CHAPTER 34
Cariprazine
Sultan Albrahim, M.D.
Joseph H. Henry, M.D.
Charles B. Nemeroff, M.D., Ph.D.
Cariprazine (Vraylar) was initially discovered by the Hungarian company Gedeon Richter Ltd. (November 2004) and was developed by Forest Laboratories and submitted to the U.S. Food and Drug Administration (FDA) in November 2012 (Forest Laboratories 2012). In September 2015, cariprazine was approved by the FDA for the treatment of schizophrenia and acute manic or mixed episodes associated with bipolar I disorder in adults (Actavis Inc. 2015) (Figure 34–1). Cariprazine is also in development in a variety of countries for use in the treatment of schizophrenia with predominantly negative symptoms (Phase III), for use as an adjunct in the treatment of major depressive disorder (Phase II/III), and for use in the treatment of bipolar depression (Phase II) (McCormack 2015).
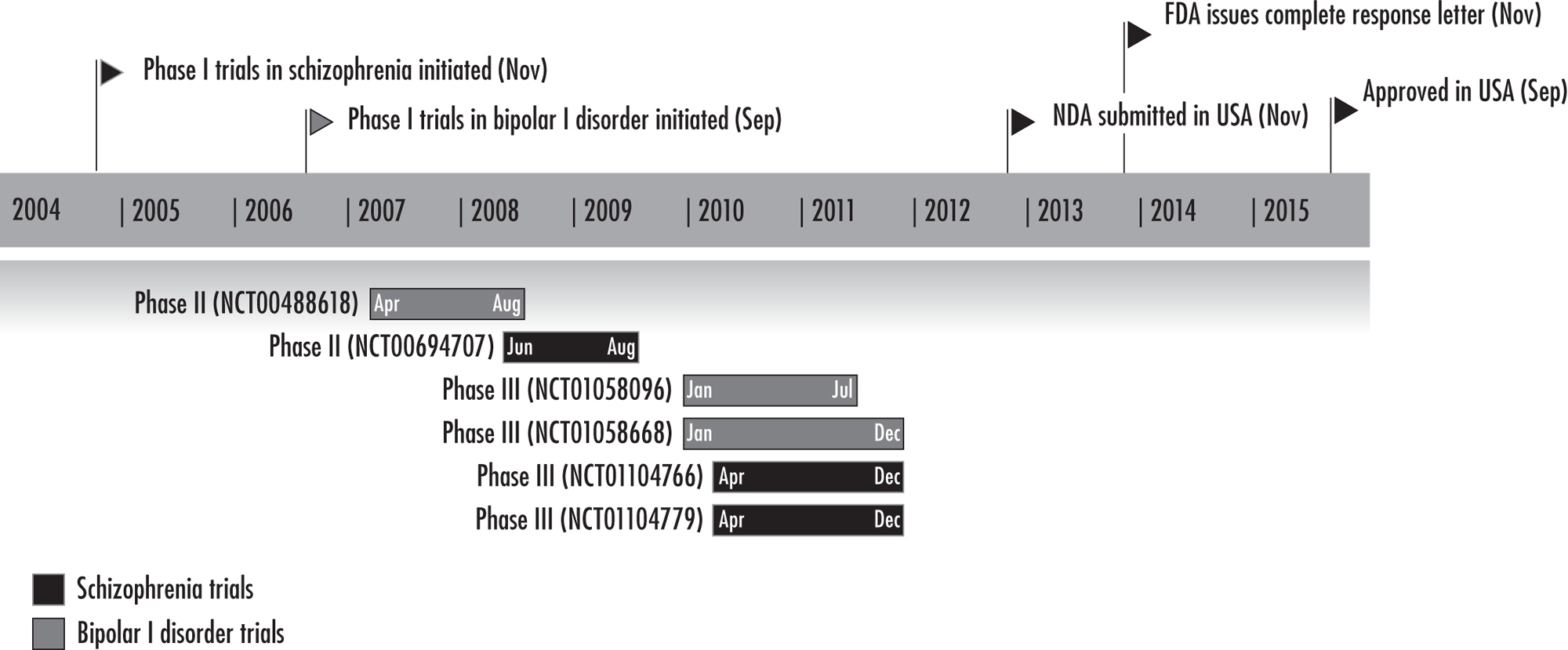
FIGURE 34–1. Key development milestones for cariprazine.
Note. FDA=U.S. Food and Drug Administration; NDA=new drug application.
Source. Reprinted from McCormack PL: “Cariprazine: First Global Approval.” Drugs 75(17):2035–2043, 2015. Copyright © 2015, Springer International Publishing. Used with permission.
Cariprazine, a piperazine/piperidine derivative, is classified as an atypical antipsychotic on the basis of its dopamine receptor partial agonist mechanism. Cariprazine is the third agent with a dopamine receptor partial agonist mechanism to become available (aripiprazole was approved in 2002, and brexpiprazole received FDA approval on July 13, 2015, both for schizophrenia; see Chapter 29 in this volume, “Aripiprazole and Brexpiprazole,” by Gonzalez and Strassnig). In common with aripiprazole and brexpiprazole, cariprazine has partial agonist effects at dopamine 2 (D2) and dopamine 3 (D3) receptors and serotonin 1A (5-HT1A) receptors and antagonist effects at serotonin 2A (5-HT2A) receptors. What is unique about cariprazine is its higher affinity for and more selective binding to D3 receptors compared with other currently marketed typical and atypical antipsychotics.
Comparison With Other Dopamine Receptor Partial Agonists
The theory behind the use of partial agonists, including cariprazine, is that these agents restore homeostatic balance to neurochemical circuits by 1) decreasing the effects of endogenous neurotransmitters (dopamine tone) in regions of the brain where their transmission is excessive, such as in the mesolimbic regions in schizophrenia or mania; 2) simultaneously increasing neurotransmission in regions where transmission of endogenous neurotransmitters is low, such as in the prefrontal cortex in schizophrenia; and 3) exerting little effect in regions where neurotransmitter activity is normal, such as the pituitary gland.
Aripiprazole and brexpiprazole are dopamine D2 receptor–preferring partial agonists, with minimal D3 effects. In contrast, cariprazine has a six- to eightfold greater affinity for D3 receptors than for D2 receptors, with a specificity for the D3 receptor that is 3–10 times greater than aripiprazole’s specificity for that receptor (Mattingly and Anderson 2016).
Aripiprazole binds more potently than cariprazine to human and rat 5-HT2A, 5-HT2C, and adrenergic receptors. In contrast, cariprazine has lesser affinity for human and rat hippocampal 5-HT1A receptors (and demonstrates low intrinsic efficacy), low affinity for human 5-HT2A receptors, moderate or low affinity for histamine H1 and 5-HT2C receptors, and negligible affinity for cholinergic or adrenergic receptors, all of which suggest a reduced propensity for side effects related to these receptors (Kiss et al. 2010).
In addition to its labeled indications for schizophrenia and manic or mixed episodes associated with bipolar I disorder, aripiprazole is approved as an adjunctive treatment for depression or bipolar maintenance, and for irritability associated with autistic disorder. In addition to its approved use in schizophrenia, brexpiprazole has an FDA indication for use as an adjunct to antidepressant treatment in major depressive disorder. Cariprazine has not been directly compared with aripiprazole, brexpiprazole, or other antipsychotic agents in clinical studies of schizophrenia or bipolar I disorder, and it has not yet received approval for indications other than schizophrenia and bipolar I disorder. In contrast to the variety of formulations available for aripiprazole, cariprazine is currently available only in a capsule for oral administration.
Although their mechanisms of action are in some ways similar, the three agents differ in terms of their pharmacodynamic profile and receptor affinities, as summarized in Table 34–1.
Affinity Kia (in vitro) |
||||||||
Receptorb |
Activity |
Cariprazine |
Aripiprazole |
Brexpiprazole |
||||
Dopamine |
D3 |
Partial agonist |
0.085 |
0.8 |
1.1 |
|||
D2L |
Partial agonist |
0.49 |
0.34 |
0.30 |
||||
D2S |
Partial agonist |
0.69 |
||||||
Serotonin |
5-HT1A |
Partial agonist |
2.6 |
1.7 |
0.12 |
|||
5-HT2A |
Antagonist |
18.8 |
3.4 |
0.47 |
||||
5-HT2B |
Antagonist |
0.58 |
0.36 |
1.9 |
||||
5-HT2C |
Antagonist |
134 |
15 |
34 |
||||
5-HT7 |
Antagonist |
111 |
39 |
3.7 |
||||
Histamine |
H1 |
Antagonist |
23.2 |
61 |
19 |
|||
Adrenergic |
α1A |
Antagonist |
155 |
57 |
15 |
|||
aReceptor binding affinity Ki [nM], inverse relationship. bCariprazine and aripiprazole demonstrated negligible (percentage displacement less than 20% at 1 μM test concentration) affinities for adrenergic (α2B, α2C), cannabinoid (CB1, CB2), dopamine (D1, D4.2, D5), histamine (H2, H3, H4), muscarinic (M1, M2, M3, M4, M5), nicotinic, and serotonin (5-HT3, 5-HT4, 5-HT5A, 5-HT6) receptors (Kiss et al. 2010). Source. Adapted from Citrome L: “The ABC’s of Dopamine Receptor Partial Agonists—Aripiprazole, Brexpiprazole and Cariprazine: The 15-Min Challenge to Sort These Agents Out.” International Journal of Clinical Practice 69(11):1211–1220, 2015. Copyright © 2015, John Wiley & Sons Ltd. Used with permission. |
||||||||
Pharmacodynamics
Structure
Cariprazine, also known as RGH-188 and MP-214, is 3-[4-{2-[4-(2,3-dichlorophenyl)piperazin-1-yl]ethyl}cyclohexyl]-1,1-dimethylurea (Figure 34–2). The active ingredient is cariprazine hydrochloride, a compound synthesized and selected for development on the basis of its high selectivity for dopamine D3 receptors over D2 receptors (Veselinovic et al. 2013). Cariprazine has two clinically relevant active metabolites: desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR) (Citrome 2013b).
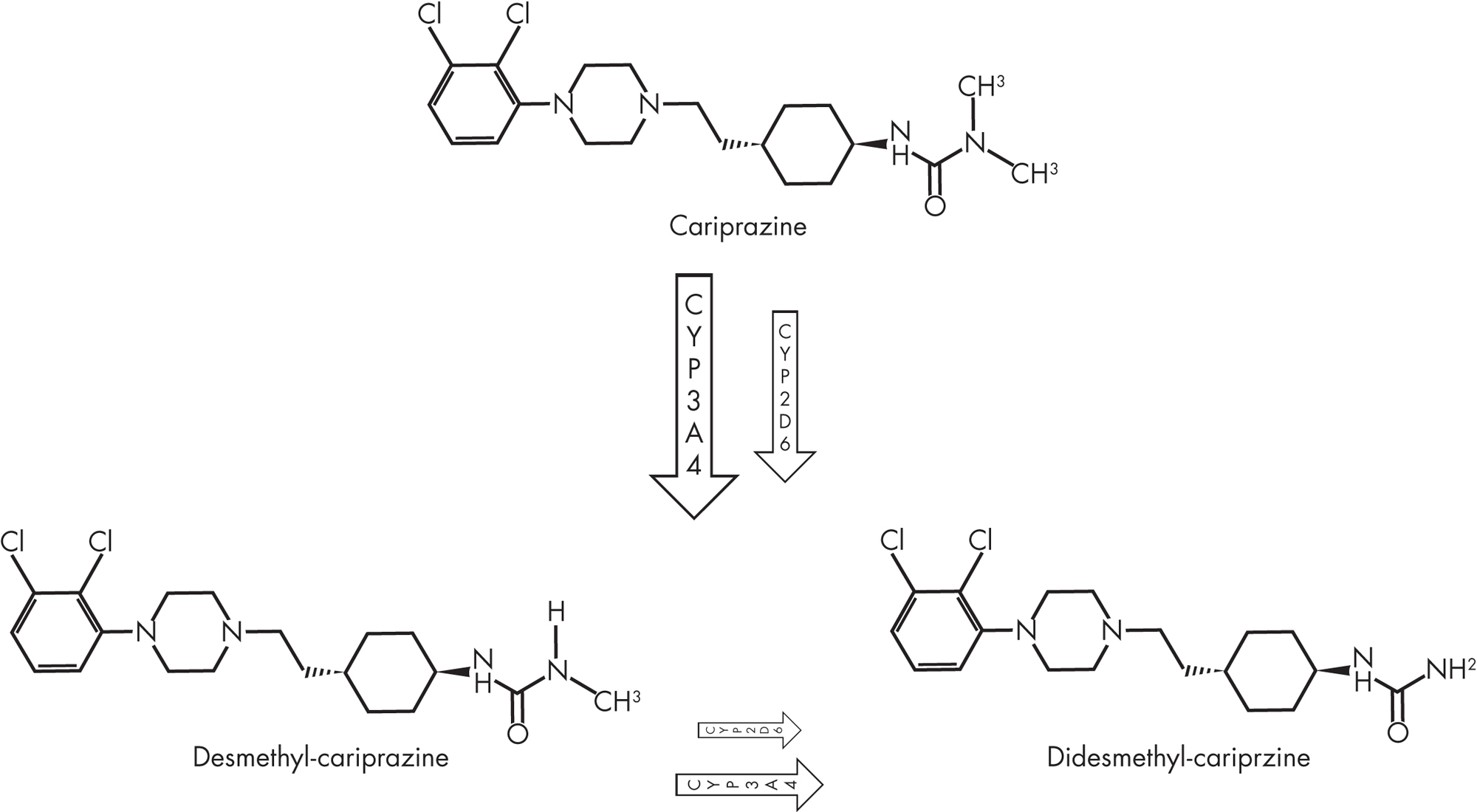
FIGURE 34–2. Chemical structures of cariprazine and its major metabolites, desmethyl-cariprazine (DCAR) and didesmethyl-cariprazine (DDCAR).
Receptor-Binding Profile
Cariprazine is a potent dopamine D3 and D2 receptor partial agonist with preferential binding to D3 receptors, which is a unique pharmacological profile among known antipsychotic medications (Kiss et al. 2010).
Whereas D2 receptor antagonism is required for antipsychotic efficacy, D3 receptor antagonism may impart beneficial effects on cognition while attenuating the risk of extrapyramidal side effects (EPS) (Veselinovic et al. 2013).
Cariprazine acts as a partial agonist at dopamine D3 and D2 receptors with very high binding affinity and at serotonin 5-HT1A receptors with high binding affinity (see Table 34–1). Cariprazine acts as an antagonist at serotonin 5-HT2B and 5-HT2A receptors with very high and moderate binding affinity, respectively, and at histamine H1 receptors with moderate binding affinity. Cariprazine shows weak binding affinity for serotonin 5-HT2C and 5-HT7 receptors, very low affinity for α1A-adrenergic receptors, and no appreciable affinity for cholinergic muscarinic receptors (Actavis Inc. 2015).
Dopamine Receptors
D3 and D2. Dopamine, the neurotransmitter primarily linked to psychosis, has very high affinity for D3 receptors. In vitro, virtually all of the first- and second-generation antipsychotics in clinical use are D2 nonselective and therefore show considerable affinity for the D3 receptor subtype. D3 receptors have pre- and postsynaptic localizations in brain stem nuclei, limbic parts of the striatum, and cortex and exert a widespread influence on dopamine release, on dopaminergic function, and on several other neurotransmitters. The D2 receptor’s assumed role as an autoreceptor is suggested by its high expression in regions such as the ventral tegmental area and substantia nigra.
The signaling pathways of D3 receptors are distinct from those of other members of the D2-like receptor family. Although the role of D3 receptor blockade in alleviating positive symptoms is still controversial, selective D3 receptor antagonism has therapeutic effects in schizophrenia and beyond, as demonstrated by several animal models: improved cognitive function, emotional processing, executive function, flexibility, and social behavior. D3 receptor antagonism seems to contribute to the atypicality of antipsychotics by reducing motor EPS; has no direct influence on prolactin release; and does not cause anhedonia, weight gain, or metabolic dysfunction (Gross and Drescher 2012).
D3, an autoreceptor primarily located in the limbic system, controls phasic (not tonic) activity of dopamine nerve cells and mediates behavioral abnormalities induced by glutamate and N-methyl-D-aspartate receptor antagonists (Veselinovic et al. 2013).
Animal models and preclinical studies have demonstrated antipsychotic-like and procognitive effects potentially attributable to dopamine D3 receptor–preferring agents. D3 receptor blockade appears to enhance—whereas D3 receptor agonism seems to impair—cognitive function in animals (Gyertyán et al. 2011).
The overall function of dopamine D3 receptors in the human brain remains incompletely understood.
Serotonin Receptors
5-HT1A. Cariprazine has a high affinity for human serotonin 5-HT1A receptors and acts as a partial agonist at these receptors. The effect of cariprazine depends on the serotonin concentration at the site.
In vivo, at lower dosages, cariprazine’s partial agonism at 5-HT1A receptors may contribute minimally to its antipsychotic-like activity and side-effect profile; at higher dosages, the 5-HT1A receptor partial agonism may contribute to cariprazine’s favorable side-effect profile (i.e., lack of EPS) (Kiss et al. 2010).
Cariprazine’s 5-HT1A receptor partial agonism may also contribute to its antidepressant efficacy (Blier and Ward 2003) and to its beneficial effects on negative symptoms and cognitive dysfunction (Bantick et al. 2001), as suggested by preclinical studies.
5-HT2B. Cariprazine has very high binding affinity for human serotonin 5-HT2B receptors (Ki=0.58 nM). Activity at these receptors may modulate dopamine release in the nucleus accumbens (Citrome 2013a).
Cariprazine’s antagonist activity at 5-HT2B receptors may contribute to its postulated effects on mood and cognition, as observed in preclinical studies.
3-HT2A. Cariprazine’s binding affinity for human serotonin 5-HT2A receptors is lower than that of aripiprazole and brexpiprazole. Theoretically, antagonist effects at serotonin 5-HT2A receptors are essential for “atypicality” of antipsychotics. It is unknown whether cariprazine’s relatively low affinity for 5-HT2A receptors has any clinical implications.
Histamine H1 Receptors
Cariprazine’s moderate affinity for H1 receptors is roughly equivalent to the affinity of aripiprazole and brexpiprazole for these receptors. Sedation and orthostatic hypotension with high dosages of cariprazine might be related to this property.
Positron Emission Tomography Studies
Although several marketed antipsychotics have shown moderate in vitro affinity for the D3 receptor, positron emission tomography (PET) studies have suggested that these compounds do not demonstrate D3 receptor occupancy in vivo at therapeutic dosages (Girgis et al. 2016).
Three PET studies have demonstrated cariprazine’s excellent brain penetration, showing high and dose-dependent in vivo occupancy of both dopamine D3 and D2 receptors in rodents (Gyertyán et al. 2011), in monkeys (Seneca et al. 2011), and in patients with schizophrenia (Girgis et al. 2016).
In a Phase I open-label three-cohort study, patients with schizophrenia (n=3) received multiple-dose cariprazine treatment for 15 days. Cariprazine treatment resulted in high D2 and D3 receptor occupancy at all dosages (range, 0.5–12.0 mg/day) and conditions (i.e., acute [day 1], day 4/5, and subchronic [day 15]). A clear dose–occupancy relationship existed for acute, day 4/5, and subchronic treatment, with the highest occupancy for both D2 and D3 receptors observed with the highest dosage (12 mg/day).
For dosages of 12 mg/day, 3 mg/day, and 1 mg/day, respectively, the mean D3 receptor occupancies were 99%, 92%, and 76%, and the mean D2 receptor occupancies were 95%, 79%, and 45%.
For acute (day 1/4) dosing, the median effective dose (ED50) was 1.52 mg for D3 receptor occupancy and 1.84 mg for D2 occupancy, with a D3/D2 selectivity ratio range of 1.21–1.31. For subchronic [day 15] dosing, the ED50 was 0.30 mg for D3 receptor occupancy and 1.03 mg for D2 occupancy, with a D3/D2 selectivity ratio range of 3.43–5.75.
Cariprazine showed high and dose-dependent in vivo occupancy of both dopamine D3 and D2 receptors in patients with schizophrenia. After subchronic (15-day) administration, cariprazine showed a threefold greater preference for D3 versus D2 receptors. The increased D3/D2 selectivity after subchronic dosing compared with acute dosing may be related to the pharmacological activity of cariprazine’s metabolites (Girgis et al. 2016).
Pharmacokinetics
In a Phase II double-blind, placebo-controlled tolerance study, the pharmacokinetic properties of cariprazine were similar in healthy volunteers and schizophrenia patients, and there were no significant clinical changes in pharmacokinetic properties by age, sex, or race/ethnicity (Kapás et al. 2008).
In premarketing studies investigating cariprazine’s tolerability in specific populations, patients with either mild or moderate hepatic impairment (Child-Pugh score between 5 and 9), in comparison with healthy subjects, had approximately 25% higher exposure (peak serum concentration [Cmax] and AUC [area under the concentration curve]) to the parent drug, and approximately 45% lower exposure to the major active metabolites (DCAR and DDCAR), following a single daily dose of 1 mg cariprazine or 0.5 mg cariprazine for 14 days). Pharmacokinetic analyses in patients with renal impairment showed no significant relationship between plasma clearance and creatinine clearance (Actavis Inc. 2015).
Absorption and Distribution
Cariprazine can be taken with or without food. Food causes a slight delay but has no significant effect on the extent of absorption (Citrome 2013a).
The effect of a meal on the pharmacokinetics of cariprazine was evaluated in an open-label randomized crossover study in 12 healthy male volunteers who received a single oral dose of 2 mg. Tmax was observed at 3–4 hours under fasting conditions. Previous consumption of a meal delayed absorption of the drug but did not affect the final extent of absorption. The plasma profile of cariprazine followed a multi-exponential disposition, with a terminal half-life of 5–6 days (Veselinovic et al. 2013).
Peak serum concentrations are reached between 3 and 6 hours. There is a linear relationship between dose and plasma concentration. Cariprazine and its major active metabolites are highly bound (91%–97%) to plasma proteins (Actavis Inc. 2015). Cariprazine and DCAR reach steady-state concentrations within 1–2 weeks (Figure 34–3); by contrast, DDCAR requires 4–8 weeks of daily cariprazine administration to reach steady-state levels.
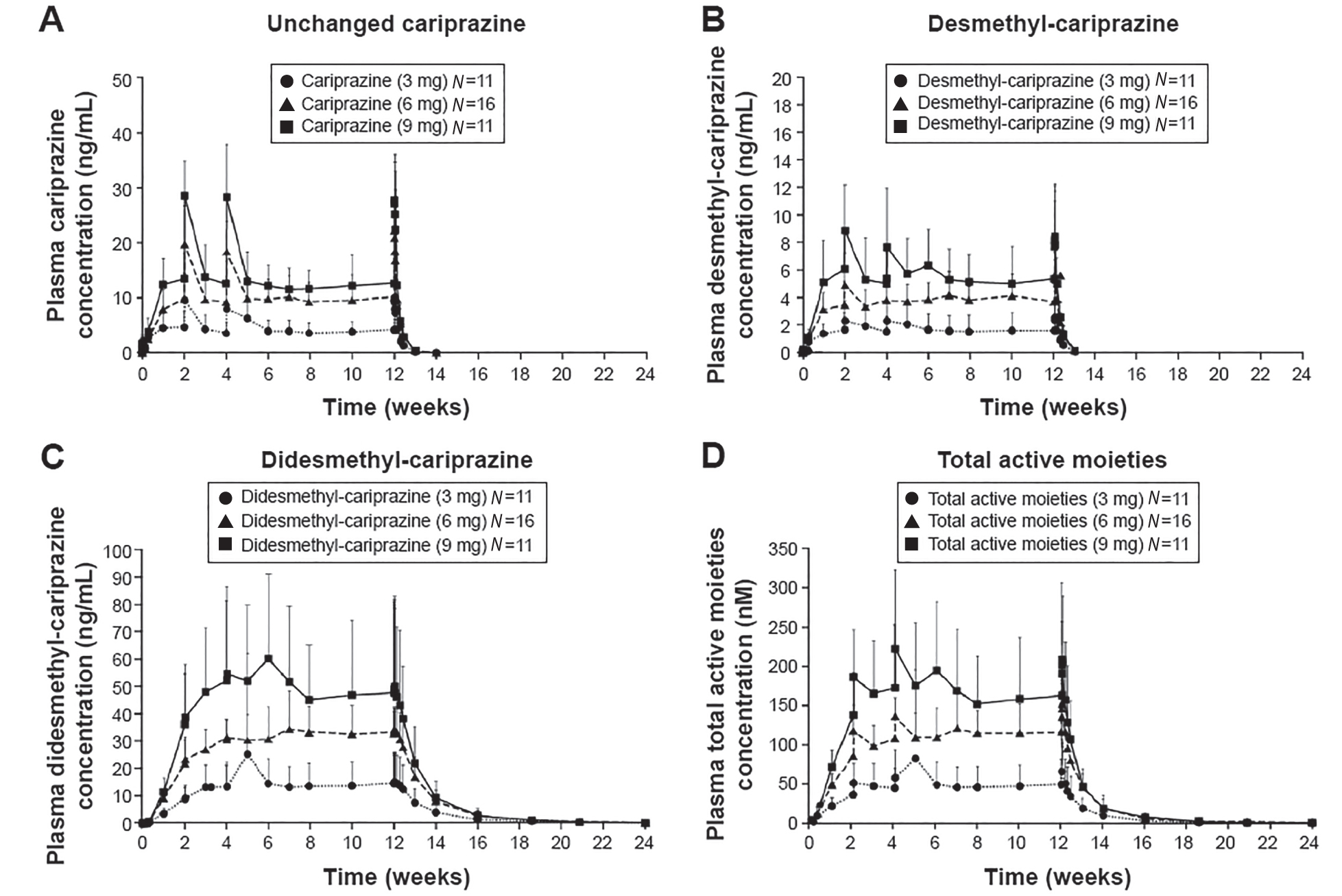
FIGURE 34–3. Plasma concentrations of cariprazine (A), desmethyl-cariprazine (B), didesmethyl-cariprazine (C), and total active moieties (sum of cariprazine, desmethyl-cariprazine and didesmethyl-cariprazine) (D) for each dosage group during the treatment and follow-up periods.
Note. Data are mean ± standard deviation. The lower limit of quantification of analytes was 0.02 ng/mL. The mean was not plotted when at least half of the results were below the lower limit of detection except at time=0.
Source. Reprinted from Nakamura T, Kubota T, Iwakaji A, et al.: “Clinical Pharmacology Study of Cariprazine (MP-214) in Patients With Schizophrenia (12-Week Treatment).” Drug Design, Development and Therapy 10:327–338, 2016. Copyright © 2016, Dove Medical Press Ltd. Used with permission.
Effective half- life (calculated from time to steady state) of total active moieties was ±1 week. Terminal half-lives of cariprazine, DCAR, and DDCAR range from 31.6 to 68.4, 29.7 to 37.5, and 314 to 446 hours, respectively (Nakamura et al. 2016).
The mean half-life of cariprazine is 2–5 days over a dosage range of 1.5–12.5 mg/day, which is considerably longer (days) than the mean half-life in laboratory animal studies (hours in rats and dogs) (Mészáros et al. 2008).
On the first day of dosing, systemic exposure to the metabolites (DCAR and DDCAR) was relatively low compared with exposure to the parent drug. However, on day 37, with the 12.5-mg/day dosage, systemic exposure to DDCAR was threefold greater than that to cariprazine for the AUC over 24 hours and sixfold greater for the AUC over 7 days, indicating a slower elimination and a substantially longer half-life for this metabolite than for the parent compound (Veselinovic et al. 2013).
After initiating cariprazine therapy or changing the dosage, response and side effects should be monitored for several weeks because of the long half-life of cariprazine’s active metabolite DDCAR.
Metabolism and Elimination
Cariprazine is extensively metabolized by hydroxylation and demethylation in the liver cytochrome P450 (CYP) enzyme system, mainly by the 3A4 enzyme and to a lesser extent by the 2D6 enzyme. Eight metabolites for cariprazine were identified in liver microsomes, but only two active metabolites, DCAR and DDCAR, were clinically relevant (equipotent to cariprazine with similar receptor partial agonism) (Veselinovic et al. 2013).
Cariprazine’s main metabolite, DCAR, is further metabolized by CYP3A4 and CYP2D6 to DDCAR, which is metabolized by CYP3A4 to a hydroxylated metabolite (Actavis Inc. 2015).
Cariprazine and DCAR levels had decreased by more than 90% at 1 week after the last dose, whereas DDCAR levels had decreased by approximately 50% at 1 week; total active moieties had decreased by approximately 90% at 4 weeks. Cariprazine and its metabolites were eliminated almost completely in the 12 weeks following the last cariprazine dose (Nakamura et al. 2016).
Following administration of cariprazine 12.5 mg/day to patients with schizophrenia for 27 days, about 21% of the daily dosage was found in urine, with approximately 1.2% of the daily dosage excreted in urine as unchanged cariprazine (Actavis Inc. 2015).
Drug–Drug Interactions
Cariprazine and its major active metabolites are weak competitive inhibitors of the human CYP2D6 and CYP3A4 isozymes. They have no induction effect on the CYP enzyme system in human hepatocytes. Cariprazine is a weak inhibitor of a number of CYP450 isoenzymes in vitro: 1A2, 2C9, 2D6, 3A4, 2C19, 2A6, and 2E1.
Because cariprazine is highly metabolized by CYP3A4 and to some degree by CYP2D6, changes in steady-state plasma concentrations would be expected if the drug were coadministered with potent CYP3A4 inhibitors or inducers. However, CYP2D6 inducers or inhibitors are not expected to have this effect on cariprazine metabolism, because CYP2D6 is known to be a poor metabolizer of cariprazine and its metabolites.
Taking a strong CYP3A4 inhibitor (e.g., ketoconazole, fluoxetine, grapefruit juice) with cariprazine will increase concentrations of the parent drug by about 3.5-fold, decrease DCAR by about one-third, and increase DDCAR by about 1.5-fold. The concomitant use of CYP3A4 inducers with cariprazine has not yet been evaluated. When cariprazine is to be added to an existing regimen of a strong CYP3A4 inhibitor, the recommended starting dosage is 1.5 mg every other day, with a maximum dosage of 3 mg/day (Actavis Inc. 2015).
The absence of any significant effect on the CYP system permits cariprazine to be used in combination with other psychotropics if needed clinically without significant drug–drug interactions. No such combination studies have been published to date.
Indications, Dosing, and Efficacy
Cariprazine has a half-life greater than 24 hours, which allows once-daily dosing, an advantage in terms of antipsychotic adherence. Cariprazine has a relatively broad therapeutic index, in that dosages in the range of 0.5–12.5 mg/day are well tolerated. The minimum cariprazine starting dosage is 1.5 mg/day. The minimum therapeutic dosage for schizophrenia is 1.5 mg/day, and that for bipolar disorder is 3 mg/day; however, the maximum recommended target dosage is 6 mg/day for both illnesses. Any dosage changes will be reflected fully in the serum level 2 weeks after initial dosing. In short-term controlled trials, dosages above 6 mg/day did not confer increased effectiveness sufficient to outweigh dose-related adverse reactions. The use of cariprazine in geriatric or pediatric patients has not been studied (Actavis Inc. 2015).
FDA-Approved Indications
Schizophrenia
In the treatment of patients with schizophrenia, the recommended dosage of cariprazine is 1.5–6 mg/day. The recommended starting dosage is 1.5 mg/day, which can be increased to 3 mg/day on day 2, with further upward dosage increments of 1.5 or 3 mg/day as necessary, depending on clinical response and tolerability.
FDA approval for the schizophrenia was based on data from three positive 6-week double-blind, randomized controlled studies of cariprazine that were conducted in adult patients with schizophrenia between 2008 and 2011. Two of the studies used fixed dosages and included active drug comparators (risperidone and aripiprazole) and placebo. All three trials consisted of a washout period of up to 1 week, 6 weeks of double-blind treatment, and a 2-week safety follow-up. The primary efficacy measure in each study was change from baseline in Positive and Negative Syndrome Scale (PANSS) Total score; assessments were conducted at screening, at baseline, and at the end of each double-blind treatment week (weeks 1–6) (Table 34–2).
Triala |
Randomization (N) |
Efficacyb |
Side effects |
NCT00694707 RGH-MD-16 Phase II |
Total N: 732 64% completed the study Placebo (151) Cariprazine fixed dosages: 1.5 mg/day (145) 3 mg/day (147) 4.5 mg/day (148) Active control: Risperidone 4 mg/day (141)c |
At week 6, statistically significant (P<0.001 [LOCF]) LSMD in favor of cariprazine versus placebo were observed for
Risperidone was superior to placebo on both measures (LSMD: PANSS=−15.1, P<0.001 [LOCF; CGI-S=−0.8, P<0.05 [LOCF]). |
The most frequent cariprazine adverse events (≥5% and twice the rate of placebo) were akathisia, extrapyramidal disorder, insomnia, sedation, nausea, dizziness, and constipation. Mean changes in metabolic parameters were small and similar between groups. |
NCT01104766 RGH-MD-04 Phase III |
Total N: 617 Placebo (153) Cariprazine fixed dosages: 3 mg/day (155) 6 mg/day (157) Active control: Aripiprazole 10 mg/day (152)c |
At week 6, statistically significant LSMD in favor of cariprazine versus placebo were observed for
Aripiprazole was superior to placebo on both measures (LSMD: PANSS–Total=−7.0, P=0.0008; CGI-S=−0.4, P=0.0001). |
Akathisia was reported (≥5%; twice the rate of placebo). Changes in metabolic parameters were small and similar to those with placebo. |
NCT01104779 RGH-MD-05 Phase III |
Total N: 446 60.5% completed the study Placebo (147) Cariprazine flexible dosing: 3–6 mg/day (151) 6–9 mg/day (148) No active control group |
At week 6, statistically significant LSMD in favor of cariprazine versus placebo were observed for
|
The most frequent cariprazine adverse events (≥5%; twice the rate of placebo) in both cariprazine groups were akathisia, extrapyramidal disorder, and tremor; most were mild to moderate in severity. Mean changes in metabolic parameters were generally small and similar between groups. Prolactin levels decreased in all groups. |
Note. CGI-S=Clinical Global Impression–Severity; LOCF=last observation carried forward; LSMD=least squares mean differences; PANSS=Positive and Negative Syndrome Scale. aTrial registration: ClinicalTrials.gov identifiers NCT00694707, NCT01104766, and NCT01104779. bIn each study, the PANSS was used as the primary measure and the CGI-S rating scale was used as the secondary efficacy measure. The primary endpoint was change from baseline in PANSS total score at the end of week 6. The change from baseline for cariprazine and for all active control groups was superior to placebo in all three trials. cThe active control medications (aripiprazole and risperidone) were included for use in assessment of assay sensitivity and not for efficacy comparisons. However, they were superior to placebo. |
|||
As indicated in Table 34–2, patients were randomly assigned to receive cariprazine (1.5 mg/day, 3 mg/day, or 4.5 mg/day); risperidone (4 mg/day); or placebo in the NCT00694707 study; cariprazine (3 mg/day or 6 mg/day), aripiprazole (10 mg/day), or placebo in the NCT01104766 study; and cariprazine (3–6 mg/day or 6–9 mg/day) or placebo in the NCT01104779 study. The efficacy of cariprazine compared with placebo was demonstrated at dosages ranging from 1.5 to 9 mg/day. There was, however, a dose-related increase in certain adverse effects (e.g., EPS), particularly at dosages above 6 mg/day. Findings on the time course of efficacy in the NCT01104766 study are shown in Figure 34–4.
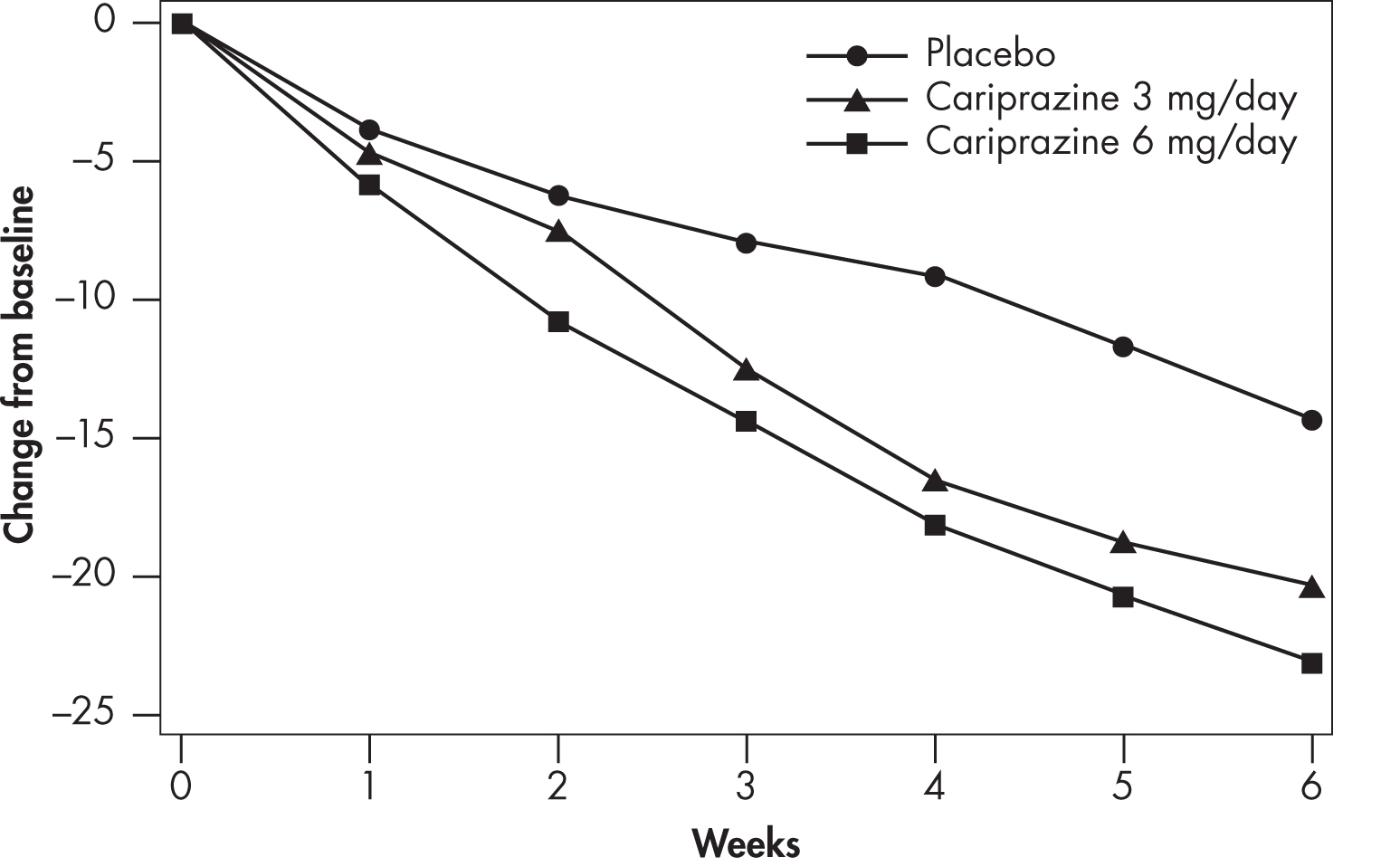
FIGURE 34–4. Change from baseline in Positive and Negative Syndrome Scale (PANSS) Total score, by weekly visit (Study ID: NCT01104766).
Source. Actavis Inc. 2015.
In Phase III clinical trials, dosages of 3–9 mg/day produced significant improvement on the PANSS and on the Clinical Global Impression scale. Higher dosages (6–9 mg/day) showed early separation from placebo (by the end of week 1) but carried a dosage-related risk of adverse events, leading the FDA to recommend 6 mg/day as the maximum dosage (Mattingly and Anderson 2016).
The effects of cariprazine on human cognition were evaluated in the NCT 01104779 study (weeks 0, 3, and 6). No significant differences were observed for the cariprazine group versus the placebo group on the Cognitive Drug Research System Attention Battery Tests or the Color Trails Test (Kane et al. 2015).
Data on direct comparisons of clinical efficacy between cariprazine and aripiprazole or risperidone are not yet available (Veselinovic et al. 2013).
A recent 6-week double-blind, placebo-controlled proof-of-concept study (NCT00404573) evaluated the efficacy, safety, and tolerability of low-dosage (1.5–4.5 mg/day) versus high-dosage (6–12 mg/day) cariprazine in patients with schizophrenia. Surprisingly, no significant differences between the two dosages of cariprazine and placebo were noted on PANSS Total score changes or any other efficacy parameters (Clinical Global Impression–Severity [CGI-S] scale or PANSS subscales) after multiplicity adjustment (Durgam et al. 2016b).
Acute Manic or Mixed Episodes Associated With Bipolar I Disorder
Cariprazine’s efficacy in the treatment of bipolar manic or mixed episodes was established at dosages ranging from 3 to 12 mg/day. Dosages above 6 mg/day did not appear to add additional benefit over lower dosages, and there was a dose-related increase in certain adverse effects (e.g., EPS). Therefore, the maximum recommended dosage is 6 mg/day. Findings on the time course of efficacy in the NCT01058668 study are shown in Figure 34–5 (Vraylar 2015).
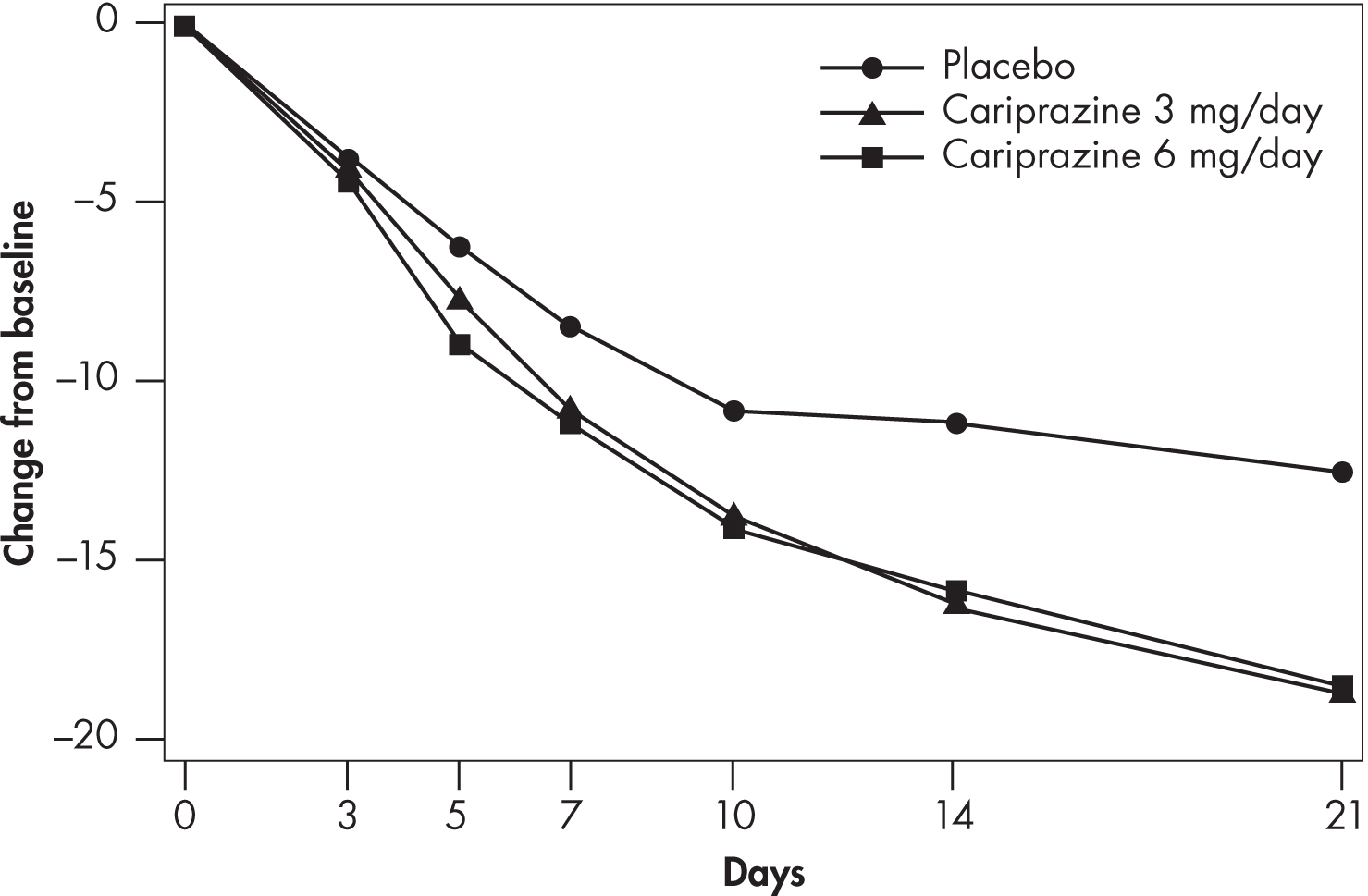
FIGURE 34–5. Change from baseline in Young Mania Rating Scale (YMRS) Total score, by study visit (Study ID: NCT01058668).
Source. Actavis Inc. 2015.
FDA approval for the bipolar disorder indication was based on data from three 3-week placebo-controlled trials in adults with manic or mixed episodes of bipolar I disorder with or without psychotic features. All three studies demonstrated cariprazine’s superiority over placebo (Table 34–3).
Triala |
Randomization (N) |
Efficacyb |
Side effects |
NCT01058668 Phase III |
Total N: 497 74% completed the study Cariprazine flexible dosing: 3–6 mg/day (169) 6–9 mg/day (167) Placebo (161) |
At week 3, statistically significant LSMD in favor of cariprazine versus placebo were observed for
|
The most common (≥5%; twice the rate of placebo) treatment-related adverse events for cariprazine were akathisia (both groups) and nausea, constipation, and tremor (6–12 mg/day only). |
NCT00488618 Phase II |
Total N: (235) 61.9% of placebo group and 63.6% of cariprazine group completed the study Cariprazine flexible dosing: 3–12 mg/day (118) Placebo (117) |
At week 3, statistically significant LSMD in favor of cariprazine versus placebo were observed for
|
The most common adverse events (>10% for cariprazine) were akathisia (cariprazine: 22%; placebo: 6%), extrapyramidal symptoms (parkinsonism) (cariprazine: 16%; placebo: 1%), headache, constipation, nausea, and dyspepsia. Changes in metabolic parameters were similar between groups, with the exception of fasting glucose change. |
NCT01058096 Phase III |
Total N: 310 68.4% completed the study Cariprazine flexible dosing: 3–12 mg/day (158) Placebo (152) |
At week 3, statistically significant LSMD in favor of cariprazine versus placebo were observed for
|
The most common cariprazine-related (>10%; twice the rate of placebo) treatment-emergent adverse events were akathisia, extrapyramidal disorder, tremor, dyspepsia, and vomiting. Mean changes from baseline in metabolic parameters were generally small and similar between groups. |
Note. CGI-S=Clinical Global Impression–Severity; LSMD=least squares mean differences; YMRS–Total=Young Mania Rating Scale total score. aTrial registration: ClinicalTrials.gov identifiers NCT01058668, NCT00488618, and NCT01058096. bIn all trials, patients were adults (ages 18–65 years; mean age=39 years) whose symptoms met DSM-IV-TR criteria for bipolar I disorder with manic or mixed episodes and with or without psychotic features (YMRS score≥20). YMRS and CGI-S were used as the primary and secondary efficacy measures, respectively; the primary endpoint was decrease from baseline in YMRS–Total score at the end of week 3. The change from baseline for cariprazine was superior to placebo in all three trials. All three trials lacked an active comparator arm and had a short duration. |
|||
As indicated in Table 34–3, patients were randomly assigned to receive flexible dosages (3–12 mg/day) of cariprazine or placebo. No active comparator arm was included.
Possible Future Indications
Bipolar Depression
Two studies failed to establish cariprazine’s efficacy in patients with bipolar depression. In a Phase II (N=233) 8-week randomized, double-blind, parallel-group, flexible-dose multicenter trial of cariprazine (0.25–0.75 mg/day or 1.5–3.0 mg/day) in bipolar depression, improvement with cariprazine versus placebo did not reach significance on the primary efficacy measure (change from baseline to week 8 on the Montgomery-Åsberg Depression Rating Scale (MADRS) using mixed model repeated measures [MMRM] analyses). The authors speculated that high placebo response may have contributed to the outcome (Ahuja et al. 2011).
In a recent 8-week multinational randomized, double-blind, placebo-controlled, parallel-group, fixed-dose (0.75, 1.5, or 3 mg/day) multicenter study of cariprazine in adults (N=571) experiencing a current major depressive episode in bipolar I disorder, improvement with cariprazine versus placebo did not reach significance on the primary assessment (MADRS change from baseline to week 6 using MMRM analyses). The 0.75 mg/day dosage did not separate from placebo; however, the 1.5 mg/day dosage produced greater improvement on the MADRS total score change compared with the 3 mg/day dosage (Durgam et al. 2016a).
Major Depressive Disorder (Adjunctive Therapy)
A Phase III trial of cariprazine as an adjunct to antidepressant treatment in adult patients with major depressive disorder (NCT01715805) is in progress. In this randomized, double-blind trial, patients will receive oral cariprazine 1.5–4.5 mg/day or placebo for 8 weeks in addition to antidepressant therapy. The primary endpoint is change on the MADRS score at 8 weeks, and the secondary endpoint is change on the Sheehan Disability Scale score at 8 weeks. The trial is recruiting approximately 1,100 patients in the United States and Puerto Rico (McCormack 2015).
Another Phase III extension trial to assess the long-term safety and tolerability of cariprazine as an adjunct to antidepressant therapy in adult patients with major depressive disorder (NCT01838876) is under way. This 26-week open-label, flexible-dose (1.5–4.5 mg/day) study has enrolled 347 patients, and the final data collection point was scheduled for July 2015 (McCormack 2015).
Substance Use Disorders
Cariprazine is being investigated for its potential anti-abuse/relapse-preventing effects in preclinical studies. In a study by Román et al. (2013), cariprazine, as well as aripiprazole and bifeprunox, reduced the rewarding effects of cocaine (minimum effective doses were 0.17, 1.0, and 0.1 mg/kg for cariprazine, aripiprazole, and bifeprunox, respectively) and delayed relapse to cocaine seeking, with ED50 values of 0.2, 4.2, and 0.17 mg/kg, respectively. The behavioral effects of cariprazine were as potent as those of bifeprunox and were more potent than those of aripiprazole (Román et al. 2013).
Hostility
Using pooled data from the three positive randomized controlled trials in schizophrenia conducted between 2008 and 2011, a post hoc analysis was performed to investigate the effect of cariprazine on hostility in patients with schizophrenia (Citrome et al. 2016).
Statistically significant improvement (as assessed by mean change from baseline to week 6 on the PANSS Hostility item) was seen in cariprazine-treated versus placebo-treated patients. Antihostility effects were partially independent of improvement on PANSS Positive symptom items, were independent of sedation effects, and were greater in cariprazine-treated patients with higher versus lower baseline levels of hostility (Citrome et al. 2016).
Side Effects and Toxicology
Cariprazine generally was well tolerated in short-term trials for schizophrenia and bipolar I disorder. No significant anticholinergic or antiadrenergic side effects were documented, because cariprazine has negligible affinity for cholinergic or adrenergic receptors.
Common Treatment-Emergent Adverse Events in Clinical Trials
12-Week Open-Label Study
In a 12-week open-label, fixed-dose (3, 6, or 9 mg/day) study of 38 adult patients with schizophrenia (Nakamura et al. 2016), the overall completion rate was 63.2%; 15.8% of patients discontinued the medication because of adverse events.
Incidence of treatment-emergent adverse events (TEAEs) was 97.4%. No abnormal laboratory values or major differences from baseline in EPS were observed. A total of 37 (97.4%) patients reported at least one TEAE, and 31 (81.6%) patients experienced at least one adverse drug reaction (defined as adverse events for which a causal relationship with cariprazine was determined to be “reasonably possible”). Side effects observed in this study were similar to those reported in previous studies. Across all dosage groups, akathisia was observed in 21% of patients during cariprazine treatment. One case of cataract (deemed mild in severity) was reported (Nakamura et al. 2016).
48-Week Open-Label Studies
In two 48-week studies (N=679) of open-label, flexible-dose cariprazine in adult patients with schizophrenia (NCT01104792, 3–9 mg/day; NCT00839852, 1.5–4.5 mg/day) (reported by Nasrallah et al. 2014), the overall completion rate was 40.1%. The mean duration of treatment with cariprazine (days±SD) was 188.4±136.8; 211 patients (31.1%) were exposed to cariprazine for at least 1 year.
Incidence of TEAEs was 81.4%. TEAEs reported in at least 10% of patients were akathisia (15.5%), insomnia (13.1%), headache (12.7%), and weight gain (10.5%) (Table 34–4). The mean increase in body weight was 2.46 kg. Mean changes from baseline to the end of study in metabolic parameters and other clinical laboratory values, blood pressure, and electrocardiographic parameters were generally small. No patients met criteria for Hy’s law (regarding risk of fatal drug-induced liver injury). Mean prolactin levels decreased from baseline to the end of study. The incidence of treatment-emergent parkinsonism (Simpson-Angus Scale score >3) was 10.7%, and the incidence of treatment-emergent akathisia (Barnes Akathisia Rating Scale score >2) was 17.8%. Ophthalmological testing revealed no significant changes.
TEAE |
n (%) |
Akathisia |
105 (15.5) |
Insomnia |
89 (13.1) |
Headache |
86 (13.1) |
Weight increase |
71 (10.5) |
Anxiety |
58 (8.5) |
Tremor |
47 (6.9) |
Extrapyramidal disorder |
45 (6.6) |
Schizophrenia |
38 (5.6) |
Nausea |
38 (5.6) |
Restlessness |
38 (5.6) |
Dyspepsia |
37 (5.4) |
Nasopharyngitis |
34 (5.0) |
aReported by ≥5% of patients. Source. Reprinted from Nasrallah HA, Cutler AJ, Wang Y, et al.: “P.3.d.025 Safety and Tolerability of Cariprazine in Long-Term Treatment of Schizophrenia: Integrated Summary of Safety Data” (poster & abstract). European Neuropsychopharmacology 24 (Suppl 2):S536, 2014, Table 2. Copyright © 2014, Elsevier. Used with permission. |
|
Serious adverse events were reported in 79 patients (11.6%), including one death (suicide) during the open-label treatment period. The most common serious adverse events were worsening of schizophrenia (4.4%) and psychotic disorder (2.1%) (Nasrallah et al. 2014).
16-Week Open-Label Study
In a 16-week study (N=402) of open-label, flexible-dose cariprazine 3–12 mg/day in adult patients with bipolar mania (Ketter et al. 2013), the overall completion rate was 33%; 16% of patients discontinued the medication because of adverse events. The mean treatment duration was 57.7 days, and the mean cariprazine dosage was 6.2 mg/day. No deaths were reported.
Incidence of TEAEs was 83%. TEAEs reported in at least 10% of patients were akathisia (33%), headache (17%), constipation (11%), and nausea (10%); overall, EPS-related TEAEs were reported in 46% of patients. Suicidal ideation and behavior (assessed with the Columbia Suicide Severity Rating Scale) occurred in 9% and 1% of patients, respectively; suicidal ideation and suicide attempt occurred in 4 and 3 patients, respectively. The mean body weight increase was <1 kg; 9% of patients had ≥7% weight gain. Mean changes in laboratory values, vital signs, electrocardiogram results, and ophthalmology parameters were generally small. Cariprazine treatment was not associated with an increase in prolactin levels.
Serious adverse events occurred in 8% of patients; most serious adverse events were associated with worsening of mania, depression, or akathisia. The most common adverse events leading to discontinuation were akathisia (5%) and depression (2%) (Ketter et al. 2013).
Atypical Antipsychotic Class Warnings
Cariprazine has not been approved for use in the treatment of pediatric or geriatric patients.
Numerous risks are associated with the use of atypical antipsychotic medications in general. As a member of this class, cariprazine carries the following risks (Actavis Inc. 2015):
Increased mortality in elderly patients with dementia-related psychosis
Increased risk of cerebrovascular adverse reactions, including stroke, in elderly patients with dementia-related psychosis
Neuroleptic malignant syndrome
Tardive dyskinesia
Leukopenia, neutropenia, and agranulocytosis
Seizures
Potential for cognitive and motor impairment
Body temperature dysregulation
Dysphagia
The boxed warnings are based on the pharmacological actions of this class. No such effects were observed in the cariprazine studies, possibly because of the short lengths of the trials.
Late-Occurring Adverse Drug Reactions
Cariprazine has a long half-life, but the half-lives of its major metabolites are even longer. Therefore, plasma levels accumulate over time, and side effects may appear weeks after the initiation of treatment. As a result, the incidence of adverse reactions in short-term trials may not reflect the rates after longer-term exposure. Clinicians are advised to monitor for adverse reactions for several weeks after starting a patient on cariprazine and after any dosage increase. If side effects are noted, dosage reduction or medication discontinuation should be considered.
Akathisia
Across clinical trials for both FDA-approved disorders, akathisia and parkinsonism were among the more common side effects of cariprazine. Both were usually mild, resulting in relatively few premature discontinuations. Parkinsonism appeared to be somewhat dosage-related, whereas akathisia had no clear relationship to dosage (Mattingly and Anderson 2016).
Changes in Metabolic and/or Clinical Laboratory Parameters
In the pooled analysis of safety and tolerability data from the two open-label long-term studies discussed earlier (Nasrallah et al. 2014; see “48-Week Open-Label Studies” subsection), mean changes from baseline in plasma lipid levels during long-term cariprazine treatment were small and not clinically meaningful (Table 34–5). The mean increase in fasting glucose (4.5 mg/dL) was similar to that observed during the 6-week lead-in studies. Mean change from baseline in weight was 1.6 kg. A potentially clinically significant increase in body weight was seen in 27% of patients; a potentially clinically significant decrease was seen in 11% of patients. Mean prolactin levels decreased during long-term cariprazine treatment (see Table 34–5). Mean alanine transaminase and aspartate transaminase values increased slightly. Creatine kinase levels increased slightly during open-label treatment; however, the large standard deviation indicates that the data were highly variable (Nasrallah et al. 2014).
Mean change (SD) |
|
Metabolic laboratory parameters |
|
Total cholesterol, mg/dL |
−5.3 (31.1) |
LDL cholesterol, mg/dL |
−3.5 (26.4) |
HDL cholesterol, mg/dL |
−0.8 (11.3) |
Triglycerides, mg/dL |
1.2 (87.2) |
Fasting glucose, mg/dL |
4.5 (23.2) |
Body weight |
|
Body weight, kg |
1.6 (5.4) |
Waist circumference, cm PCS changes (≥7%) in body weight, %: ≥7% increase from baseline: 27.2 ≥7% decrease from baseline: 10.9 |
1.6 (7.4) |
Prolactin |
|
Prolactin, ng/mL |
−15.4 (39.6) |
Creatine kinase |
|
Creatine kinase, U/L |
18.5 (264.0) |
Liver function |
|
ALT, U/L |
2.4 (27.8) |
AST, U/L |
0.5 (15.5) |
Total bilirubin, mg/dL |
0.02 (0.28) |
Note. ALT=alanine aminotransferase; AST=aspartate aminotransferase; PCS=potentially clinically significant; SD=standard deviation. Source. Reprinted from Nasrallah HA, Cutler AJ, Wang Y, et al.: “P.3.d.025 Safety and Tolerability of Cariprazine in Long-Term Treatment of Schizophrenia: Integrated Summary of Safety Data” (poster & abstract). European Neuropsychopharmacology 24 (Suppl 2):S536, 2014, Tables 4 and 5. Copyright © 2014, Elsevier. Used with permission. |
|
In schizophrenia clinical trials that included active control medications, the proportion of patients with potentially significant (≥7%) weight gain was greater for aripiprazole and risperidone than for cariprazine (Durgam et al. 2014).
High Dosage and Overdose
In clinical trials, the 12.5 mg/day dosage was well tolerated in most cases. Several cases of overdose were reported (Trials RGH-PK-15 and RGH-MD-36), at dosages of up to 48 mg/day in one accidental overdose, but in none of those cases was more than supportive measures required (Citrome 2013a, 2013b).
Use During Pregnancy and Lactation
There have been no well-controlled studies of cariprazine in pregnant women, but neonates whose mothers have been exposed to other antipsychotic medications during the third trimester of pregnancy are at risk for extrapyramidal and withdrawal symptoms following delivery.
Administration of cariprazine to rats during the period of organogenesis caused malformations, reduced pup survival, and developmental delays at drug exposures less than the human exposure at the maximum recommended human dosage (MRHD) of 6 mg/day. However, cariprazine was not teratogenic in rabbits at doses up to 4.6 times the MRHD of 6 mg/day. Based on animal data, cariprazine may cause fetal harm. Pregnant women should be advised of the potential risk to the fetus (Actavis Inc. 2015).
Ongoing Cariprazine Trials
Numerous trials of cariprazine are currently ongoing or under way (McCormack 2015):
Phase III trial of cariprazine in the treatment of schizophrenia or bipolar disorder in some countries of the European Union, India, Russia, South Africa, Serbia, Colombia, and Ukraine
Phase III trial of cariprazine in the treatment of schizophrenia with predominant negative symptoms in the Czech Republic, Hungary, Spain, Poland, Croatia, France, Serbia, Romania, Russia, Ukraine, and Bulgaria
Phase III trial of cariprazine in the prevention of relapse of schizophrenia in India, Romania, Slovakia, the United States, and Ukraine
Phase II/III trial of cariprazine in the treatment of schizophrenia in Japan, South Korea, and Taiwan
Phase III trial of cariprazine as an adjunct to antidepressant treatment of major depressive disorder in Puerto Rico and the United States
Phase II trial of cariprazine as an adjunct to antidepressant treatment of major depressive disorder in Estonia, Finland, Slovakia, Sweden, Ukraine, and the United Kingdom
Phase II trial of cariprazine in the treatment of bipolar depression in Bulgaria, Canada, Colombia, Russia, Ukraine, and the United States
Conclusion
Cariprazine combines functional selectivity at dopamine D3 and D2 receptors, partial agonist activity at serotonin 5-HT1A receptors, and antagonism at serotonin 5-HT2B and 5-HT2A receptors. Cariprazine’s receptor-binding profile is unique, with the highest affinity and selectivity for D3 receptors of any second-generation antipsychotic. It has antipsychotic and mood-stabilizing effects, as well as possible antidepressant, procognitive, anti-abuse, and antihostility effects. Cariprazine has the potential to improve the cognitive and negative symptoms of schizophrenia, as shown in preclinical trials.
References
Actavis Inc: VRAYLAR™ (cariprazine) capsules, full prescribing information. Parsippany, NJ, Actavis Inc, 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204370lbl.pdf. Accessed May 15, 2016.
Ahuja S, Bose A, Lu K, et al: A multicenter, randomized, double-blind trial to evaluate the effect of cariprazine in bipolar depression (no 23).. Poster presented at the International Society for CNS Clinical Trials and Methodology (ISCTM) 2011 Autumn Conference, Amelia Island, FL, October 3–4, 2011. Available at: https://isctm.org/public_access/Oct_2011/PosterAbstracts.pdf. Accessed May 14, 2016.
Bantick RA, Deakin JF, Grasby PM: The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics? J Psychopharmacol 15(1):37–46, 2001 11277607
Blier P, Ward NM: Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry 53(3):193–203, 2003 12559651
Calabrese JR, Keck PE Jr, Starace A, et al: Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 76(3):284–292, 2015 25562205
Citrome L: Cariprazine in schizophrenia: clinical efficacy, tolerability, and place in therapy. Adv Ther 30(2):114–126, 2013a 23361833
Citrome L: Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol 9(2):193–206, 2013b 23320989
Citrome L: The ABC’s of dopamine receptor partial agonists—aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract 69(11):1211–1220, 2015 26477545
Citrome L, Durgam S, Lu K, et al: The effect of cariprazine on hostility associated with schizophrenia: post hoc analyses from 3 randomized controlled trials. J Clin Psychiatry 77(1):109–115, 2016 26845266
Crocq MA, Mant R, Asherson P, et al: Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. J Med Genet 29(12):858–860, 1992 1362221
Durgam S, Starace A, Li D, et al: An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res 152(2–3):450–457, 2014 24412468
Durgam S, Starace A, Li D, et al: The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord 17(1):63–75, 2015 25056368
Durgam S, Earley W, Lipschitz A, et al: An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am J Psychiatry 173(3):271–281, 2016a 26541814
Durgam S, Litman RE, Papadakis K, et al: Cariprazine in the treatment of schizophrenia: a proof-of-concept trial. Int Clin Psychopharmacol 31(2):61–68, 2016b 26655732
Forest Laboratories: Forest Laboratories submits new drug application for cariprazine for the treatment of both schizophrenia and manic or mixed episodes associated with bipolar I disorder. Press Release, November 28, 2012. Available at: http://news.frx.com/press-release/corporate-news/forest-laboratories-submits-new-drug-application-cariprazine-treatment-. Accessed May 15, 2016.
Girgis RR, Slifstein M, D’Souza D, et al: referential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology (Berl) 233(19–20):3503–3512, 2016 27525990
Gross G, Drescher K: The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handb Exp Pharmacol (213):167–210, 2012 23027416
Gyertyán I, Kiss B, Sághy K, et al: Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem Int 59(6):925–935, 2011 21767587
Hellstrand M, Danielsen EA, Steen VM, et al: The ser9gly SNP in the dopamine D3 receptor causes a shift from cAMP related to PGE2 related signal transduction mechanisms in transfected CHO cells. J Med Genet 41(11):867–871, 2004 15520413
Jeanneteau F, Funalot B, Jankovic J, et al: A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc Natl Acad Sci U S A 103(28):10753–10758, 2006 16809426
Kane JM, Zukin S, Wang Y, et al: Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol 35(4):367–373, 2015 26075487
Kapás M, Mészáros GP, Yu B, et al: P.3.c.051 Comparison of the pharmacokinetic behaviour of RGH-188 in schizophrenic patients and healthy volunteers (abstract). Eur Neuropsychopharmacol 18 (suppl 4):S433, 2008
Ketter M, Sachs GS, Lu K, et al: Long-term safety and tolerability of open-label cariprazine in patients with bipolar I disorder. Paper presented at the 10th International Conference on Bipolar Disorder, Miami Beach, FL, June 13–16, 2013
Kiss B, Horváth A, Némethy Z, et al: Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 333(1): 328–340, 2010 20093397
Laszlovszky I, Lu K, Debelle M, et al: EPA-0870—Efficacy and safety of cariprazine in patients with acute exacerbation of schizophrenia: results of two phase III trials (abstract). Eur Psychiatry 29 (suppl 1): S1, 2014
Lundstrom K, Turpin MP: Proposed schizophrenia-related gene polymorphism: expression of the Ser9Gly mutant human dopamine D3 receptor with the Semliki Forest virus system. Biochem Biophys Res Commun 225(3):1068–1072, 1996 8780735
Ma G, He Z, Fang W, et al: The Ser9Gly polymorphism of the dopamine D3 receptor gene and risk of schizophrenia: an association study and a large meta-analysis. Schizophr Res 101(1–3):26–35, 2008 18295456
Mattingly G, Anderson R: Cariprazine for schizophrenia and bipolar I disorder. Current Psychiatry 15(2):34–39, 2016
McCormack PL: Cariprazine: first global approval. Drugs 75(17):2035–2043, 2015 26510944
Mészáros GP, Bolf ET, Gemesi L: P.3.d.015 Pharmacokinetics of RGH-188, a novel dopamine D3/D2 antagonist/partial agonist atypical antipsychotic, in rats and dogs (abstract). Eur Neuropsychopharmacol 18 (suppl 4):S456–S457, 2008
Nakamura T, Kubota T, Iwakaji A, et al: Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des Devel Ther 10:327–338, 2016 26834462
Nasrallah HA, Cutler AJ, Wang Y, et al: P.3.d.025 Safety and tolerability of cariprazine in long-term treatment of schizophrenia: integrated summary of safety data (poster & abstract). Eur Neuropsychopharmacol 24 (suppl 2):S536, 2014
Román V, Gyertyán I, Sághy K, et al: Cariprazine (RGH-188), a D3-preferring dopamine D3/D2 receptor partial agonist antipsychotic candidate demonstrates anti-abuse potential in rats. Psychopharmacology (Berl) 226(2):285–293, 2013 23138433
Sachs GS, Greenberg WM, Starace A, et al: Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord 174:296–302, 2015 25532076
Seneca N, Finnema SJ, Laszlovszky I, et al: Occupancy of dopamine D2 and D3 and serotonin 5-HT1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacology (Berl) 218(3):579–587, 2011 21625907
Tadori Y, Forbes RA, McQuade RD, Kikuchi T: Functional potencies of dopamine agonists and antagonists at human dopamine D2 and D3 receptors. Eur J Pharmacol 666(1–3):43–52, 2011a 21658377
Tadori Y, Forbes RA, McQuade RD, Kikuchi T: In vitro pharmacology of aripiprazole, its metabolite and experimental dopamine partial agonists at human dopamine D2 and D3 receptors. Eur J Pharmacol 668(3):355–365, 2011b 21816144
Veselinovic T, Paulzen M, Gründer G: Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother 13(11): 1141–1159, 2013 24175719