I. CLASSIFYING REDOX REACTIONS
A. DISPROPORTIONATION
1. Disproportionation is a reaction where there is a simultaneous oxidation and reduction of a single species. See the following example.
![]()
In this reaction, Cl+ is oxidized to Cl5+ and Cl+ is simultaneously reduced to Cl−.
B. SYNTHESIS (OR COMBINATION)
1. Synthesis (or combination) is a reaction where a compound is formed by the reaction of simpler materials, often its elements. See the following example.
![]()
In this reaction, H0 is oxidized to H− and O0 is reduced to O2−.
C. DECOMPOSITION
1. Decomposition is a reaction where a compound is broken down into simpler substances. It is the reverse of a synthesis reaction. See the following example.
![]()
In this reaction, Hg2+ is reduced to Hg0 and O2− is oxidized to O0.
D. SINGLE DISPLACEMENT (SINGLE REPLACEMENT)
1. Single displacement (or single replacement) is a reaction where an atom or ion in a compound is displaced (replaced) by another atom or ion.
i. Metal displacement is shown in the following example.
![]()
In this reaction, Zn0 is oxidized to Zn2+ and Cu2+ is reduced to Cu0.
ii. Hydrogen displacement:
a) Hydrogen displacement from water is shown in the following example.
![]()
In this reaction, Na0 is oxidized to Na+ and H+ is reduced to H0.
b) Hydrogen displacement from acids is shown in the following example.
![]()
In this reaction, Zn0 is oxidized to Zn2+ and H+ is reduced to H0.
The reactions in (i) and (ii) can be replicated using many different metals and solutions but not all combinations result in a reaction. See Activity Series later in this chapter.
iii. Halogen displacement is shown in the following example.
![]()
In this reaction, Br− is oxidized to Br0 and Cl0 is reduced to Cl−. Once again, not all combinations lead to a reaction. Some halogens will displace other halogens from solutions of their ions but some will not. A halogen high in group 17 will displace one below it from a solution of its ions, but the reverse is not possible.
1. Combustion is a reaction where a compound or element burns in oxygen. Very often this is a hydrocarbon (a compound of hydrogen and carbon) that reacts with oxygen to form carbon dioxide and water. See the following example.
![]()
In this reaction, O0 is reduced to O2− and carbon and hydrogen gain oxygen and are said to be oxidized.
F. REDOX REACTIONS AS GAS-PRODUCING REACTIONS
1. Acid + Metal Salt + Hydrogen. See the following example.
![]()
In this reaction, zinc is oxidized, and hydrogen is reduced.
i. A simple lab test for hydrogen is the “squeaky pop” test with lighted splint.
2. Oxygen is produced by the decomposition of hydrogen peroxide with a MnO2 catalyst. See the following reaction.
![]()
In this reaction, is oxygen is both oxidized and reduced.
i. A simple lab test for oxygen is the relighting of a glowing splint.

Being able to recognize the oxidation states of individual atoms is crucial. It will allow you to quickly identify what is being oxidized and what is being reduced. Practice that skill!
II. ACTIVITY SERIES
A. DISPLACEMENT REACTIONS
1. Some metals will displace other metals from solutions, some will not.
2. Some metals will undergo displacement reactions with water, some with acids, some with both, and some with neither.
3. You can use the activity series to make predictions regarding which metals will (and which will not) undergo the displacement reactions discussed earlier. Metals are arranged in order of increasing ability to displace hydrogen, with the most reactive at the top.
i. All metals above hydrogen in the series will displace it from an acid.
ii. All metals below hydrogen will not displace it from an acid or water.
iii. A metal relatively high in the series will displace one below it from a solution of its ions but the reverse process is not possible.
Activity Series of Metals
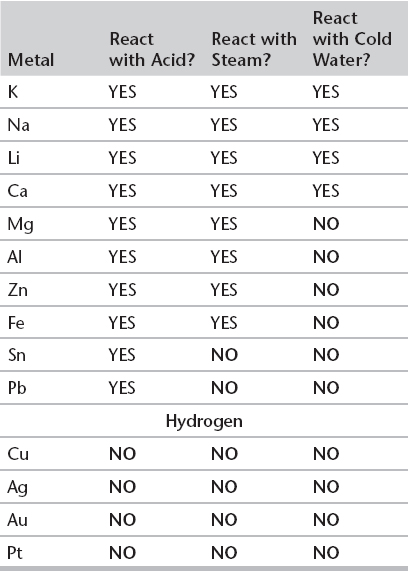

Make up your own mnemonic to remember the activity series.
III. ELECTROCHEMISTRY
A. STANDARD ELECTRODE REDUCTION POTENTIALS (SERPs)
1. The relative likelihood of a REDOX half equation occurring is summarized in the following standard electrode reduction potential table.
Note: All half equations are shown as reductions.
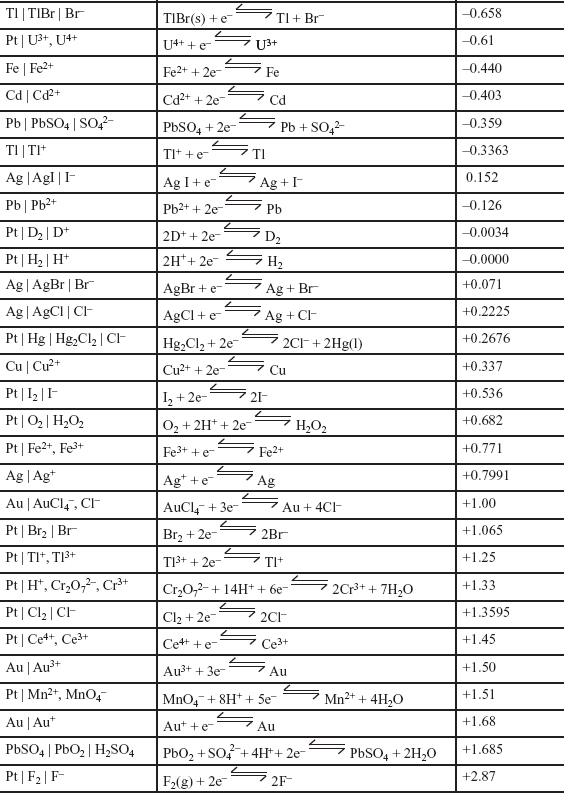
i. Half equations with negative electrode reduction potentials lose electrons most readily and tend to go in reverse. These species are best reducing agents.
ii. Half equations with positive electrode reduction potentials gain electrons most readily and tend to go forward. These species are best oxidizing agents.
2. The standard electrode reduction potential of a half cell, E θ, is defined as the electrode potential of a half cell, measured relative to a standard hydrogen electrode, which has a value of 0.00V, measured under standard conditions.
i. Standard conditions are 25°C (298K), any gases at a pressure of 1 atm, and all solutions at concentrations of 1M.
B. GALVANIC CELLS
1. A galvanic cell generates electrical energy from a spontaneous REDOX reaction. Connecting two half cells that have different electrode potentials forms an electrochemical cell (battery). For example, see the following diagram.
2. A high-resistance voltmeter is used to measure the voltage.
3. A salt bridge connects the two half cells. It can be made from a piece of filter paper soaked in an inert ionic solution, often KCl or KNO3. It allows the flow of ions.
4. The electrons flow through the wire, toward the more positive half cell where reduction takes place. This is the cathode.
5. The electrons flow through the wire, from the more negative half cell where oxidation takes place. This is the anode.
6. The voltage in the cell can be calculated using E θ cell = Eθreduced − Eθoxidized.
7. The relationship between Gibbs Free Energy and Eθcell is summarized by the expression ΔGθ = − n F Eθ, where F = Faraday constant = 96,500 J/V mol, and n = number of moles of electrons transferred.
8. The Nernst equation can be used to calculate the voltage in a cell when conditions are not standard. One form of the
equation is ![]() where R = 8.314 J/K mol,
where R = 8.314 J/K mol,
T = Kelvin temperature, n = number of electrons transferred, F = Faraday constant, Eθ = the voltage generated if the conditions were standard, ln represents the natural logarithm, and Q = reaction quotient (see Chapter 29).
1. Electrolysis is the process in which electrical energy is used to cause a nonspontaneous REDOX reaction to occur. Electrolysis occurs in an electrolytic cell and it is the opposite of a galvanic cell. See the following diagram.
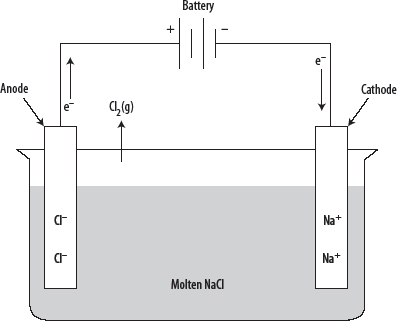
2. Anions in the electrolyte solution are attracted toward the anode, where they undergo oxidation. Electrons flow from the anode to the cathode, where cations undergo reduction.
3. The electrolysis of aqueous solutions can be more complicated because water is present that can ALSO undergo REDOX processes. See the following examples.

When this is the case, decisions based upon SERP values must be made about the relative likelihood of one process over another.
4. The amount of a substance produced in an electrolytic cell can be calculated using Faraday’s law. This quantitative aspect of electrolysis can be approached as follows.
i. Calculate the number of Faradays passed by applying the following formula: q = I t, where I = current in Amps, q = charge in Coulombs, and t = time in s.
ii. Secondly, convert q to Faradays.
Number of Faradays
![]()
iii. Use the stoichiometry of the electrode process, remembering that a process that produces a product by the transfer of one electron will require one Faraday, and that a process that produces a product by the transfer of two electrons will require two Faradays, and so on.