I. IONIC BONDING
A. IONIC BONDS ARE STRONG INTRABONDS FORMED BETWEEN METALS AND NONMETALS
1. Atoms have equal numbers of protons and electrons and consequently have no overall charge.
2. When atoms lose or gain electrons, (in order to achieve full s and p sub-shells or a “noble gas structure” and stability), the proton/electron numbers are unbalanced, causing the particles to become charged. These charged particles are called ions.
3. The strong electrostatic forces between the charged particles are called ionic bonds.
4. Because the electrostatic forces are large, the ionic bond is a strong one.
5. Metal atoms have a tendency to lose electrons to form positive ions and nonmetal atoms have a tendency gain electrons to form negative ions; therefore, the ionic bond is usually formed between metals and nonmetals.
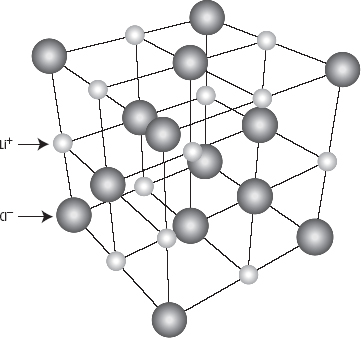
6. Ionic substances form regular, cubic arrangements called giant ionic lattices, as shown in the following diagram.

7. The lattice energy is a quantitative measure of the attraction between ions in an ionic lattice.
II. COVALENT BONDING
A. COVALENT BONDS ARE STRONG INTRABONDS FORMED BETWEEN TWO NONMETALS
1. Covalent bonding involves the sharing of electrons between atoms to form discrete molecules.
2. In covalent bonding, atoms once again want to achieve full s and p sub-shells, this time by joining together and sharing valence electrons. Full s and p sub-shells are called octets.
3. One shared pair of electrons represents a single covalent bond; two shared pairs represent a double bond; and three shared pairs represent a triple bond.
4. Covalent bonds usually occur between atoms that are nonmetals.
5. Any electron pairs that occur in the valence shell of an atom but do not form a bond with another atom are called nonbonding electrons or lone pairs.
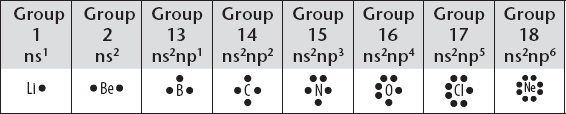
6. The structures of molecules that contain covalent bonds are described by the use of Lewis diagrams where “dots” are used to represent valence electrons, as shown in the following diagram. Also, see Chapter 6.

Ionic and covalent bonds are both strong bonds that can be described as intrabonds, i.e., bonds that exist within a compound. However, covalently bonded, molecular substances (i.e., those that do not form giant networks) have low melting and boiling points because they are only attracted to one another with weak intermolecular forces. For example, when water boils, only relatively weak intermolecular forces are being broken, NOT the very strong covalent bonds between hydrogen and oxygen atoms within the molecule. It is very important to understand the distinctions between intrabond breaking and interbond breaking. Many questions will expect you to make this distinction.
A. METALLIC BONDING REFERS TO THE BONDING AND STRUCTURE PRESENT IN METALS
1. A metal’s structure can be considered to be a close-packed lattice of positive ions surrounded by a “sea” of moving electrons, as shown in the following diagram.
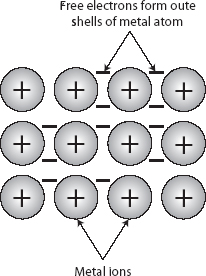
2. These electrons and their movement cause metals to be good conductors of electricity.
3. The close-packed ions make them good conductors of heat.
4. The metallic bond is the electrostatic attraction between the positive and negative charges.
5. The flexibility of these bonds makes metals malleable and ductile.

Look out for questions that ask about the properties of metals related to their bonding and structure. Good conduction of heat and electricity, malleability and ductility, along with luster (shininess) are all typical properties of metals. Recognizing these traits in test questions will help you raise your score.