom individual is a female and higher individuals are younger males with whom she mates. The mother then broods offspring underneath her caplike shell, packaged in dozens of capsules, each containing dozens to hundreds of embryos (Proestou et al. 2008).
Factoid: Did you know? As a part of the mating process, many snails spear each other with calcareous “love darts” containing a mucus that decreases sperm digestion in the recipient and thereby increases the chance of fertilization by the successful dart thrower.
FIGURE 4.7 Stack of slipper limpets, Crepidula fornicata, a mollusk that broods larvae under its shell.
Factoid: Did you know? In slipper limpets an individual typically begins life as a male but later becomes a female, in a reproductive system known as protandry. In other animal species showing sequential hermaphroditism, an individual may begin life as a female but later becomes a male, in a reproductive system known as protogyny.
Somewhat like the Streblospio worms and the Acropora corals described earlier, periwinkles and slipper limpets provide examples of poecilogony because even closely related populations or species in each of these genera sometimes show different strategies of larval development, including planktotrophy and brooding (Hoagland 1986; Collin 2004). Thus, these species again demonstrate that larval ontogeny can be labile during marine invertebrate evolution. The genus Crepidula is also of special interest because well-preserved fossils, complete with brooded larvae, have been discovered in some of its species (Herbert and Portell 2004).
An elegant form of off-body larval brooding occurs in many whelk species (Buccinidae and Melongenidae) such as Busycon carica (fig. 4.8), in which a previously mated female lays a beautiful string of about 80 leathery capsules, each of which houses several dozen embryos (Walker et al. 2005, 2007; Avise et al. 2004). Many other marine gastropods, including wentletraps (Epitoniidae), turritellas (Turritellidae), trivias (Eratoidea), moon snails (Naticidae), bonnets (Cassidae), dove shells (Columbellidae), tulip conchs (Fasciolariidae), and cowries (Cypraeidae), similarly lay benthic egg capsules of diverse and often lovely structural designs.
FIGURE 4.8 A female knobbed whelk (Busycon carica) and her string of egg cases.
Factoid: Did you know? In some marine snails such as the Scotch Bonnet (Phalium granulatum), a female extrudes a cylindrical tower of egg cases, upon which she sits like a queen on her throne.
The terrestrial snail Cantareus (formerly Helix) aspersus (fig. 4.9) illustrates another form of off-body brooding (Herzberg and Herzberg 1962). In this hermaphroditic species, copulation is sometimes reciprocal, with each individual simultaneously inseminating its partner. Several days later, each “pregnant” parent digs a 1-inch nest in moist soil, wherein it deposits about 80 fertilized eggs. About two weeks later, the embryos hatch and crawl away.
FIGURE 4.9 The brown garden snail, Cantareus aspersus, a nest-brooding species.
Internal brooding in bivalve mollusks is taxonomically widespread and can take many forms (Hain and Arnaud 1992). In such species, internal fertilization typically occurs when a gravid female or a gravid hermaphrodite “inhales” sperm through a siphon that it also uses to filter water for food and oxygen. Depending on the species, the resulting zygotes and embryos may be released quickly or perhaps retained either in the body cavity or gill chambers for varying lengths of time. In the marine species Adacnarca nitens, each female broods 50– 70 offspring internally, in clusters of embryos housed within her branchial chamber (Higgs et al. 2009). In freshwater mussels in the family Unionidae (fig. 4.10), an adult may brood thousands to millions of larvae in its gills, where the “glochidial embryos” are initially enclosed in a vitelline membrane that provides intimate physical contact with the parent and is suspected to play a nutritive role early in embryonic development (Schwartz and Dimock 2001). After this gestational phase, the discharged larvae typically attach to a host fish, where they continue developing and eventually metamorphose into juvenile mussels (Rogers-Lowery and Dimock 2006).
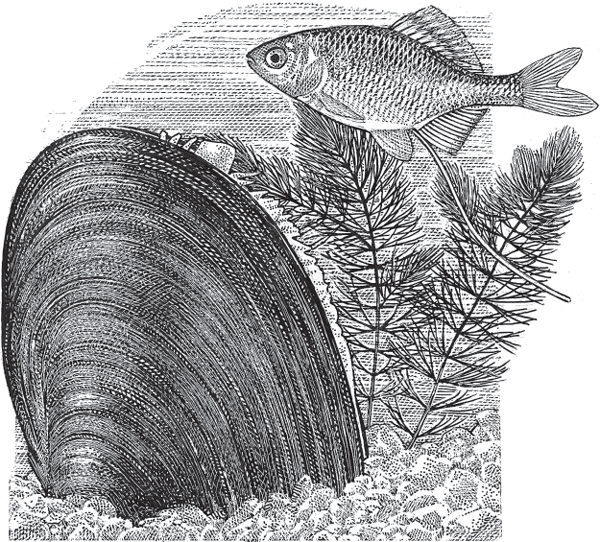
FIGURE 4.10 Freshwater unionid mussel and bitterling fish. These mussel species brood their larvae internally until the latter transfer to a secondary host (a fish) to continue their development.
Factoid: Did you know? Some unionid mussels have evolved special wormlike protuberances that they actively wave to attract a host fish, who is then colonized by the bivalve’s shed larvae.
In general, larval brooding in bivalves and other benthic (bottom-dwelling) invertebrates is presumed to be a useful reproductive tactic especially in harsh environments, where embryonic survival might otherwise be extremely low (Poulin et al. 2002; Galley et al. 2005; Heilmayer et al. 2008). One potential disadvantage of brooding (in contrast to planktotrophy) is that the relatively advanced larvae born of a “pregnant parent” may have rather limited opportunities for dispersal (Carlon 2002). However, in some cases even brooding species can be effective colonizers (Johannesson 1988), especially when they take advantage of some other dispersal mechanism such as floating (High-smith 1985), “kelp rafting” (Helmuth et al. 1994), or hitching rides on secondary hosts (as in the freshwater mussels).
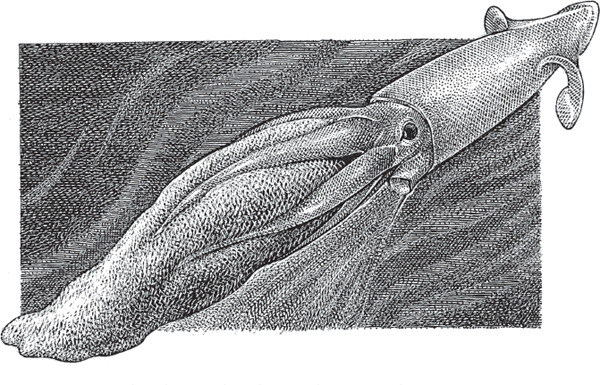
FIGURE 4.11 Egg-brooding female of the giant deep-sea squid, Gonatus onyx.
A few mollusks in the class Cephalopoda also brood their young. In squids and octopuses, internal fertilization follows mating in which the male transfers a spermatophore to his mate’s mantle cavity. Although biologists used to think that each female then deposits her fertilized eggs on the sea floor and leaves them to develop on their own, recent observations reveal that individuals in some squid species hold such eggs in their arms (attached to small hooks) until the embryos hatch (Seibel et al. 2005). In the giant deep-sea squid, Gonatus onyx (fig. 4.11), females have been photographed while arm-carrying thousands of egg-encased embryos in huge clusters. A female octopus also guards and tends her fertilized eggs, which she typically lays by the thousands in strings that she hangs from the ceiling of her lair.
Many species in this largest invertebrate phylum brood their young in one fashion or another. For example, females in many crustacean species (subphylum Crustacea) carry embryos in special pouches inside or outside their bodies. In the suitably named “opossum shrimps” (Mysida) and in many species of isopods (Isopoda) and amphipods (Amphipoda), that pouch is called a marsupium, and it is located on the female’s thorax. Many other crustaceans, including fairy shrimps (Anostraca), water fleas (Cladocera), and copepods (fig. 4.12), also brood their eggs in special dorsal, ventral, or lateral sacs before releasing them to the outside world (Ohman and Townsend 1998; Logerwell and Ohman 1999). Even many sessile barnacles (Cirripedia) manage to mate and brood internally fertilized eggs (Crisp 1959; O’Riordan et al. 1992). In these typically hermaphroditic species, an individual copulates with close neighbors using an extendable penis, after which the eggs are brooded and later released as nauplii larvae, which may spend weeks in the plankton.

FIGURE 4.12 Copepods (Copepoda) are among the many arthropod groups in which various species brood their young.
In many species of crabs, shrimps, crayfish, and lobsters (Decapoda), a gravid female typically cups her abdomen under her cephalothorax and thereby forms a spawning chamber into which she lays and then fertilizes her eggs, typically using dissolved spermatophore packages that she had received from earlier matings with one or more males (Walker et al. 2002; Toonen 2004; Gosselin et al. 2005; Yue et al. 2010). She then carries the eggs and juveniles (fig. 4.13) on her pleopods (swimming legs) for as long as several months, all the while fanning and protecting the hatchlings until they are mature enough to depart and fend for themselves.
FIGURE 4.13 Mother and brood in a freshwater crayfish, Orconectes placidus.
Factoid: Did you know? A lobster mother may carry as many as 30,000 embryos.
In several species of land crabs from Jamaica, females show perhaps even more parental attention by actively tending their broods in off-body sites. In the bromeliad crab, Metopaulias depressus (fig. 4.14), each mother raises her young in the base of a water-filled bromeliad leaf. There, she circulates the water, removes detritus, feeds her young, and protects them from predators. She even hauls in empty snail shells that provide calcium to her offspring and serve as a pH buffer. In another Jamaican endemic (Sesarma jarvisi), the adult crab actively delivers water to an empty snail shell or turns the adopted shell upside down to collect rainwater. For several months, the snail shell then becomes a relatively safe nursery for the crab’s embryos.
Factoid: Did you know? Although Jamaica’s bromeliad crab is fully non-marine, many other so-called land crabs around the world liberate their hatched larvae into the sea, where they continue their development.
FIGURE 4.14 The bromeliad crab, Metopaulias depressus, a terrestrial crustacean in which mothers tend their brooded young in special off-body incubators.
Insects (Insecta) are the most species rich of extant arthropods, and although most insects lay eggs (i.e., are oviparous), females in some taxa retain fertilized eggs within the uterus and later give birth to live young (Meier et al. 1999; Agarwala and Bhadra 2010). Thus, such species are ovoviviparous. A few insects even show facultative ovoviviparity, wherein a female either lays fertilized eggs or delivers already-hatched young, depending on the environmental circumstances (Markow et al. 2008).
Factoid: Did you know? Researchers have managed to promote the evolution of ovoviviparity in experimental fruit-fly populations by selecting for females with increased live-bearing behavior.
In a select few invertebrates, males (rather than females) tend the broods (Clutton-Brock 1991; Tallamy 2001). Among the arthropods, the two most famous examples are giant water bugs in the insect family Belostomatidae (Smith 1976, 1997) and the 1,200-plus sea spider species in the subphylum Pycnogonida (King 1973; Shuster and Wade 2003; Bain and Govedich 2004). In the belostomatids, a male water bug typically carries 30–160 fertilized eggs that his mate has cemented to his back (fig. 4.15).
In the pycnogonid sea spiders (fig. 4.16), each male typically totes dozens to hundreds of fertilized eggs in masses glued either to his ventral surface or to a pair of specialized legs known as ovigers. In the sea spiders and giant water bugs alike, males may retain the developing eggs and embryos for several weeks or even months (Smith 1974; Tomaschko et al. 1997).
FIGURE 4.15 A male giant water bug carrying fertilized eggs cemented to his back.
FIGURE 4.16 A male sea spider (Ammothea hilgendorfi) carrying masses of fertilized eggs on his legs.
One prediction of selection theory is that a male should offer substantial care of this sort only if the members of his brood carry copies of his own genes (Trivers 1972; Thornhill 1976). For water bugs, observations suggest that each “pregnant” male does indeed have high assurance of paternity for his brooded young (Smith 1979a, 1979b), in part because a male copulates repeatedly with a female before receiving her eggs; in addition, for sea spiders, genetic-parentage analyses have confirmed that each brood-carrying male is indeed the biological sire of the embryos that he tends (Barreto and Avise 2008, 2010, 2011).
Factoid: Did you know? Although paternal care of offspring is relatively rare in invertebrates, such behavior has evolved independently on at least 17 occasions in these animals (Tallamy 2001).
Many of the 1,000-plus species in this subphylum of Chordata consist of hermaphroditic individuals that are male and female simultaneously. Sperm are shed into the sea, whereas eggs typically retained by an adult are fertilized internally by male gametes sucked into a hermaphrodite’s body through special incurrent siphons. The resulting larvae then brood internally before being released live by the viviparous parent.
Factoid: Did you know? Urochordates are also known as tunicates, or sea squirts, the latter name deriving from the fact that these small, sessile, baglike animals often squirt seawater when stepped on or otherwise disturbed.
A botanical analogue of pregnancy also exists (Farnsworth 2000). Vivipary is the precocious development of sexual progeny directly on the parent plant (unlike the germination of a seed elsewhere, such as in the soil). True vivipary entails the germination of sexual seeds that for a time remain attached to and develop directly on the dam. In other words, viviparous seeds typically germinate and grow within the fruit prior to abscission from the maternal parent. This phenomenon is rare in plants, having been described in only about 50 species (Elmqvist and Cox 1996). Examples are mangrove trees in the genus Rhizophora (Farnsworth and Farrant 1998); alpine bluegrass, Poa alpina (fig. 4.17); spreading schiedea, Schiedea diffusa (Wagner et al. 2005), which is endemic to Hawaii; and several coastal cacti (fig. 4.18) native to northwestern Mexico (Cota-Sánchez et al. 2007). Another type of “live-bearing” in plants, sometimes called pseudovivipary, transpires when asexual (rather than sexual) propagules grow on the parent (Beetle 1980; Elmqvist and Cox 1996).
One could also argue that all seed plants are in effect viviparous in the sense that during seed development an embryo is brooded and nurtured within an ovule, initially while attached to the maternal plant. The seed coat (testa) of the mature seed is in fact maternal tissue that encases the gestating conceptus. From this biological perspective, it is perhaps unfortunate that vivipary has acquired a much more restricted meaning in botany.
As was true for vertebrate pregnancies (chapter 2), standard prerequisites for larval brooding by invertebrates are the retention of ova by females and internal fertilization. This means that during any evolutionary transition from nonbrooding ancestors to internal-brooding descendents, one of the parents (typically the dam) assumes added responsibility for offspring care. How this comes about in particular instances undoubtedly varies across taxa. Here are merely a few of the evolutionary considerations and some empirical examples.
FIGURE 4.17 The alpine bluegrass, Poa alpina, a “pregnant” plant species in which sexually produced seeds germinate while still attached to the mother.
Conventional wisdom for marine invertebrates held that planktotrophy was the ancestral lifestyle from which lecithotrophy recurrently evolved in various taxonomic groups. For example, lecithotrophy probably evolved from planktotrophy at least four independent times in asterinid starfish (Hart et al. 1997). This evolutionary directionality can be rationalized by supposing that whenever complex feeding structures are lost by larvae in a planktotrophic lineage, such adaptations may be difficult to regain. On the other hand, several probable instances have been identified (e.g., in littorinid and calyptraeid snails) in which larval feeding (planktotrophy) appears to have reevolved in nonfeeding (lecithotrophic) lineages (Reid 1990; Collin 2004). Overall, studies on species representing several invertebrate phyla, including Mollusca and Echinodermata, have revealed (as in the Streblospio polychaetes and Acropora corals described earlier) that modes of larval development in marine invertebrates can switch back and forth quite rapidly in geological time and thus are not always tightly constrained in evolution. Compared to planktotrophy, lecithotrophy often registers an increased investment by the mother in her progeny, albeit indirectly via a richer yolk that she has provisioned to each egg. However, brooding (and, in the extreme, matrotrophy) can take parental investment to even greater heights.

FIGURE 4.18 The Mexican columnar cactus, Pachycereus pringlei. Some species in this genus have viviparous fruits containing sexual progeny that continue to grow directly on the parent plant.
Freshwater jawless leeches (family Glossiphoniidae, phylum Annelida) are remarkable not only for their bloodsucking behavior but also for their refined parental care of offspring. All glossiphoniids use their flattened bodies to cover their developing egg capsules, but some species, such as Marsupiobdella africana (fig. 4.19), go at least one step further by carrying their eggs and young in a special internal brood pouch.
Sawyer (1971) used evolutionary logic in conjunction with a suspected phylogeny for these leeches to deduce the probable history of this transition to internal pregnancy. In Sawyer’s reconstruction (fig. 4.20), the process began in an ancestor that laid unattended eggs from which larvae hatched and simply crawled away. Then, in stage II of the evolutionary progression, the parent presumably tended the substrate-attached eggs by covering them with its body. In stage III, the egg capsules became cemented to the parent’s ventral surface. Finally, in evolutionary step IV, the parent’s body became reshaped to curve around the egg capsules and thereby form an internal pouch, within which larvae later hatch and are “live-born.” In the Annelid subclass Hirudinea as a whole, extant species are known that represent all of these presumed stages in the evolutionary progression from oviparity to viviparity.
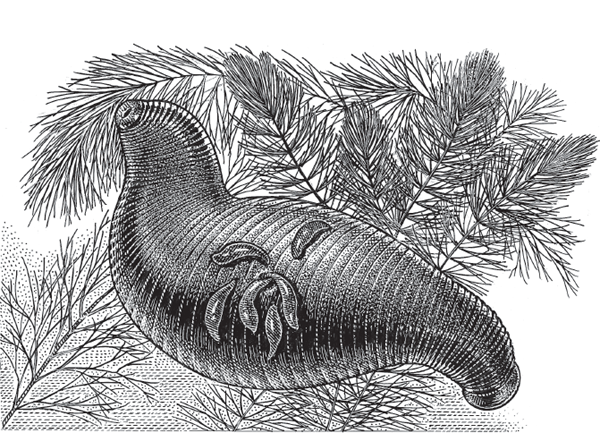
FIGURE 4.19 A South African leech (Marsupiobdella africana) giving birth to larvae. This species broods its young internally.
FIGURE 4.20 Four presumptive stages (see the text) in the evolutionary transition from egg laying to live-bearing in the leech family Glossiphoniidae (after Sawyer 1971).
Similar kinds of evolutionary scenarios can be envisioned for various other viviparous marine invertebrates. In gastropod mollusks, for example, in many oviparous species a female simply lays unattended egg capsules, but each female cowry (Cypraeidae) sits on her clump of eggs for many days until they hatch, and females in several viviparous groups retain their egg capsules internally until they hatch and the young are live-born.
Evolutionary reconstructions can be similarly tailored for some viviparous invertebrates that inhabit the terrestrial realm. For example, most Drosophila fruit flies are oviparous, but developmental profiles vary greatly within and among species, with females in a few species, including sechellia and yakuba, sometimes retaining fertilized eggs within their uteri before giving birth to live young (Markow et al. 2008). Such ovoviviparity might be adaptively advantageous under particular environmental circumstances, such as when oviposition sites are scarce or when fertilized eggs might need the added protection that internal brooding can provide. More generally, as is also true for various vertebrate taxa (chapter 3), ovoviviparity in invertebrates might be a rather common intermediate step during evolutionary interconversions between oviparity and viviparity (Sellier 1955; Markow et al. 2008). Indeed, examples of viviparity in insects have long been known (Hagan 1948), and Meier et al. (1999) suggest that viviparity (including ovoviviparity) has evolved from oviparity more than 60 times within the dipteran flies alone. Minimally, all that need be entailed in such transitions is the retention of fertilized eggs that hatch within the female prior to (rather than after) oviposition.
In a small minority of invertebrate and vertebrate animal species, males take the prime responsibility for offspring brooding. How such paternal investment might have evolved has been debated extensively (Goodwin et al. 1998; Dulvy and Reynolds 1997; Andersson 2005; Reynolds et al. 2002), with evolutionary scenarios often differing depending on the taxa (such as birds versus fishes) being considered (Reynolds et al. 2002). (Some of the evolutionary scenarios for fishes are presented in chapter 7.)
Larval brooding by invertebrate males is also an uncommon phenomenon; it is displayed by only a few groups, including water bugs (Belostomatidae) and sea spiders (Pycnogonidae). For the water bugs, a combination of field observations and phylogenetic (PCM) analyses led Smith (1997) to create the following scenario for the evolution of the male’s peculiar habit of carrying fertilized eggs on his back. In many nonbrooding belostomatid species, females typically lay their zygotes on emergent aquatic vegetation, where the embryos develop and respire using atmospheric oxygen that diffuses to them. According to Smith (1997), some gravid females may have accidentally laid their eggs on mate-guarding males instead. Furthermore, such males may not have been unduly inconvenienced by this burden because any egg-toting costs (such as impaired swimming or visibility to predators) might have been outweighed by an increase in the male’s genetic fitness through increased progeny survival (Kraus et al. 1989). After males have been “captured” in such a paternal-care tactic, females would probably come under increased selection pressure to put energy into producing more eggs and eventually surrendering all postzygotic care to their mates (Tallamy 1995). The water bugs’ habit of alternating copulation with oviposition probably gives each male a high assurance of paternity for the brood he tends, so this particular mating behavior, too, likely arose during this evolutionary transition to exclusive paternal care of offspring.
For invertebrate animals, brooding almost invariably entails packaging many full-sib or half-sib embryos in cramped quarters within, on, or adjacent to the body of one parent (usually the dam). Such communal housing in conjunction with the particular genetic relationships among broodmates sets the evolutionary stage for various conflicts of interest over finite resources (Parker et al. 2002). The following sections introduce these topics, which we then explore further in part II.
Sibling rivalries (Mock and Parker 1997) are likely to be exacerbated in vertebrate pregnancies and invertebrate broods alike because the offspring are in close contact and have ample opportunities to interact. The intensity of such conflicts can vary according to many factors, such as the size of the brood relative to the availability of resources (e.g., space, oxygen, nutrients), and to the broodmates’ genetic relationships, which themselves are a function of the reproductive mode (e.g., sexual vs. asexual) and the mating system (e.g., monogamy vs. polygamy) of the parent(s). The resolution of such intra-brood conflicts may be reflected in outcomes as subtle as differential growth rates among the broodmates or as overt as sibling cannibalism (see box 2.1).
From their own selfish genetic perspectives, a parent and its offspring typically have different “ideas” about how best to invest finite parental resources (Trivers, 1974). Typically, from a parent’s perspective the optimal strategy is to supply less than what an offspring would ideally desire. This fact yields an inherent intergenerational conflict of interests among family members, the magnitude of which depends on many additional factors, such as the mating system (which can influence genetic relationships within each family), the prospects for future reproduction by the parent, and the relative contributions of dam and sire(s) to the rearing of progeny (Parker 1985). An extensive body of evolutionary theory discusses how parent-offspring conflicts might be resolved in particular biological circumstances (Trivers 1972; Maynard Smith 1977; Parker and Macnair 1978; Macnair and Parker 1978, 1979; Charnov 1982; Mock and Forbes 1992; Godfray 1994; Lessels and Parker 1999; Parker et al. 2002). In general, vertebrate pregnancy and invertebrate brooding both tend to heighten the intergenerational tension because in such circumstances a parent and its offspring interact directly and often intimately (Zeh and Zeh 2000; Crespi and Semeniuk 2004).
From their own selfish genetic perspectives, males and females in sexual species typically have very different approaches to how best to optimize personal fitness (see chapter 7) despite the fact that they must somehow collaborate to produce successful offspring. This sexual conflict of interests routinely leads to sexually antagonistic selection that can operate at any stage (or all stages) of the reproductive process, ranging from prezygotic mate choice and courtship behaviors, to fertilization modes, to tactics of postzygotic parental care (Arnqvist and Rowe 2005). In general, the phenomena of pregnancy (in vertebrates) and larval brooding (in invertebrates) tend to heighten the intersexual tensions because huge disparities then characterize the mode and magnitude of personal investment in progeny by sires and dams. Thus, what is best for the genetic fitness of the goose is not necessarily what is best for the gander.
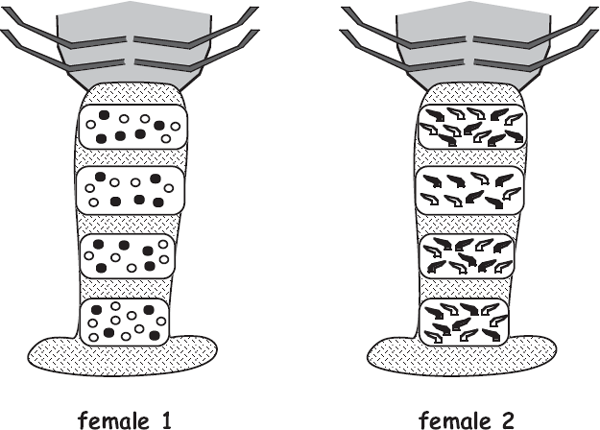
All forms of invertebrate brooding in which the female parent remains associated with the brood (and can be collected with it) offer researchers wonderful opportunities to determine who sired the embryos and thereby to deduce the genetic mating system of a species. Under the “one-parent-known” statistical model (Selvin 1980), the process works generally as follows (see the appendix). Using suitable molecular techniques in a genetics laboratory, a researcher assays the female brooder and her known biological offspring at multiple marker genes (loci). At each locus, each diploid embryo inherited one allele from its known dam so the other allele that it is observed to carry must have come from its (otherwise unknown) sire. The researcher then accumulates such genetic data for several polymorphic marker loci and for multiple embryos within the brood and thereby deduces the multilocus genotype of each brood’s sire(s). By repeating this protocol for many pregnancies or broods, a researcher can unveil the genetic mating system of a population or species. Sometimes it is possible to deduce not only the number of sires and their relative contributions to each brood but also to map the spatial positions of their embryos within a female’s egg case or her brood pouch. Examples involving the egg capsules of a marine whelk (Busycon carica) and the brood chambers of a freshwater crayfish (Orconectes placidus) are diagrammed respectively in figure 4.21 and figure 4.22.
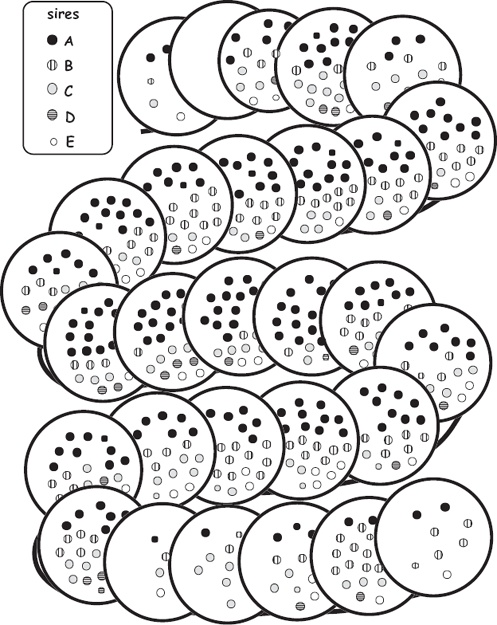
FIGURE 4.21 Genetically deduced sires for several hundred embryos along 29 successive egg capsules in an egg-case string from a female knobbed whelk (after Walker et al. 2007).
An analogous but gender-reversed process is employed to deduce maternity and the mating system for species in which the brooding parent is the sire rather than the dam. An empirical example involving the sea spider, Ammothea hilgendorfi, is presented in figure 4.23.
FIGURE 4.22 Diagrams of the abdomens of two “pregnant” crayfish showing in each case the spatial arrangements of progeny from two genetically deduced sires (open versus closed symbols). Left, unhatched eggs in the brood of one female; right, hatchlings in the brood of a second female (after Walker et al. 2002).
In principle, similar kinds of genetic analyses can be conducted on non-brooding species in which neither biological parent is known at the outset, but the detective process is far easier when one known parent can be collected together with its brooded young.
1. Internal brooding by viviparous invertebrates is the evolutionary analogue of pregnancy in vertebrate animals. Many invertebrate phyla ranging from Annelida (polychaetes and other worms) to Cnidaria (corals) to Echinodermata (sea stars and their allies) to Mollusca (snails and their allies) to Arthropoda (insects and their allies) include at least some species that give birth to live offspring after having brooded their larvae internally. Indeed, even a few plant species are in effect viviparous.
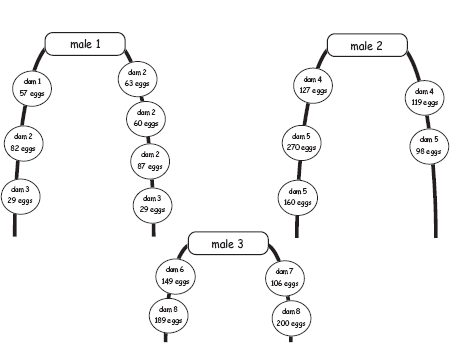
FIGURE 4.23 Diagrammatic representation of broods carried on the specialized legs (heavy lines) of three male sea spiders (after Barreto and Avise 2010). Each circle represents an egg cluster composed of the indicated numbers of eggs (embryos). Genetic analyses of the sires and each of their progeny were used to decipher the dams of these embryos. Note that all of the embryos within a cluster proved to have the same mother but that each male had received egg clusters from at least two females. Overall, this parentage study involving nearly 1,400 embryos from 13 “pregnant” males indicated that this population of Ammothea hilgendorfi has a polygynandrous mating system because members of both sexes proved to have had multiple sexual partners during each breeding episode.
2. In addition to the internal brooders, females (and, far more rarely, males) in various other invertebrate taxa carry their developing young on the outside of their body or brood their larvae in special off-body nests or capsules. Altogether, the wide diversity of brooding and feeding modes for invertebrate larvae parallels the great diversity of gestational phenomena for vertebrate embryos.
3. For several invertebrate taxa, phylogenetic character mapping (PCM) in conjunction with other lines of evidence has been used to reconstruct the probable course of evolutionary interconversions among alternative reproductive modes. Some such analyses have even been conducted across microevolutionary timescales because several invertebrate taxa display the phenomenon of peociliogony, wherein alternative modes of larval brooding and feeding coexist as polymorphisms within particular species.
4. Some of the evolutionary transitions provisionally reconstructed for various invertebrate groups are as follows: (a) from ancestral planktotrophy (plankton feeding) to lecithotrophy (yolk feeding) by larvae; (b) from off-body larval brooding to on-body larval brooding to within-body brooding by dams; and (c) from off-body larval gestation without parental care to on-body brooding by sires. The latter transition (to a tactic of high paternal investment) has been rare and apparently has been accompanied by a high assurance of genetic paternity for the brooding males.
5. For sexually reproducing species, invertebrate brooding (like vertebrate pregnancy) tends to exacerbate several inherent evolutionary conflicts of interest: between siblings within a brood, who compete for finite resources; between the two parents, who have different optimal tactics for enhancing personal genetic fitness; and between brooding parents and their progeny, who have different perspectives on the optimal magnitude and distribution of parental investment in offspring.
6. Another important feature of invertebrate brooding that is analogous to that of vertebrate pregnancy relates to genetic parentage analyses. Because full-sib and half-sib embryos are associated physically with their brooding parent (typically the dam), molecular markers can be employed to deduce the sire(s) of each brood and thereby reveal the genetic mating system of a population. For species in which the brooding sex is the male, analogous determinations of genetic maternity and mating systems can likewise be accomplished. In some cases the arrangements of different full-sibships within a brood can even be microspatially mapped inside each brooding chamber.