CHAPTER 24
Classic Antipsychotic Medications
Henry A. Nasrallah, M.D.
Rajiv Tandon, M.D.
History and Discovery
Prior to the introduction of classic antipsychotic medications in the 1950s, treatment for psychotic disorders primarily consisted of institutional confinement and supportive care with minimal control of psychotic symptoms. The discovery of chlorpromazine, the first of the “classic” antipsychotics, was serendipitous and owes much to the observations of a French surgeon, Henri Laborit, who noted that chlorpromazine, when used as an adjunct to anesthesia, calmed patients significantly following surgery. Laborit recommended its use to two French psychiatrists, Jean Delay and Pierre Deniker, who used it successfully in psychotic patients. Heinz Lehmann was the first to use chlorpromazine in North America (in Montreal, Canada). In the early 1960s, the first large-scale placebo-controlled trials were conducted within the U.S. Veterans Administration system.
After the successes of chlorpromazine, numerous other phenothiazines were synthesized by modifying the side chains on the phenothiazine rings. Subsequently, several nonphenothiazine antipsychotics, such as haloperidol, were introduced. The last of these drugs approved by the U.S. Food and Drug Administration (FDA) was molindone, introduced in 1975. Of 51 classic antipsychotic drugs (representing eight different chemical classes) available in the world, 9 are currently available in the United States.
The ability of chlorpromazine and other conventional antipsychotics to suppress psychotic symptoms (delusions, hallucinations, and disorganization) had a profound impact on chronically hospitalized psychiatric populations worldwide. Massive numbers of psychiatric patients were discharged from state hospitals to the community. This period of deinstitutionalization led to a decrease in the number of patients in state and county mental hospitals in the United States from 559,000 in 1955 to 338,000 in 1970 to 107,000 in 1988, an 80% decrease over 30 years. Initially, this led to enthusiasm about the possibility that patients with schizophrenia and other psychoses would be able to function well in the community. However, it soon became apparent that improvement with antipsychotic treatment was incomplete. In addition, poor treatment adherence—with subsequent relapses—led to frequent rehospitalization (i.e., the “revolving door” phenomenon).
As psychiatrists began using chlorpromazine, they observed that treated patients frequently manifested signs and symptoms of parkinsonism. Along with other side effects such as dystonia and akathisia, these symptoms are collectively referred to as extrapyramidal side effects (EPS). Because of the high prevalence of these movement disorders in patients receiving antipsychotic treatment, many psychiatrists believed EPS to be an unavoidable accompaniment of antipsychotic action; in fact, the onset of some EPS was considered to indicate the minimal effective antipsychotic dose (i.e., the “neuroleptic threshold”). The first report of persistent orobuccal movements (later labeled tardive dyskinesia) came from France in 1959. The pervasiveness of debilitating short-term and long-term motor side effects associated with classic antipsychotic drugs led to a search for agents that would be at least as efficacious but without the risk of EPS.
Clozapine, a dibenzodiazepine synthesized in 1959, was the first antipsychotic drug without EPS (i.e., atypical). It was initially marketed in Europe in 1972 but was withdrawn in some countries in 1975 after several reports of fatalities secondary to agranulocytosis. Clozapine was not introduced in the United States until 1989, after convincing studies demonstrated its efficacy in antipsychotic-refractory schizophrenia. Other atypical agents (also referred to as second-generation antipsychotics [SGAs]) have been launched in the past 15 years and are reported to be associated with lower levels of EPS and a broader spectrum of efficacy; of the 16 SGAs currently being marketed, 12 are available in the United States (see Chapters 25–34 in this volume). Since the introduction of these newer agents, use of the classic antipsychotics (also referred to as traditional, conventional, or first-generation antipsychotics [FGAs]) has declined, especially in the United States—FGAs currently aggregate approximately 5% of all antipsychotic prescriptions in the country. Results of several government-sponsored effectiveness studies—for example, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)—in the past decade have challenged the prevailing worldview of the greater effectiveness of SGAs over FGAs and have reinvigorated interest in the utility and clinical applicability of classic antipsychotics.
Structure–Activity Relations
Phenothiazines
Members of the phenothiazine class of classic antipsychotics share the same basic phenothiazine ring but differ in substitutions at both their R1 and R2 positions (Figure 24–1). Based on the side chain attached to the nitrogen atom in the middle ring (R1), the phenothiazines are further subdivided into three subtypes: aliphatic, piperidine, and piperazine phenothiazines.
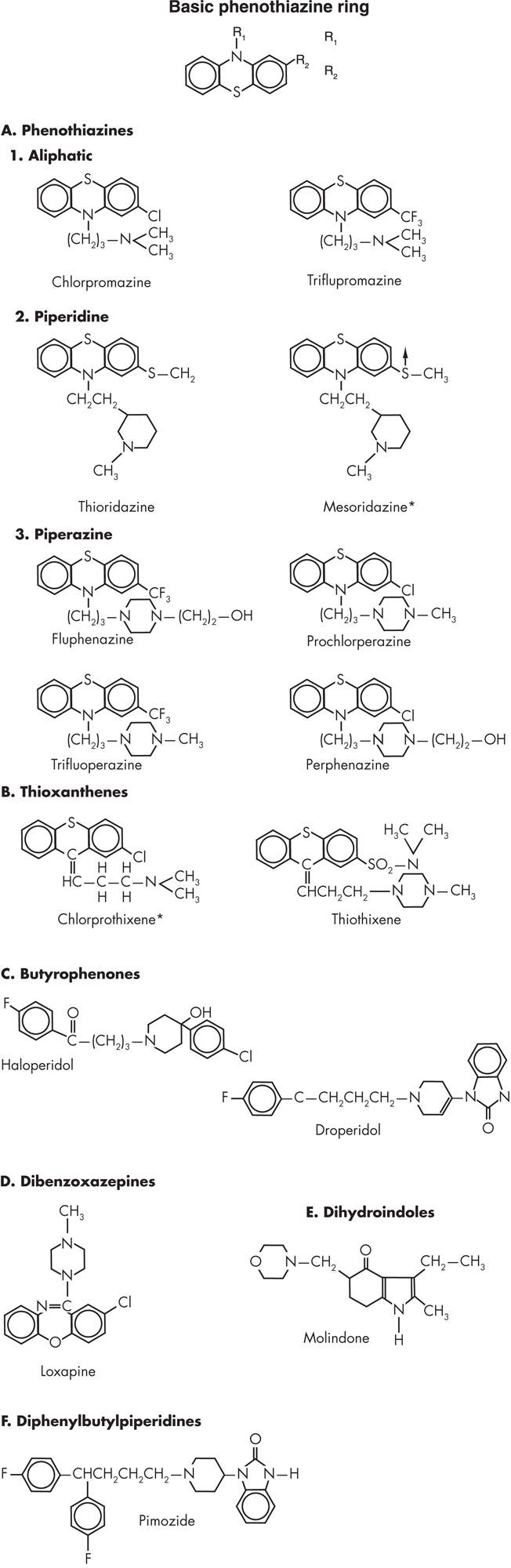
FIGURE 24–1. Chemical structures of various classic antipsychotics.
*No longer available in the United States.
Aliphatic Phenothiazines
The aliphatic phenothiazines share a dimethylamide substitution at their tenth carbon. Chlorpromazine (Thorazine or Largactil) is the prototypical member of this class and remains the aliphatic phenothiazine most widely used throughout the world. With a chlorine atom attached to its second carbon, chlorpromazine is heavily sedating because of its high level of anticholinergic, anti-α-adrenergic, and antihistaminergic actions.
Piperidine Phenothiazines
Piperidine phenothiazines—for example, thioridazine (Mellaril) and its metabolite, mesoridazine (Serentil)—are named for the presence of a piperidine ring at their tenth carbon. Although members of this group have similar efficacy and side effects compared with aliphatic phenothiazines, they are notable for having a less potent effect on nigrostriatal dopamine2 (D2) receptors and a higher level of anticholinergic activity; consequently they are associated with a lower frequency of EPS. The use of these agents has been virtually extinguished by a black box warning about significant QTc prolongation that was added to their product label in 2000.
Piperazine Phenothiazines
With a substitution of a piperazine group at the tenth carbon of a phenothiazine, the piperazines have greatly increased dopamine type 2 (D2) receptor blockade and a lower affinity for muscarinic, α-adrenergic, and histaminergic receptors. Some of the most potent conventional antipsychotics available in the United States, including fluphenazine (Prolixin), perphenazine (Trilafon), and trifluoperazine (Stelazine), belong to this class. The well-known antiemetic prochlorperazine (Compazine) is also part of this class; although approved for the treatment of psychosis, it is rarely utilized as an antipsychotic.
Thioxanthenes
Structurally and pharmacologically similar to the phenothiazines, the thioxanthenes also differ widely in their pharmacological profiles based on similar side-chain substitutions (see Figure 24–1). For instance, chlorprothixene shares the same dimethylamide and chloride substitution as chlorpromazine, with which it also shares its pharmacological profile. Thiothixene (Navane) has both a piperazine side chain and a strongly electrophilic substitution [SO2N(CH3)CH3], thus sharing the pharmacological profile of the piperazines.
Butyrophenones
The butyrophenone class has a piperidine ring with a three-carbon chain ending in a carbonyl-substituted p-fluorobenzene ring. Haloperidol, arguably the best-known classic antipsychotic, is the most widely used member of this class. Haloperidol and other members of this class are strong dopamine receptor antagonists and show little antimuscarinic, antihistaminergic, and antiadrenergic activity.
Dibenzoxazepines
Loxapine, the only FDA-approved agent within the dibenzoxazepine class, is composed of a tricyclic ring structure with a seven-member central ring. It has a piperazine side chain and chlorine at position R2 (see Figure 24–1). It exhibits an intermediate level of D2 blockade, as well as some serotonin2 (5-HT2) antagonism. Its side-effect profile is characterized by intermediate sedation and autonomic effects. Loxapine has the distinction of being the most “atypical” of the classic antipsychotics because it is structurally similar to the dibenzodiazepine clozapine. Another notable feature of loxapine is that one of its metabolites, amoxapine, is marketed as an antidepressant.
Dihydroindoles
Molindone is the only member of the dihydroindoles available in the United States. Sharing a similar structure with the indoleamines (see Figure 24–1), such as serotonin, molindone has the distinction of being the only classic antipsychotic not associated with any weight gain or a lowering of the seizure threshold.
Diphenylbutylpiperidines
Pimozide, the only agent within the diphenyl-butyl-piperidine class available in the United States, is approved only for the treatment of Tourette syndrome and has the distinction of possessing the highest selectivity and potency for dopamine D2 receptors among the conventional antipsychotics. It significantly prolongs the QTc interval, and this has limited its utilization. Derived from benperidol, pimozide shares many characteristics of the butyrophenones (see Figure 24–1).
Benzamides and Iminodibenzyl Agents
Sulpiride, the prototypical substituted benzamide, is a relatively selective dopamine D2 antagonist and lacks significant activity on cholinergic, histaminergic, or noradrenergic receptors. Because of this relative selectivity and a lower propensity to cause EPS, sulpiride is one of the more common classic antipsychotics utilized in Europe. No classic antipsychotic agent from either the benzamide or the iminodibenzyl class is available in the United States.
Pharmacological Profile
The classic conventional antipsychotic drugs have a multitude of effects on various physiological variables through their actions on different neurotransmitter systems. The antipsychotic effects of these agents are believed to occur primarily through antagonism of D2-type dopaminergic receptors. Therapeutic and adverse effects of D2 antagonism have been conceptualized in the context of the major dopaminergic tracts present in the brain, which include the mesocortical, mesolimbic (A10), tuberoinfundibular (A12), and nigrostriatal (A8 and A9) tracts.
The effect of D2 receptor blockade on the mesolimbic dopaminergic systems is believed to represent the putative mechanism of action of conventional antipsychotics, but D2 blockade in other tracts is believed to result in a number of adverse cognitive and behavioral side effects. Such side effects are frequently observed in both animals and human subjects. D2 receptor antagonism in the mesocortical dopaminergic pathway leads to a blunting of cognition (bradyphrenia) and avolition-apathy (sometimes referred to as the neuroleptic-induced deficit syndrome), which can be difficult to differentiate from the primary negative symptoms of schizophrenic illness itself.
Blockade of the tuberoinfundibular tract projecting to the hypothalamus and pituitary gland results in multiple neuroendocrine side effects processed through the pituitary gland. Although dopamine is involved in enhancing the release of most pituitary hormones, it is actually responsible for the tonic inhibition of prolactin release. With significant dopaminergic blockade of the tuberoinfundibular tract, prolactin release is no longer prevented, and the release of other pituitary hormones is no longer enhanced. High levels of prolactin combined with decreased levels of follicle-stimulating hormone and luteinizing hormone often result in amenorrhea, galactorrhea, gynecomastia, decreased bone density, impaired libido, and erectile dysfunction.
High levels (exceeding 78%) of D2 dopaminergic blockade within the nigrostriatal system, which projects to the basal ganglia and caudate, produce some of the most undesirable side effects of conventional antipsychotics. Movement disorders or EPS such as akathisia, rigidity, and hypokinesia were once believed to be necessary “evidence” of a therapeutic antipsychotic dosage. However, the advent of the new-generation antipsychotics that are associated with minimal EPS conclusively dispensed with this misconception. At higher levels of D2 blockade, one may also observe dystonia, catalepsy, and a rigid, immobile catatonic state.
Classic antipsychotic agents have varying degrees of activity at serotonergic, cholinergic, noradrenergic, histaminergic, and other nondopaminergic receptors. Although it is unclear whether any of these activities contribute to or interfere with their effectiveness in the treatment of psychotic symptoms, they clearly result in a variety of adverse effects. Because of differences in the pharmacological activity of different classic antipsychotic agents at these receptors, there are predictable differences in their side-effect profiles.
Pharmacokinetics
Generally, the pharmacokinetic profiles of the conventional antipsychotics remain poorly understood. Even for some of the more extensively studied agents, many hundreds of potential metabolites remain undiscovered, and the physiological activity of several metabolites has yet to be adequately defined. Nonetheless, certain general statements can be made concerning the classic antipsychotics as a group.
Administration and Absorption
Many of the conventional antipsychotics are available in both oral and intramuscular formulations. Although relatively common in the past, intravenous usage of parenteral formulations of antipsychotics is not FDA-approved. Peak plasma levels with oral preparations are generally reached in 1–4 hours, with these levels being reached slightly more rapidly with liquid concentrates. Oral preparations are extensively metabolized in the liver during their first pass through portal circulation by undergoing a range of transformations, including glucuronidation, oxidation, reduction, and methylation. Steady-state levels are reached in a period of four to five times the half-life of the drug in question.
Intramuscular administration results in faster, more predictable absorption, with peak plasma levels in 30–60 minutes and clinical efficacy as rapidly as 15 minutes. With intramuscular or intravenous administration, plasma levels may be as much as four times the levels of the oral route because of circumvention of the hepatic first-pass metabolism.
Although 10 classic antipsychotics are available in a long-acting (depot) formulation around the world, haloperidol and fluphenazine are the only classic antipsychotics currently available in such a formulation in the United States. The currently available decanoate forms of both haloperidol and fluphenazine are administered through injection into a major muscle, and the drug is slowly released to the bloodstream over time. As the esterified version of the drug diffuses into other tissues, the ester chain is hydrolyzed, resulting in the smooth release of the drug in question. Fluphenazine decanoate can be given every 2–3 weeks on the basis of its half-life of 7–10 days, whereas haloperidol decanoate may be given every 4 weeks because of its longer half-life. The bioavailability of intramuscular relative to oral administration is twofold greater.
Distribution
Most of the conventional antipsychotics are highly protein bound (85%–90%). This feature is of importance when other highly protein-bound medications are used concomitantly because of the risk of increasing levels of free or unbound drugs into the toxic range. The antipsychotic drugs are highly lipophilic, which allows unbound portions of the drug to readily cross the blood–brain barrier, with concentrations twofold higher in the brain than in the peripheral circulation. The drugs also readily cross the placenta to the fetus in pregnancy.
Metabolism
The conventional antipsychotics are metabolized in the liver by hydroxylation and demethylation to forms that are more soluble and readily excreted by the kidneys and in the feces. Many of these compounds undergo further glucuronidation and remain active as dopamine receptor antagonists. Because of the many active metabolites of the antipsychotic agents, it has not been possible to obtain meaningful correlations between plasma levels and clinical response. Variables such as age, genetic variability among individuals, and coadministration of other drugs cause plasma levels to vary 10- to 20-fold across individuals. The majority of conventional antipsychotics are metabolized by the cytochrome P450 (CYP) enzyme subfamilies. Since CYP2D6 is important for the metabolism of many of these antipsychotics, genetic variation in the rate of 2D6 metabolism should be considered. CYP1A2 and 3A4 subfamily enzymes are also involved in the metabolism of some classic antipsychotics, and this may be relevant to understanding drug–drug interactions of those agents.
Excretion
The major routes of excretion of the classic antipsychotics are through urine and feces by way of bile. These drugs are also excreted in sweat, saliva, tears, and breast milk. Elimination half-life varies from 18 to 40 hours for these drugs. Lower doses of antipsychotics are generally needed in elderly patients because of decreased renal clearance. Because of the long elimination half-lives of the classic antipsychotics, once-a-day dosing is possible for each of these agents following stabilization.
Mechanism of Action
Dopamine has been at the center of neurobiological theories of psychosis for the past half-century, and even today, all agents approved as antipsychotics share the single common attribute of dopamine D2 receptor antagonism. Amphetamine intoxication served as a drug-induced model of the positive symptoms of schizophrenia. Drugs that blocked dopaminergic receptors, specifically the D2 receptor, were noted to have greater efficacy and potency as antipsychotics. Since dopaminergic agonists exacerbate psychosis and dopaminergic blockade treats it, dopamine has held central importance in our conceptualization of the neuropharmacology of schizophrenia.
Indications and Efficacy
Schizophrenia and Schizoaffective Disorder
Classic antipsychotics are best known for the acute and maintenance treatment of the psychotic (also known as positive) symptoms of schizophrenia and schizoaffective disorder. The major putative mechanism of action is through D2 blockade of the mesolimbic tract. In many individuals, this blockade results in a measurable decrease in the positive symptoms of schizophrenia, including hallucinations, delusions, and disorganization (Tandon et al. 2010, 2013). However, negative and cognitive symptoms of schizophrenia respond less robustly. In fact, they may be worsened by blockade of mesocortical tracts that play roles in cognition and hedonic reinforcement.
The failure to improve the negative symptoms of schizophrenia is one of the major drawbacks of the classic antipsychotics. In fact, the EPS induced by the FGAs can worsen negative and cognitive symptoms by inducing bradykinesia and bradyphrenia. Another major limitation is the lack of improvement of positive symptoms (i.e., refractoriness) in about 25% of schizophrenia patients and partial response (i.e., treatment resistance) in another 25%.
Substance-Induced Psychosis
As noted previously, conventional agents can reverse the psychosis associated with acute and chronic amphetamine intoxication as well as that associated with cocaine use. However, the risk of acute dystonia must be considered in these populations, as dopamine receptor downregulation is common, resulting in greater sensitivity to rapid D2 blockade. Results in treatment of psychosis secondary to drugs acting in non-dopaminergic mechanisms (such as hallucinogens) are less satisfactory, although there may be some role for the classic antipsychotics in treating phencyclidine (PCP) psychosis.
Personality Disorders
Although any personality disorder can be associated with transient psychotic features emerging under stressful conditions, Cluster B disorders are most often associated with this phenomenon. Treatment for transient psychotic episodes has included short-term use of low doses of a high-potency antipsychotic. Although some symptoms of personality disorders may be amenable to such pharmacological treatment, long-term conventional antipsychotic treatment is not recommended.
Mood Disorders
The utility of antipsychotic agents in the treatment of mood disorders with psychotic features is well known. However, their utility in the treatment of nonpsychotic depression and bipolar disorder is described as well. Several conventional antipsychotics (such as thioridazine) are FDA approved for the treatment of depression and anxiety without overt psychosis. However, they are no longer used for this purpose because of the availability of other, more effective and better-tolerated agents. The utility of conventional agents as adjuncts to mood stabilizers in the treatment of patients with bipolar and related disorders has been well described, both in the acute management of mania and in the maintenance treatment of bipolar disorder with severe mood disturbance and/or psychotic features. However, the newer atypical antipsychotics have largely replaced conventional antipsychotics in the management of bipolar disorder.
Tourette Syndrome
The tics present within Tourette syndrome are believed to be due to a hyper-dopaminergic state that is amenable to treatment by dopamine receptor antagonists. Pimozide is the only conventional antipsychotic with this indication, which is its only FDA-approved indication.
Huntington’s Disease
Although there is no cure for Huntington’s disease, the psychosis and choreiform movements associated with this disease may be ameliorated by dopamine receptor antagonism. Several conventional antipsychotics carry FDA indications for treatment of this disease.
Nausea, Emesis, and Hiccups
The lower-potency antipsychotics exert a potent antiemetic effect through histamine1 (H1) receptor antagonism. This effect is closely related to their original role in reducing perioperative stress and emesis. Many well-known antiemetics, such as promethazine (Phenergan), are phenothiazines with a short-chain substitution. In addition, chlorpromazine is approved for oral or intramuscular therapy of intractable hiccups.
Side Effects and Toxicology
As noted earlier, side-effect profile—rather than efficacy in treating psychosis—is used to differentiate the conventional antipsychotics (Dodd et al. 2015). These agents serve as antagonists at four major neurotransmitter receptor systems in the central nervous system (CNS): the dopamine type 2 receptor family (D2, D3, and D4), muscarinic cholinergic receptors (M1), α-adrenergic receptors (α1 and α2), and histamine receptors (H1) (Table 24–1).
Chlorpromazine |
Thioridazine |
Perphenazine |
Trifluoperazine |
Fluphenazine |
Thiothixene |
Haloperidol |
Loxapine |
Molindone |
|
D1 |
High |
— |
High |
High |
High |
Moderate |
High |
High |
Low |
D2 |
High |
Very high |
Very high |
Very high |
Very high |
Very high |
Very high |
Very high |
Very high |
D3 |
High |
Very high |
Unknown |
Unknown |
Very high |
Unknown |
Very high |
Unknown |
Unknown |
D4 |
High |
Very high |
Unknown |
High |
Very high |
Unknown |
Very high |
Very high |
Low |
H1 |
High |
High |
Moderate |
Moderate |
High |
Moderate |
Low |
High |
Very low |
M1 |
High |
High |
Low |
Low |
Low |
Low |
Low |
Moderate |
None |
α1 |
Very high |
Very high |
High |
High |
High |
Moderate |
Low |
High |
Low |
α2 |
Moderate |
Very high |
Moderate |
Low |
Low |
Moderate |
Low |
Low |
Moderate |
5-HT1 |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
5-HT2 |
High |
High |
Moderate |
Moderate |
Moderate |
Moderate |
Moderate |
Very high |
Low |
Note. Receptor families: dopamine (D1, D2, D3, D4), histamine (H1), muscarinic cholinergic (M1), α-adrenergic (α1, α2), and serotonergic (5-HT1, 5-HT2). |
|||||||||
The therapeutic action of classic antipsychotics in ameliorating the positive symptoms of schizophrenia is believed to be due to D2 blockade in the mesolimbic dopamine tract. Blockade of D2 receptors in the mesocortical, nigrostriatal, and tuberoinfundibular systems leads to the tract-related side effects described earlier in this chapter (see section “Pharmacological Profile”). Lower-potency agents have greater antihistaminergic, anticholinergic, and antiadrenergic actions. However, they have fewer D2-related side effects because of their lower affinity to D2 receptors and relatively high anticholinergic activity. Higher-potency agents, such as haloperidol, produce more D2-related movement disorders and prolactin elevation but otherwise have a cleaner side-effect profile, having fewer anticholinergic, antiadrenergic, and antihistaminergic side effects. Anticholinergic action often leads to dry mouth (xerostomia), blurred vision (mydriasis), constipation, urinary retention, sinus tachycardia, confusion, impaired cognition, paralytic ileus, exacerbation of open-angle glaucoma, and drowsiness. Antagonism of α1-adrenergic receptors is associated with orthostatic hypotension, QTc prolongation, reflex tachycardia, dizziness, incontinence, and sedation. Antagonism of α2 receptors can be associated with retrograde ejaculation and priapism. Antagonism of H1 receptors leads to sedation, drowsiness, and weight gain.
The frequencies of adverse reactions to classic antipsychotic agents are summarized in Table 24–2.
Phenothiazines |
Butyrophenone |
Dibenzoxazepine |
Dihydroindole |
Diphenylbutylpiperidine |
Thioxanthene |
|||||||
Chlorpromazine |
Mesoridazine |
Thioridazine |
Fluphenazine |
Perphenazine |
Trifluoperazine |
Haloperidol |
Loxapine |
Molindone |
Pimozide |
Thiothixene |
||
Cognitive effects |
||||||||||||
Drowsiness, sedation |
High |
High |
High |
Low |
Moderate |
Low |
Low |
High |
High |
Moderate |
Moderate |
|
Insomnia, agitation |
Low |
Low |
Low |
Low |
Low |
Low |
Moderate |
Low |
Low |
Low |
Moderate |
|
Extrapyramidal effects |
||||||||||||
Parkinsonism |
Low to Moderate |
Low to Moderate |
Low |
High |
Moderate |
Moderate to High |
High |
Moderate |
Moderate to High |
Moderate |
High |
|
Akathisia |
Low |
Moderate |
Low |
High |
Moderate |
High |
High |
Moderate |
High |
Moderate |
High |
|
Dystonic reactions |
Low |
Low |
Low |
High |
Moderate |
Moderate |
High |
Moderate |
Moderate |
Low |
Low |
|
Cardiovascular effects |
||||||||||||
Orthostatic hypotension |
High |
High |
High |
Low |
Low |
Low to Moderate |
Low |
Low |
Low |
Low |
Low |
|
Tachycardia |
Moderate |
Moderate |
High |
Low |
Low |
Low |
Low |
Moderate |
Low |
Low |
Low |
|
ECG abnormalities |
Moderate |
Low |
Moderate |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Cardiac arrhythmias |
Low |
Moderate |
Moderate |
Low |
Low |
Low |
Moderate |
Low |
Low |
Low |
Low |
|
Anticholinergic effects |
||||||||||||
High |
High |
High |
Low |
Low |
Low |
Low |
Moderate |
Moderate |
Low |
Low |
||
Endocrine effects |
||||||||||||
Sexual dysfunction |
Moderate |
Moderate |
High |
Moderate |
Moderate |
Moderate |
Moderate |
Low |
Low |
Low |
Low |
|
Galactorrhea |
Moderate |
Moderate |
Moderate |
High |
Moderate |
Moderate |
High |
Moderate |
Moderate |
Low |
Low |
|
Weight gain |
High |
High |
High |
Moderate |
Moderate |
Moderate |
Low |
Moderate |
Low |
Low |
Moderate |
|
Skin reactions |
||||||||||||
Photosensitivity |
Moderate |
Low |
Moderate |
Low |
Low |
Low |
Low |
Low |
Low |
— |
Low |
|
Rashes |
Moderate |
Low |
Moderate |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Pigmentation |
High |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
— |
Low |
|
Ocular effects |
||||||||||||
Lenticular pigmentation |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Pigmentary retinopathy |
Low |
Low |
Moderate |
Low |
Low |
Low |
Low |
Low |
Low |
— |
Low |
|
Other effects |
||||||||||||
Blood dyscrasias |
Low to Moderate |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Hepatic disorder |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Seizures |
Moderate |
Moderate |
Moderate |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
Cognitive Side Effects
CNS side effects of classic antipsychotics can be subclassified into cognitive and neuromuscular side effects. Cognitive effects include sedation, confusion, disturbed concentration, memory impairment, and delirium (Himelhoch et al. 1996). Antihistaminergic and anticholinergic actions lead to sedation and slowed mentation. These effects, which are most pronounced with lower-potency agents (e.g., chlorpromazine), are most severe earlier in treatment, with some tolerance developing over time. Anticholinergic delirium is the most common cause of medication-induced delirium. Because delirium results in high rates of morbidity and mortality (over 20% mortality), this potential side effect is important, especially in populations of individuals who are more sensitive to anticholinergic medications, such as the elderly. In addition, every antipsychotic—especially the low-potency agents—can potentially lower the seizure threshold.
Extrapyramidal Side Effects
Neuromuscular CNS side effects are due to antagonism of D2 receptors in the nigrostriatal dopaminergic pathway. Generally, antipsychotics manifest EPS when dopaminergic blockade exceeds 75%–80% of D2 receptors. EPS are most frequent with the high-potency agents such as haloperidol. A greater risk of EPS differentiates the classic from the atypical antipsychotics—in fact, the term atypical was originally coined to highlight this ability to produce an antipsychotic effect equivalent to that of the typical high-potency agents with lower EPS.
Acute-Onset EPS
Acute-onset EPS include medication-induced parkinsonism, acute dystonia, and akathisia. Antipsychotic-induced parkinsonism occurs in 15% of patients after several weeks of treatment. It is more common in patients older than 40 years, although it can occur at any age. Symptoms are identical to those of Parkinson’s disease and include muscle stiffness (“lead-pipe” rigidity), cogwheel rigidity, shuffling gait, stooped posture, drooling, bradykinesia, resting tremor, masked facies, and akinesia. Slowed, restricted movements of the body and face (akinesia) may be mistakenly diagnosed as being due to depression or the negative symptoms of schizophrenia.
It is estimated that up to 10% of patients may experience an acute dystonic episode, which usually occurs within the first few hours or days of treatment. It is more common in youth, in recent cocaine users, and with intramuscular doses of high-potency antipsychotics. Dystonia is an acute, sustained, painful muscular contraction. Potential areas of involvement include the tongue (protrusions, twisting), jaw, neck (spasmodic retrocollis or torticollis), and back (opisthotonos). If the dystonia involves the eyes, it results in a symmetrical or unilateral upward lateral movement called an oculogyric crisis. Laryngeal dystonia can result in sudden death secondary to a patient’s inability to breathe. Dystonia can be extremely uncomfortable and frightening for patients and can lead to noncompliance with medication for fear of recurrence. Treatment of dystonia requires rapid diagnosis and intravenous administration of antihistaminergic or anticholinergic agents. Anticholinergic agents are often initiated with high-potency antipsychotics in an effort to avoid this side effect.
Akathisia is a subjective feeling of motor restlessness in which patients feel an irresistible urge to move continuously. It is described as an unpleasant sensation and may result in dysphoria. Akathisia can occur at any time during treatment and is the most prevalent of the EPS. It frequently leads to noncompliance with medications and is believed to increase suicide risk in some patients.
Late-Onset EPS
Tardive dyskinesia is characterized by a persistent syndrome of involuntary choreoathetoid movements of the head, limbs, and trunk. It generally takes at least 3–6 months of exposure to antipsychotics before the disorder develops. Perioral movements involving buccolingual masticatory musculature are the most common early manifestation of tardive dyskinesia. Tardive dyskinesia has an estimated yearly incidence of 5% among adults and as high as 25% in the elderly who receive continuous conventional antipsychotic therapy and has been a major source of litigation in past psychiatric practice. The risk of developing tardive dyskinesia is reported to increase with age and to be higher in certain ethnic groups; female gender, presence of mood disorders, and early onset of EPS have also been associated with increased risk of tardive dyskinesia.
Tardive dyskinesia may be masked by continuing dopamine blockade and has a variable course following development. Over time, spontaneous resolution or improvement has been described in some individuals. There is no single effective treatment, although treatment with clozapine has been reported to improve symptoms. Cases of tardive dyskinesia have been described with every antipsychotic, although classic antipsychotics are associated with a much greater risk of tardive dyskinesia than second-generation or atypical agents (Correll et al. 2004). Other tardive syndromes include tardive dystonia, tardive akathisia, and tardive pain.
Neuroleptic Malignant Syndrome
Neuroleptic malignant syndrome (NMS) is a poorly understood syndrome that usually appears within hours or days of initiation of antipsychotic treatment. This syndrome is characterized by muscular rigidity, hyperpyrexia, autonomic instability (hypo- or hypertension, tachycardia, diaphoresis, pallor), and altered consciousness. NMS has an estimated incidence of 0.02%–2% and carries a mortality rate of 20%–30%. Death most often occurs secondary to dysrhythmias, renal failure secondary to rhabdomyolysis, aspiration pneumonia, or respiratory failure. Laboratory findings include elevated creatine phosphokinase, elevated white blood cell count, elevated liver enzymes, myoglobinemia, and myoglobinuria. The syndrome can last up to 10–14 days.
Treatment requires immediate discontinuation of the offending antipsychotic and supportive care with aggressive intravenous hydration. In the past, mild cases of NMS were treated with intravenous bromocriptine, while more severe cases were treated with intravenous dantrolene. However, randomized controlled clinical trials of NMS treatment have never been performed because of its infrequent occurrence.
Cardiac Effects
α-Adrenergic antagonism is associated with orthostatic hypotension with reflex tachycardia, with tolerance possibly developing later in the treatment course. Orthostasis is important because of an increase in falls and related injuries.
Recent studies involving several antipsychotics have drawn attention to the risk of cardiac dysrhythmias, which is especially prominent with use of lower-potency conventional antipsychotics. High dosage, rapid titration, intramuscular administration, and especially intravenous administration may be associated with a lengthening of the QTc interval, with resulting risk of serious dysrhythmias such as torsades de pointes and ventricular fibrillation. Studies with thioridazine have raised concerns about piperidine antipsychotics, leading to a decrease in the use of this class. In reality, torsades de pointes is rarely encountered during treatment with conventional antipsychotics, although some have speculated that a syndrome of unexplained sudden death described with all conventional antipsychotics may be related to sudden dysrhythmias.
Gastrointestinal Side Effects
The anticholinergic actions of conventional agents include dry mouth, nausea, vomiting, and constipation that can progress to paralytic ileus. Antihistaminergic action is associated with medication-related weight gain, which greatly increases the patient’s risk of developing diabetes.
Cholestatic jaundice is a hypersensitivity reaction described with the aliphatic phenothiazines, especially chlorpromazine (incidence of 0.1%). This reaction typically manifests during the first 1–2 months of treatment and presents with nausea, malaise, fever, pruritus, abdominal pain, and jaundice, with resulting elevations in levels of bilirubin and alkaline phosphatase. This condition rarely lasts more than 2–4 weeks after discontinuation.
Weight Gain, Diabetes Mellitus, and Dyslipidemia
With the introduction of atypical antipsychotics, several of which cause significant weight gain, there is renewed awareness of the metabolic side effects associated with antipsychotic therapy such as obesity, elevated cholesterol and triglyceride levels, and an increased risk of diabetes mellitus. These metabolic changes increase the risk of ischemic heart disease and contribute to the increased mortality observed in schizophrenia. Antihistaminergic action is associated with medication-related weight gain, which greatly increases the patient’s risk of developing diabetes. Diabetes is currently described as a worldwide epidemic. Serotonin 5-HT2C receptor blockade also significantly contributes to weight gain (Tandon and Halbreich 2003). There are significant differences among classic antipsychotics with reference to their propensity to cause these metabolic adverse effects. Molindone is the least likely to cause weight gain, whereas thioridazine and chlorpromazine are among the most likely to do so. In general, high-potency agents cause less weight gain than do low-potency agents.
Genitourinary Side Effects
Renal effects secondary to blockade of M1 receptors include urinary hesitancy or retention, which can lead to a comparable increase in urinary tract infections in both genders. As mentioned previously, antagonism of tuberoinfundibular dopaminergic tracts increases prolactin secretion. Clinical manifestations of hyperprolactinemia include gynecomastia, galactorrhea, diminished libido, erectile dysfunction, amenorrhea, decreased bone density, menstrual irregularities, infertility, delayed ovulation, and possibly increased risk of breast cancer. Other sexual side effects, such as erectile disorder, retrograde ejaculation (due to blockade of α2-adrenergic receptors), anorgasmia, and occasionally priapism, can also occur with conventional antipsychotics.
Hematological Side Effects
Hematological effects of conventional antipsychotics include transient leukopenia (white blood cell [WBC] count <3,500/mm3), which is common but not usually problematic, and agranulocytosis (WBC count <500/mm3), a life-threatening problem. Agranulocytosis occurs most often during the first 3 months of treatment, with an incidence of 1 in 500,000. Aliphatic and piperidine phenothiazines are the most common causal agents among the conventional antipsychotics. Rarely, thrombocytopenic or nonthrombocytopenic purpura, hemolytic anemia, and pancytopenia may occur.
Ocular Side Effects
In addition to direct anticholinergic ocular effects such as blurred vision (mydriasis and cycloplegia) and exacerbation of open-angle glaucoma, direct optic toxicity has been described. The conventional antipsychotics are associated with several kinds of optical pathology involving the lens, cornea, and retina. Lenticular opacities have been reported with some phenothiazines, including perphenazine, chlorpromazine, and thioridazine. An irreversible increase in retinal pigmentation has been described with thioridazine when high dosages (>800 mg/day) are used. This retinal pigmentation, which can progress even after drug discontinuation, can lead to reduced visual acuity and even blindness. Early symptoms include poor night vision and secondary nocturnal confusion.
Dermatological Side Effects
Cutaneous side effects of conventional antipsychotics involve hypersensitivity rashes—most commonly maculopapular erythematous rashes of the trunk, face, neck, and extremities—and photosensitivity reactions that can lead to severe sunburn. Care must be taken with injectable versions of many antipsychotics because of direct dermatological toxicity if the skin or subcutaneous layers are exposed. Prolonged use of chlorpromazine can lead to blue-gray discoloration in body areas exposed to sunlight.
Drug–Drug Interactions
Careful consideration of a patient’s existing drug regimen should be given prior to the initiation of antipsychotic therapy.
Protein Binding
Because conventional antipsychotics are tightly protein bound, care must be taken when these medications are administered with other highly protein-bound medications. Mutual displacement of medications such as phenytoin, digoxin, warfarin, and valproate could lead to a short-term increase in serum levels of these drugs and of the conventional antipsychotic. However, protein binding has not been of serious clinical significance.
Cytochrome P450 Inhibition
As mentioned previously, conventional antipsychotics are primarily hepatically metabolized through the CYP2D6 and 3A4 enzymes. In addition, each inhibits the 2D6 enzyme to some degree. Care must be taken when conventional agents are co-administered with potent CYP2D6 inhibitors such as fluoxetine, paroxetine, cimetidine, erythromycin, and certain class IC antiarrhythmics (e.g., quinidine). Similarly, potent CYP3A4 inhibitors such as nefazodone, fluvoxamine, and ketoconazole should be used with care. Inhibitors of CYP2D6 and 3A4, as well as competitive substrates, should be used carefully with conventional antipsychotics because of their potential to increase plasma levels. CYP1A2, induced by nicotine and inhibited by estrogen, plays a role in metabolizing some antipsychotics.
Conclusion
The classic antipsychotics revolutionized the practice of psychiatry and the treatment of the severely mentally ill throughout the world. Second-generation “atypical” antipsychotics have commercially eclipsed these first-generation agents to a large extent, in that more than 90% of patients with schizophrenia and related psychoses in the United States are currently receiving one of the atypical oral agents. In regard to long-acting (depot) agents, second-generation injectable agents are gradually displacing classic antipsychotic injectables such as haloperidol decanoate and fluphenazine decanoate. The landmark National Institute of Mental Health–funded CATIE study, which compared four SGA oral agents (olanzapine, quetiapine, risperidone, and ziprasidone) against one FGA oral agent (perphenazine), showed that there was no difference between the two generations in clinical effectiveness (defined as all-cause discontinuation) (Lieberman et al. 2005). However, the CATIE study excluded subjects with tardive dyskinesia from random assignment to perphenazine and instead assigned them to one of the atypical agents. This methodological stipulation may have confounded the findings, because the 231 subjects with tardive dyskinesia were later found to have a higher severity of psychopathology and a much greater likelihood of substance use (Nasrallah 2006). Additionally, the subjects’ low propensity to develop EPS led to a “ceiling effect” finding of no EPS differences between perphenazine and the SGAs. Nonetheless, both classic antipsychotics and atypical antipsychotics can cause serious side effects, with neurological adverse events being much more likely with the FGAs and metabolic complications being more common with the SGAs.
Conventional antipsychotics will always be remembered for their critical role as the foundation of antipsychotic pharmacotherapy and as the main impetus for the remarkable neuropharmacological progress in psychiatric neuroscience over the second half of the twentieth century. They retain an important, if limited, role in the antipsychotic armamentarium of the twenty-first century.
Suggested Readings
Glazer WM: Review of incidence studies of tardive dyskinesia associated with typical antipsychotics. J Clin Psychiatry 61 (suppl 4):15–20, 2000 10739326
Janicak PA, Marder SR, Tandon R, Goldman M: Schizophrenia—Recent Advances in Diagnosis and Treatment. Springer, Berlin, 2014
Meyer JM, Nasrallah HA (eds): Medical Illness and Schizophrenia, 2nd Edition. Washington, DC, American Psychiatric Publishing, 2010
Nasrallah HA, Smeltzer D: Contemporary Diagnosis and Management of Schizophrenia, 2nd Edition. Newtown, PA, Handbooks in Health Care, 2011
Sachdev PS: Neuroleptic-induced movement disorders: an overview. Psychiatr Clin North Am 28(1):255–274, x, 2005 15733622
Smith D, Pantelis C, McGrath J, et al: Ocular abnormalities in chronic schizophrenia: clinical implications. Aust N Z J Psychiatry 31(2):252–256, 1997 9140633
Tandon R, Belmaker RH, Gattaz WF, et al; Section of Pharmacopsychiatry, World Psychiatric Association: World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 100(1–3):20–38, 2008 18243663
References
Correll CU, Leucht S, Kane JM: Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 161(3):414–425, 2004 14992963
Dodd M, Samara MT, Tardy M, et al: Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst Rev 1:CD009831, 2015 25592299
Himelhoch S, Taylor SF, Goldman RS, et al: Frontal lobe tasks, antipsychotic medication, and schizophrenia syndromes. Biol Psychiatry 39(3):227–229, 1996 8837987
Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353(12):1209–1223, 2005 16172203
Nasrallah HA: CATIE’s surprises: In antipsychotics’ square-off, were there winners or losers? Curr Psychiatr 5(2):49–65, 2006
Tandon R, Halbreich U: The second-generation ‘atypical’ antipsychotics: similar improved efficacy but different neuroendocrine side effects. Psychoneuroendocrinology 28 (suppl 1):1–7, 2003 12504068
Tandon R, Nasrallah HA, Keshavan MS: Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res 122(1–3):1–23, 2010 20655178
Tandon R, Gaebel W, Barch DM, et al: Definition and description of schizophrenia in the DSM-5. Schizophr Res 150(1):3–10, 2013 23800613