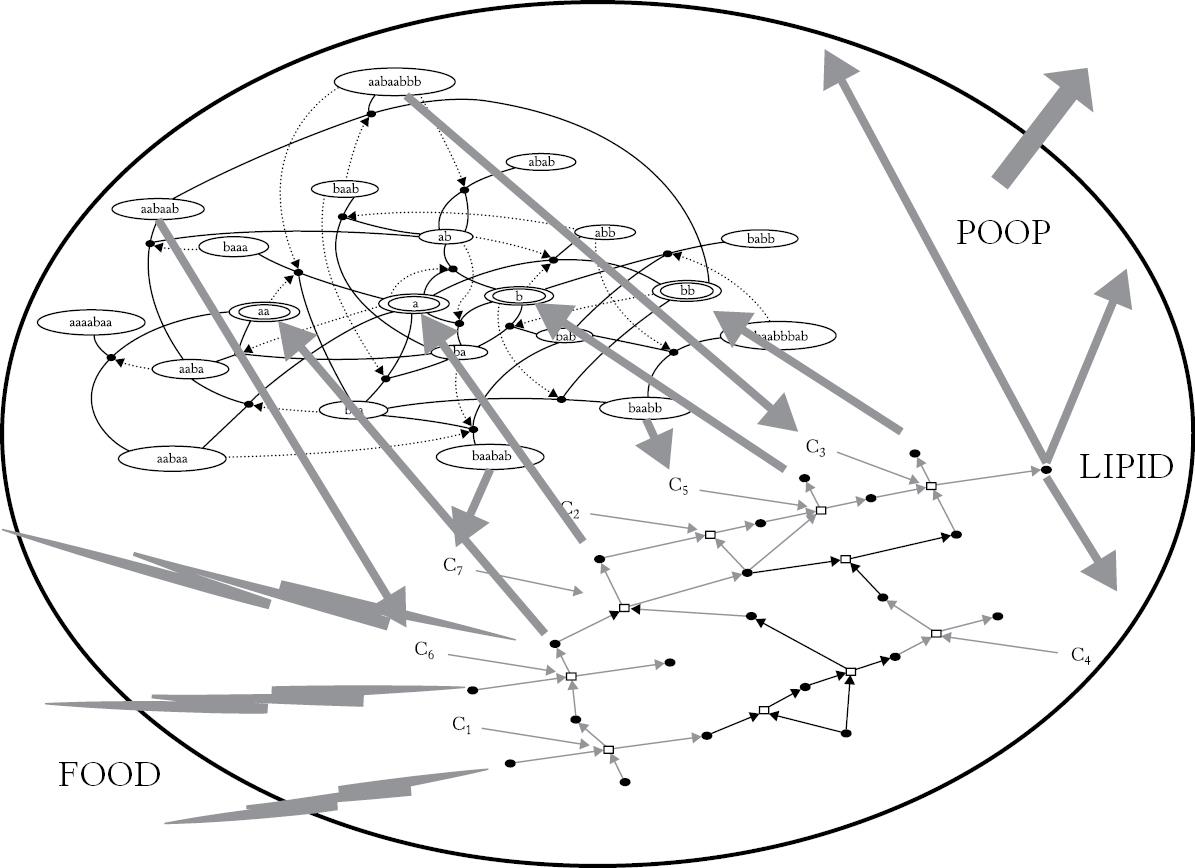
FIGURE 6.1 The protocell.
No one knows how life started. But many workers think that early life began with what they call “protocells.” A protocell is imagined to be some kind of self-reproducing molecular system, perhaps coupled to a metabolism and housed inside a hollow lipid vesicle called a liposome. The self-reproducing system might be a collectively autocatalytic set of RNA, peptides, or both kinds of molecules.
Figure 6.1 sketches our hypothetical protocell. A collectively autocatalytic set is coupled with a metabolism of small molecules whose products include lipid molecules themselves. The lipids can enter the liposome shell, driving its growth. When it becomes large enough it buds into two liposomes—a primitive kind of cell division. Food enters from the outside through the semipermeable membrane forming the liposome. Similarly, waste is excreted.

FIGURE 6.1 The protocell.
How might protocells arise? No one knows. There are two broad visions of where such an emergence might have occurred: hydrothermal vents in the oceans and fluctuating hot spring pools on land. Hydrothermal vents have been found to harbor rich simple life, and many hope that early life may have blossomed there. Computer scientist Bruce Damer and chemist David Deamer propose instead that the first protocells arose in interconnected pools of water on volcanic landscapes such as would have existed over four billion years ago resembling Iceland or Hawaii today (Damer and Deamer 2015). Evidence for such a hot spring rife with life in a 3.5-billion-year-old rock formation was recently discovered in Western Australia (Djokic et al. 2017). Wet–dry cycling—in which the water of the pool, or its edge, evaporates and recollects—occurs and, with an abundance of organic molecules, might have driven the process that I will sketch in the following.
A central part of the scenario pictures multilamellar, or multilayered, liposomes in a hot pool. Near the shores of this pool, wet–dry cycles would occur. As the day heats up, the pool dries by evaporation and refills in the cool night, as water seeps in from a nearby spring, or from rains. There are three stages: (1) When wet, the liposomes are hollow vesicles that float in the water; (2) when nearly dry, the liposomes form a gel-like aggregate among themselves; and (3) when dried onto mineral surfaces, they fuse together into layers, spilling their contents into the 2D spaces between the layers.
As wet–dry cycles follow one after another, this system cycles repeatedly between these states.
In their scenario, Damer and Deamer make use of the Plastine reaction, first studied in 1932. Large proteins are incubated with trypsin, a gut enzyme that during digestion cleaves large proteins into smaller ones. When a peptide bond is formed between two amino acids, one water molecule is released into the medium. Thus, if water is removed from the medium in such a system—for example, by evaporation—the reaction will thermodynamically reverse. New peptide bonds (and also nucleic acid bonds) can be formed, creating populations of polymers having initially random sequences. Now consider what happens in a wet–dry cycle: large polymers can be cleaved apart in an aqueous environment. Then when the system dries out, the parts of that polymer can ligate (join together) back into longer polymers. Repeated periods of wet and dry drive cycles of polymer formation from basic building blocks, cleavage, and re-synthesis—in which polymer fragments are randomly reshuffled with one another—are cleaved, and re-ligated, creating a soup of various polymers.
In the case of the Plastine reaction, if the trypsin catalyst is removed, the same thermodynamic forces are still at play, and the same reactions will happen, but more slowly. On the prebiotic Earth, there were no catalysts or enzymes, so it is reassuring that simple dehydration can do the same job, but more slowly. When water leaves through the stacked membranes layered in their “bathtub ring” on the mineral edge of the pool, the building blocks of polymers, already squeezed together by the sheets of membrane, line up and, like a zipper closing, form increased numbers of potentially functional polymers.
Thus, Damer and Deamer generate protocells, each housing a rich stew of different peptides or RNA sequences or both. During wet cycles, trillions of liposomes bud off when the layers of dried membranes bulk up with water. Some of these contain random sets of the aforementioned polymers, forming protocells. Cleavage will occur, during wet cycles, random shuffling and re-synthesis of peptide or RNA, but now this includes the peptide or RNA sequences inside the multilamellar liposomes. Together the liposomes and peptide soup inside them constitute the protocell that will ultimately evolve into the universal common ancestor
Damer and Deamer propose that as wet–dry cycles repeatedly occur over millions of years, a kind of natural selection comes into play—what Pross (2012) calls “Dynamic Kinetic Stability.” Upon drying, each protocell crowds together with thousands of others that survived to this point, sharing their collective contents. Upon rehydration, the contents are captured in new protocells that bud off, and another cycle of cleavage occurs. Damer and Deamer suggest that those systems whose molecules somehow encourage stability will “survive” or “propagate” more efficiently, leading in due course to populations of increasingly robust protocells.
They refer to these vesicle populations as “progenotes,” a term originally put forth by Carl Woese and George Fox (1977) and adopted by Damer (2016). If they are right, such progenotes are the ancestors of all life on the Earth.
Part of what I admire about this scenario is that Damer and Deamer seem to have cleaved the Gordian knot of how to get something like heritable variation without having self-reproducing cells in the first place. If selection for Dynamic Kinetic Stability can happen in their system, they have achieved a form of variation and selection that might accumulate useful variant progenotes.
I wonder, however, just where this propagating stability that is selected might come from, and I now hope to build on their ideas.
Damer and Deamer envisage selection for liposomes with useful mixtures of peptides and RNA-like polymers. But the random reshuffling due to cleavage and ligation will scramble these useful sequences in each cycle. Consider 103 useful peptides of length 10 amino acids in a liposome. There are 2010 or about 1013 such sequences. During each cleavage and re-ligation cycle, an initial 1,000 useful peptides will splay out into this sequence space at some rate, randomizing the useful peptides. It is not clear how heritable variation for useful polymers can happen, but it may arise first through template-driven replication.
I note next that hopefully the conditions envisioned by Damer and Deamer are just those that might give rise to collectively autocatalytic sets of peptides and nucleic acids within the gel aggregate or the multilamellar structure. So suppose such a set is stumbled on. And if the autocatalytic set is a stable reproducing set of polymers, then it could be selected for.
And that would give us a way to get the same set of polymers over and over. So suppose a collectively autocatalytic set forms inside a liposome. It dries out on the bottom or side of the pool, passing first through the gel phase, along with thousands of other liposomes, and then drying altogether. Somewhere along the way it fuses and spills its contents into the collective gel or dried layers, and the same polymers are taken up by its neighbors, which then rehydrate and begin the cycle anew. If any of these neighbors or the original one has a set of polymers able to regenerate the collectively autocatalytic set, that set proliferates to the neighbors in the gel or passes via the layered state to the newly forming neighbors rehydrating from the dried gel. In the next cycle, they can pass this property on to more neighbors. And so liposomes with “fit” autocatalytic chemistries proliferate.
Moreover, these systems should achieve dynamic kinetic stability. We have already seen that collective autocatalytic sets achieve the three closures: work task closure, constraint closure, and catalytic closure. These mean that the same system is recreated at each cycle. This proliferation constitutes dynamic kinetic stability. Compared to the random shuffling of polymers, progenotes with self-reproducing stable molecular systems in them will win and go on to cycle another day.
In short, the collectively autocatalytic set, by producing the same polymers, gives a selectable set on which selection can act and can also act on the help given to the multilamellar form to stabilize it in the environment—aqueous, gel, or dried—as it propagates. And the multilamellar forms help the set by confining and preserving their contents against the vicissitudes of the harsh Hadean environment.
A molecular mutualism is underway. This may allow selection not only for advantageous polymers in the set but for species of lipids in the propagating multilamellar progenote forms.
In chapter 5, we supposed that a collectively autocatalytic set could catalyze and graft to itself a connected catalyzed metabolism. The Damer–Deamer environment might just show how this could have happened on the early Earth—or anywhere in the universe with similar conditions. Such systems on the newly formed Earth would be rich in organic molecules, supplied by infalling organics as were shown to be present on the equally ancient Murchison meteorite, able to link into a catalyzed metabolism.
Some metabolisms would be better than others in supporting the reproduction of their associated collectively autocatalytic sets, so they would be selected and amplified through the Darwinian process.
Somewhere along the way, suppose that the metabolism came to form lipids as byproducts of no use to the autocatalytic set itself. Ultimately, such a union of a collectively autocatalytic set, a connected catalyzed metabolism producing lipids, and the capacity of those very lipids to form liposomes housing the autocatalytic set and metabolism could yield a form of “protocell mutualism.”
One pathway to a more complex protocell might be the gel phase, where specific lipids could be of use to the local patch of protocells in the gel forming the multilamellar structures, so they might be selected. This might afford a co-selection of metabolism, lipids, and collectively autocatalytic set functioning well in the concentrating gel stage as the pool level drops.
Then one can envisage that these three “coupled phases” working together can now form a sophisticated protocell, with a boundary membrane, lipid synthesis, autocatalytic set, and metabolism as in Figure 6.1, able to divide by budding in the pool without dependence on wet–dry cycles and capable of free living in solution. At some stage in the “late progenote” world, protocells combine innovations until, accidentally (or fortuitously), they learn the trick of division, with all of their protogenetic and metabolic autocatalytic sets securely copied and divided between daughter cells representing—and voilà . . . you have the transition to life (as we know it).
This brave scenario requires that the collectively autocatalytic set divide at the same rate as the housing liposome so they divide synchronously. Serra and Villani (2017) have shown that this arises easily.
Is this how life began? Maybe it is. It is all rather facile. Serra and Villani’s recent book (2017) describes the difficulties of getting a protocell to work at all, given low concentrations.
At most, the preceding is a hopeful beginning and a call for further study.
A deep issue is how the biosphere builds up complexity in face of the second law of thermodynamics. This law states that in a closed system, disorder, or entropy, can only increase. In a system open to the input and efflux of matter and energy, entropy increases; but thermodynamic work can be done, building up complexity. In our hoped for protocell, lipids are built up. In plants, photosynthesis builds up glucose molecules from carbon dioxide and water. Fine, but if the second law degraded this order faster than it was created, no order could accumulate! How does order accumulate?
A sufficient answer to how order accumulates seems available to us thanks to the three closures: constraint, work cycle, and catalytic. In a system with constraint and work-cycle closure, the constraints on the release of energy in the non-equilibrium processes does work, and this work is used to construct yet more of the same constraints. This is the harnessing of energy to build up further order. That these systems are also parts of reproducing molecular systems, due to autocatalysis, means that they can reproduce and further construct order faster than the second law dissipates that order. There is a persistent self-construction. As we see soon, such systems can evolve by heritable variation and selection. A biosphere can now build itself.
Getting to protocells from dumb, dead earth is already a lot. But current life is based on something more: DNA that codes for the production of proteins including the ones, like DNA polymerase, that the DNA needs to replicate itself and very much more (prokaryotes, eukaryotes, multicellular organisms, sex . . .).