Well-Being
Heritable and Changeable
Espen Røysamb1, Ragnhild Bang Nes2 and Joar Vittersø3, 1University of Oslo, Oslo, Norway, 2Norwegian Institute of Public Health, Oslo, Norway, 3University of Tromsø, Tromsø, Norway
Well-being has been found to be heritable and changeable. How do these pieces of evidence fit together? Intuitively, models of change and models of genetic influences may seem contradictory and paradoxical. In this chapter, we aim to unite these findings into an integrated understanding of human happiness. We start out by briefly reviewing some of the current evidence of temporary and lasting change in well-being from different strands of research. Second, we present the main methods of contemporary genetically informative studies and review key findings from this research on well-being. The concept of heritability is thoroughly discussed and central misunderstandings, criticism, and caveats of genetically informative studies addressed. Finally, we present some thoughts toward an integrative model of genes, environment, stability, and change. Genetics is highly likely to facilitate development of increasingly successful tailored interventions, and we propose a notion of positive gene-environment interplay as one path toward increased and sustained happiness.
Keywords
Well-being; twins; heritability; genetic; change; environment
Introduction
Research on human happiness and well-being has flourished in recent years, and several subfields within the domain have emerged. One area of research has focused on the stability and change of well-being. Longitudinal and intervention-based studies provide evidence of the plasticity of well-being and the potential of increasing happiness (Dyrdal, Røysamb, Nes, & Vittersø, 2011; Lucas, 2007; Lyubomirsky & Layous, 2013; Sheldon & Lyubomirsky, 2012). Another line of research has examined genetic and environmental contributions to individual differences in well-being using quantitative and molecular genetic techniques. The collective findings from these studies indicate both genetic and environmental influences on most well-being measures (Bartels & Boomsma, 2009; Lykken & Tellegen, 1996; Nes, Røysamb, Tambs, Harris, & Reichborn-Kjennerud, 2006; Røysamb, Tambs, Reichborn-Kjennerud, Neale, & Harris, 2003).
Are these findings on changeability and heritability mutually contradictory and paradoxical? Does recognition of genetic influences on happiness preclude optimism regarding change potentials? Based on the current evidence, we argue that well-being is both heritable and changeable. Our aim in this chapter is first to review some of the exciting evidence of both changeability and genetic influences. Second, we discuss the concept of heritability along with criticism and caveats of behavior genetic findings. Finally, we present an integrative framework of well-being and change processes, and propose the concept of positive gene-environment interplay as a path to increased happiness.
Well-being is a broad term, typically referring to a general idea of goodness in life or what it means to live well (Crisp, 2005). To operationalize well-being for research purposes, researchers have proposed a number of more specific constructs, including subjective well-being (SWB), psychological well-being (PWB), mental well-being (MWB), emotional well-being (EWB), life satisfaction (LS), and emotional happiness (David, Boniwell, & Conley Ayers, 2013; Vittersø & Soholt, 2011). These constructs refer to partly overlapping and partly different phenomena, in the sense that they reflect some common and some unique variance (Chen, Jing, Hayes, & Lee, 2012). Thus, in the present chapter, we sometimes debate these subconstructs specifically, whereas we generally refer to well-being, and—in this context—its synonym happiness, in a broad sense.
Can Happiness Change?
The unequivocal answer to this question is positive. Happiness can change, and happiness does change—during a single day and during a lifetime. Nevertheless, several questions pertaining to change are important to consider. How much can happiness change? Are changes in happiness short-term or lasting? Does happiness fluctuate around given set points? How do genetic and environmental factors contribute to stability and change?
There is a wealth of evidence supporting the notion of well-being as changing and changeable (Headey, Muffels, & Wagner, 2010; Lucas, 2007; Lyubomirsky, Sheldon, & Schkade, 2005). This volume contains reports from key researchers in the field, providing various types of compelling evidence for the dynamic nature of well-being. Thus, we primarily refer to other parts of this book for theories and studies of the changeability of happiness. Here, we briefly summarize overall findings from partly separate fields of inquiry, including longitudinal studies, intervention studies, natural quasi-experiments, national comparison studies, and clinical psychology/psychotherapy research.
Longitudinal studies of well-being typically report moderate stability (Dyrdal et al., 2011; Eid & Diener, 2004; Lucas & Diener, 2008; Lucas & Donnellan, 2007; Nes et al., 2006). Although cross-time correlations for well-being vary depending on the given measure, sample and timespan, they rarely exceed 0.6 and rarely drop below 0.3 (Diener, Inglehart, & Tay, 2013). A time1–time2 correlation of around 0.5 suggests that at any given time point, 50% of the variance is accounted for by a stability factor with the remaining 50% representing change or time-specific variation. Longer timespans typically yield lower stability than shorter timespans, and long-term change is therefore substantial. Knowing a person’s well-being level today thus provides some, yet only some, basis for predicting the same person’s well-being in 10 years’ time.
Happiness intervention studies have been crucial in testifying to human change potentials and in identifying effective factors for generating increased well-being (Lyubomirsky & Layous, 2013; Seligman, Steen, Park, & Peterson, 2005; Sheldon & Lyubomirsky, 2012), as also evidenced by recent meta-analyses (Bolier et al., 2013; Sin & Lyubomirsky, 2009). Interventions such as gratitude exercises, cultivation of optimism, and use of character strengths represent novel strategies that have been shown to affect the happiness level of participants—and also contribute to more than temporary change. Although it is rarely feasible to adhere fully to the experimental ideal of double-blind randomized controlled trials (RCTs) in this field, the evidence converges on a substantial benefit of several intervention strategies. Of note, interventions appear to differ in their effectiveness (Seligman et al., 2005), indicating that the changes recorded do not mainly reflect a general training effect. With some interventions found particularly effective, and others not, more credibility is established for specific intervention strategies, and further understanding of their potent change mechanisms is likely to result from such studies.
Natural quasi-experiments represent another set of relevant studies for examining stability and change in well-being over time. Both positive (e.g., lottery winning, marriage) and negative life events (e.g., accidents, divorce, unemployment) are associated with temporary, and, to some extent lasting, changes in well-being (Diener, Lucas, & Scollon, 2006; Lucas, 2007; Luhmann, Hofmann, Eid, & Lucas, 2012). Because exposures to life events are not random in the population (Kendler & Karkowski-Shuman, 1997), evidence of causality is not entirely conclusive. Yet, recent studies have shown a nuanced picture of short- and long-term changes following various life events. One promising design for this research field can be found in co-twin control studies. By studying well-being in identical (monozygotic) twins discordant for a certain exposure (e.g., life event), one is able to approach a randomized matched-pair design and generate evidence of causal effects. For example, in a co-twin control study of SWB and longevity, Sadler and colleagues found environmental exposures, rather than genes, to account for the increased longevity associated with high well-being (Sadler, Miller, McGue, & Christensen, 2009).
National differences in well-being also provide evidence for the changeability of well-being. There is substantial variation in mean level well-being across different countries (Diener, Tay, & Oishi, 2013; Veenhoven, 2009), even between neighboring countries with similar populations. This suggests a change potential in most countries. Because national and individual differences might be explained by partly different factors, research into the predictors of national differences is crucial to understand the role of governance, economy, health care, and culture in generating well-being. Although national differences typically are smaller than individual differences within nations (Diener, Helliwell, & Kahneman, 2010; Vittersø, Røysamb, & Diener, 2002), the notion of lifting an entire nation by only a fraction of a standard deviation implies a huge total gain.
Clinical psychology and therapy research has not played a central role in the well-being field. Nevertheless, important lessons may be learned from this area. The lifetime prevalence of any mental disorder is roughly 50% (Kessler et al., 2005; Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012), yet many of those who experience such problems return to flourishing lives. Mental health problems such as depression, anxiety, substance dependence, and eating disorders are negatively correlated with various well-being measures, yet ill-being and well-being are typically not seen as polar opposites (Keyes, 2013; Nes et al., 2013). Recent genetically informative studies confirm that genetic vulnerability for depression and internalizing disorders is inversely related to the genetic disposition for well-being, but also that there are unique genetic and environmental components of ill-being and well-being (Kendler, Myers, Maes, & Keyes, 2011; Nes et al., 2013). Studies on the development and treatment of mental disorders and subclinical psychological problems testify to a general human plasticity and a potential to change. Following trauma, conflicts, stressors, and major negative life events, onset of anxiety or depression may occur in previously healthy individuals. Furthermore, psychotherapy comprises an array of interventions aiming to generate a shift from ill-being to well-being and well-functioning, and numerous studies provide evidence of their effectiveness (Nieuwsma et al., 2012; Weisz, Weiss, Han, Granger, & Morton, 1995). Thus, our knowledge about the onset and treatment of ill-being also represents an important basis for understanding the changeability of well-being. In summary, several different strands of research provide evidence of both stability and change in well-being and suggest a relatively dynamic nature of human happiness.
Heritability
Before reviewing empirical findings from twin and family studies on well-being and happiness, we briefly outline some of the key concepts, methods, and logic of genetically informative studies (i.e., twin and family studies).
Twin Research and Biometric Modeling
Family resemblance can be attributable to both nature and nurture. Basic genetically informative designs use the known genetic relationship between family members—usually monozygotic (MZ) and dizygotic (DZ) twins—to estimate the contribution of unknown genes and environmental factors to the observed variation in a given characteristic—or phenotype—such as well-being. Some studies have included data also from other types of relatives such as non-twin siblings (Bartels & Boomsma, 2009; Stubbe, Posthuma, Boomsma, & De Geus, 2005) and parents and offspring (Nes, Czajkowski, & Tambs, 2010). Other studies include both twins reared together and twins reared apart (Tellegen et al., 1988).
Because well-being constitutes a multifactorial and polygenetic characteristic (i.e., many different genes are involved), statistical methods can be employed to estimate the relative proportions of variation attributable to genetic and environmental factors. The resulting estimates are calculated without specifying any DNA sequences or any specific environmental influences. Rather, their contributions are inferred and modeled as latent variables.
The most basic design explores whether and to what extent genes are involved (i.e., estimates heritability), whereas the more advanced designs allow for further exploration of how genes and environmental influences are involved (e.g., whether the genetic and environmental influences are specific to a given characteristic or shared with other traits, or contingent on specific circumstances).
Two types of genetic influences (additive and nonadditive) and two types of environmental influences (shared and nonshared) can be estimated in standard twin studies. Additive genetic influences comprise effects from an unknown number of individual genetic loci whose effects combine additively. By contrast, nonadditive genetic influences reflect interaction among alleles (gene variants) at the same locus (dominance) or across loci (epistasis). The overall genetic effect is termed heritability and reflects the part of the total variation attributable to genetic factors. Broad-sense heritability (H²) refers to effects from both additive and nonadditive genetic influences, and narrow-sense heritability (h²) refers only to the additive ones.
The shared, or common, environment includes environmental influences that contribute to similarity in reared-together family members and constitutes a measure of environmental effects rather than the environment as such. Whereas objective environments refer to environmental circumstances as they might be observed, effective environments are defined by the outcomes they produce. Standard twin studies estimate only the effective environment, not the objective environment. When objectively shared family factors (e.g., conflict) affect siblings in a family differently (e.g., increasing risk of anxiety in one sibling and not the other), they are classified as nonshared. This latter effect, the nonshared environment, reflects all the nongenetic sources of differences among family members as well as measurement error and stochastic chance effects.
The two environmental and two genetic components are usually derived by specifying a mathematical model according to the differential degree to which pairs of MZ and DZ co-twins are correlated for genetic and environmental effects. Because MZ co-twins have identical DNA, both types of genetic effects are perfectly correlated in these pairs. By contrast, DZ co-twins (and siblings) share on average 50% of their segregating genes, giving a genetic correlation of 0.5 for additivity and 0.00–0.25 for nonadditivity. The shared environment includes all environmental influences causing twin resemblance regardless of zygosity and is correlated 1.0 in all zygosity groups, whereas the nonshared environment is, by definition, uncorrelated.
The difference in observed similarity between MZ and DZ twins is central to estimates of heritability, and a crude estimate of narrow heritability is given by the following formula: h2=2×(rMZ – rDZ), that is, two times the difference between MZ and DZ correlations.
Multivariate analyses extend the basic univariate model by decomposing the variance and the covariance between different indicators or different time points into genetic and environmental components. This enables investigation of whether the same factors contribute to several correlated characteristics (e.g., well-being and optimism) or a given characteristic over time (e.g., in childhood and adolescence). These multivariate models are more statistically powerful than univariate models and consequently provide more precise estimates of the latent effects.
Findings: Univariate Studies
In recent years we have witnessed an increasing number of genetically informative studies on well-being (Archontaki, Lewis, & Bates, 2013; Bartels & Boomsma, 2009; Caprara et al., 2009; De Neve, Christakis, Fowler, & Frey, 2012; Franz et al., 2012; Gigantesco et al., 2011; Keyes, Myers, & Kendler, 2010; Mosing et al., 2012; Nes et al., 2006; Røysamb et al., 2003; Schnittker, 2008), including twin studies, adoption studies, family studies, and molecular genetic studies. We will shortly review the main findings, yet for a start, it seems fair to summarize the cross-sectional findings as typically revealing heritabilities in the range of 0.25–0.55. This implies a substantial genetic contribution to human well-being. Importantly, however, the findings also represent robust evidence of strong environmental influences.
Table 2.1 displays an overview of some key studies that have examined genetic and environmental influences on well-being. Collectively, these studies are based on samples of more than 80,000 twins and family members, from several different countries, and covering the life span from early adolescence through senior years. A number of different well-being constructs have been studied, including subjective well-being, psychological well-being, mental well-being, and life satisfaction. Despite some potentially interesting differences across measures and samples, the findings converge on a moderate heritability of well-being. Of note, the magnitude of the genetic influences corresponds to that typically found for common mental disorders such as anxiety and depression, and is lower than those reported for schizophrenia, bipolar disorder, and general intelligence (Bouchard, 2004; Plomin, DeFries, Craig, & McGuffin, 2003).
Table 2.1
Key Studies of Genetic and Environmental Factors in Well-Being, with Heritability Estimates
| Happiness Type | Reference & Year | N | Age | Sample Type | Phenotype | Instrument | H2/h2 | c2 | e2 |
| Momentary | |||||||||
| Riemann et al., 1998 | 600 | 18–70 | Twins reared together | Positive mood | Mood scale | .00–.21 | .07–.16 | .71–.84 | |
| Eid et al., 2003 | 278 | 18–70 | Twins reared together | PA | PA scale | .36 | – | .64 | |
| Menne-Lothmann et al., 2012 | 520 | M=27 | Female twins reared together | Momentary PA, reward | Four items | – | .00–.34 | .66–1.0 | |
| Time-specific | |||||||||
| Tellegen et al., 1988 | 804 | Twins reared together/apart | WB | MPQWB | .40–.48 | .13–.22 | .38–.40 | ||
| Røysamb et al., 2002 | 5,140 | 18–25 | Twins reared together | SWB | SWB index | .46–.54 | – | .46–.54 | |
| Røysamb et al., 2003 | 6,576 | 18–31 | Twins reared together | SWB | SWB index | .44 | – | .56 | |
| Stubbe et al., 2005 | 5,668 | 14–88 | Twins and siblings | LS | SWLS | .38 | – | .62 | |
| Steger et al., 2007 | 336 | M=49 | Twins reared together | Character strengths | VIA | .12–.58 | – | .42–.88 | |
| Nes et al., 2008 | 8,045 | 18–31 | Twins reared together | LS | LS | .17–.35 | .00–.11 | .66–.71 | |
| Schnittker, 2008 | 2,330 | 25–74 | Twins and siblings | Happiness (PA) | PA scale | .36 | .06 | .57 | |
| Caprara et al., 2009 | 856 | 23–24 | Twins reared together | SWB | SWLS | .59 | – | .41 | |
| Bartels & Boomsma, 2009 | 5,024 | 13–28 | Twins and siblings | SWB, subhappiness, QoL | SWLS, SHS, Cantril | .36–.47 | – | .53–.64 | |
| Nes et al., 2010 | 60,000 | 18–80 | Twins, siblings, parents | SWB | SWB index | .33–.36 | .00–.12 | .64–.67 | |
| Gigantesco et al., 2011 | 742 | 23–24 | Twins reared together | PWB dimensions | PWB | .37–.64 | .36–.63 | ||
| De Neve, Christakis, Fowler,& Frey, 2012 | 1,098 | Twins reared together | LS | LS | .33 | – | .67 | ||
| Archontaki et al., 2013 | 1,674 | M=45 | Twins reared together | PWB | PWB | .30–.52 | – | .48–.70 | |
| Franz et al., 2012 | 1,226 | 51–55 | Male twins reared together | PWB, LS, WB | PWB, MPQWB, LS | .19–.50 | .01–.02 | .49–.79 | |
| Mosing et al., 2012 | 2,937 | 61–66 | Twins reared together | Flow proneness | SFPQ | .41 | – | .59 | |
| Stable | |||||||||
| McGue et al., 1993 | 254 | 17–37 | Twins reared together/apart | Dispositional WB | MPQWB | .95 | – | .05 | |
| Lykken & Tellegen, 1996 | 254 | 17–37 | Twins reared together/apart | Dispositional WB | MPQWB | .80 | – | .20 | |
| Nes et al., 2006 | 8,045 | 18–31 | Twins reared together | Dispositional SWB | SWB index | .80 | – | .20 | |
| Nes et al., 2013 | 8,045 | 18–36 | Twins reared together | Dispositional LS | LS | .72 | – | .28 | |
Notes: SWLS=Satisfaction With Life Scale (Diener et al., 1985); MPQ=Multidimensional Personality Questionnaire (Tellegen, 1982); SHS=Subjective Happiness Scale (Lyubomirsky & Lepper, 1999); Cantril=Cantril’s ladder (Cantril, 1965); SWB index=Subjective Well-Being index (Moum et al., 1990); PWB=Ryff Psychological Well-Being Scale (Ryff & Keyes, 1995); SWB Questionnaire=Subjective Well-Being Questionnaire (King & Landau, 2003); AP Scale=The Aggregated Positive Affect Scale (Riemann, Angleitner, Borkenau, & Eid, 1998); VIA=Values in Action (Peterson & Seligman, 2003); BABS=Bradburn Affect Balance Scale (Bradburn, 1969); LS=Life Satisfaction single item; PAS=Positive Affect Scale (MIDUS, Mroczek & Kolarz, 1998); QoL=Quality of Life; SFPQ=Swedish Flow Proneness Questionnaire (Ullén et al., 2012); PE=Positive Emotionality; PA=Positive Affect.
Momentary: current/in-situation measures; Time-specific: general measure at single time point (e.g., SWLS); Stable: longitudinal data with two or more measurements. H2/h2=heritability; c2=common environment variance; e2=nonshared environment variance.
In Table 2.1, the upper part shows studies examining momentary states of well-being or positive affect. The middle section (“time-specific”), comprising the bulk of studies, reports findings based on general measures (e.g., SWLS) at single time points. Finally, the lower section comprises longitudinal studies investigating the genetic contribution to the stable component of well-being. As can be seen, genetic factors appear to play a limited role only in momentary states, a moderate role in cross-sectional general experiences, and a substantial role in long-term well-being.
In addition to genetically informative research focusing explicitly on the well-being measures mentioned previously, several studies have reported genetic effects for related constructs. For example, substantial heritabilities have been reported for optimism (Caprara et al., 2009; Mosing, Zietsch, Shekar, Wright, & Martin 2009; Plomin et al., 1992), positive emotionality (e.g., Johnson, McGue, & Krueger, 2005; Krueger, South, Johnson, & Iacono, 2008; Tellegen et al., 1988), and resilience (Waaktaar & Torgersen, 2012).
Another noteworthy finding from genetically informed studies of well-being is the general absence of common environmental effects (Nes, 2010). That is, family resemblance in well-being seems to be mainly due to shared genes. Environmental factors are evidently important but seem to be nonshared (i.e., unique to the individual), implicating that the environment tends to influence family members differently. Shared family factors such as parenting and family events may still be relevant to well-being, but do not usually contribute to resemblance within families or twin pairs (i.e., do not have general effects). A certain factor may thus be shared in terms of exposure, but not in terms of effects. For example, two twins may be exposed to the same parenting style yet react differently and not become similar because of this parenting.
Findings: Bi- and Multivariate Studies
Whereas the initial twin studies primarily focused on disentangling the genetic and environmental contributions to single well-being measures at single time points, the field has moved forward in several important directions. One set of studies explored the genetic and environmental contributions to associations between well-being and various health-related constructs. Twin data enable estimation of the extent to which genes and environments explain the observed correlations between two or more phenotypes. For example, given a negative correlation between well-being and depression, it would be pertinent to examine the sources of this association. Are there partly overlapping genetic factors, implying that genes that contribute to well-being also act as protective factors against depression? Or do certain environmental factors simultaneously contribute to well-being and protect against depression? Genetic factors have been found to play an important role in the associations between well-being and sleep problems (Nes, Røysamb, Reichborn-Kjennerud, Tambs, & Harris, 2005), perceived health (Røysamb et al., 2003), depression (Franz et al., 2012; Nes et al., 2013), internalizing and externalizing disorders (Bartels, Cacioppo, van Beijsterveldt, & Boomsma, 2013; Kendler, Myers, & Keyes, 2011; Kendler et al., 2011) as well as subclinical levels of anxiety and depressive symptoms (Nes, Czajkowski, Røysamb, Reichborn-Kjennerud, & Tambs 2008). Typically, environmental factors also contribute to the examined associations, but often to a lesser degree. Moreover, both genes and environmental factors represent unique influences that contribute to the specific variance in well-being.
Another group of recent studies has applied multivariate modeling to disentangle the genetic and environmental structure underlying a set of different well-being constructs. One study modeled the factors underlying psychological well-being, trait well-being, life satisfaction, self-esteem, and depression (Franz et al., 2012) and found two general factors with substantial genetic variance and a set of mostly environmental factors explaining the unique variance of each specific phenotype. In a similar vein, the underlying structure of quality of life in general, satisfaction with life, quality of life at present, and subjective happiness was found to be accounted for by additive and nonadditive genetic, as well as nonshared environmental, factors (Bartels & Boomsma, 2009). Correlations among the indicators were largely attributable to shared genes, however, suggesting that these four indicators do not differ at the genetic level. Another study reported high heritability (72%) for a latent factor of mental well-being, which accounted for the covariance among emotional, social, and psychological well-being (Keyes et al., 2010). Finally, high genetic correlations were found for the six subcomponents of psychological well-being, with the environmental effects being mostly trait specific (Archontaki et al., 2013).
Findings: Stability and Change
The multivariate twin model has also been successfully applied to examine genetic and environmental contributions to stability and change in well-being (Kendler et al., 2011; Lykken & Tellegen, 1996; McGue, Bacon, & Lykken, 1993; Nes et al., 2013; Nes et al., 2006). Whereas environmental influences are commonly assumed to be related to both stability and change, genetic factors are often implicitly assumed to represent static influences throughout the life span. However, genetic influences imply neither immutability nor stability (Pedersen & Reynolds, 1998), and new genetic effects may emerge at different developmental stages throughout the life span. Genes contributing to well-being among adolescents are not necessarily the same as those contributing to well-being later in life. Even moderately stable traits might be influenced by differing sets of genes at different life stages (Plomin, 1986). In theory, both genetic and environmental factors might therefore represent sources of both change and continuity.
Yet the studies published to date collectively indicate that genes primarily generate stability, whereas environmental influences generate both stability and change, although mainly the latter (Johnson, McGue, & Krueger, 2005; Lykken & Tellegen, 1996; McGue, 1993; Nes et al., 2006). Stability in well-being commonly accounts for 50% of the cross-sectional variation (Lucas & Donnellan, 2007; Schimmack, Krause, Wagner, & Schupp, 2010), and two longitudinal studies on subjective well-being have indicated that as much as 80% of this stable level is attributable to genes (Lykken & Tellegen, 1996; Nes et al., 2006) (see Table 2.1). Likewise, stability in positive emotionality as measured by the Multidimensional Personality Questionnaire (Johnson et al., 2005) and dispositional life satisfaction (Nes et al., 2013) appear to be largely attributable to genes. Conjointly, the findings are in line with a theory that posits a general well-being set point or set range (see more below), or a general readiness to perceive and interpret the world more or less positively, which is fairly stable across time and strongly influenced by genes. By contrast, environmental influences seem to be as important for change as genetic factors are to stability. Around 80% of the change variance appears to be accounted for by environmental factors.
Of note, however, environmental influences might also generate stability over time. Johnson and colleagues (2005) have, for example, reported that when accounting for measurement error, 40% of the variation in personality, including the higher-order factor positive emotionality, was attributable to stable environmental factors. Also, Nes and colleagues (2006) reported 20%–25% of the phenotypic cross-time correlations for subjective well-being to be due to environmental sources. Whether these stable environmental influences reflect long-term effects from past or persistent events or consistently occurring factors in the environment was not explored.
Lastly, there is also evidence for some new genetic variance emerging over time. Cross-time correlations between genetic factors for well-being have been estimated to 0.78–0.85 in a sample of young adult Norwegian twins (Nes et al., 2006). Although new genetic variance may reflect changes at the molecular level, it may also reflect alterations in psychosocial circumstances of sufficient magnitude to elicit new genetic influences for SWB. The genetic effects on change thus suggest that despite the DNA not undergoing change, different life situations, circumstances, or developmental stages are likely to make different genetic factors for well-being salient.
Gene-Environment Interplay
In addition to operating as sources of main effects, genes and environment may interact and correlate in several ways. Four specific types of gene environments interplay, all of which may be highly relevant for well-being, have been outlined in the field, including (i) heritability-environment interaction, (ii) gene-environment correlation (rGE), (iii) gene-environment interaction (GxE), and (iv) epigenetics (Caspi & Moffitt, 2006; Moffitt, Caspi, & Rutter, 2006).
Heritability-environment interaction refers to variation in the magnitude of the genetic and environmental influences across population strata. Several studies have explored heritability-environment interaction for well-being related phenomena and shown that family factors such as marital status, socioeconomic factors, and parenting may moderate genetic and environmental influences on well-being. The heritability of subjective well-being has, for example, been reported to vary across marital status, with lower heritability estimates for individuals with a marital or equivalent partner (Nes, Røysamb, Harris, Czajkowski, & Tambs, 2010). Another study examining the impact of financial standing found environmental influences on life satisfaction to decrease with increasing income, whereas the proportion of the variation due to genetic factors increased with increasing income (Johnson & Krueger, 2006). Heritability and both shared and nonshared environmental factors for positive emotionality have also been shown to vary as a function of adolescents’ perceived relationship with their parents (Krueger et al., 2008).
Gene-environment correlation (rGE) implicates that the probability of exposure to environmental influences is not random, but partly dependent on an individual’s genotype (i.e., the nature of nurture). rGE is commonly classified as passive, active, or evocative (McGue, 2010; Rutter, Moffitt, & Caspi, 2006; Scarr & Weinberg, 1983). Passive rGE refers to situations in which an individual inherits both genes and environmental circumstances that mutually reinforce each other. A relevant example to well-being research would be when children of happy parents inherit genes associated with happiness as well as experience emotionally stable, happy, and supportive parents (i.e., double advantage). Individuals are also active agents in selecting their environments (active rGE) and, in turn, trigger responses (evocative rGE) that commonly boost and strengthen their genetic propensities. Children with temperaments high in positivity and sociability tend to actively seek out circumstances matching their (partly genetic) disposition (active rGE) and may elicit more positive and supportive responses in parents and others (evocative rGE). For example, in the study by Krueger and colleagues (2008), adolescents with a genetic propensity to positive emotionality were reported to elicit more positive regard in their parents.
Gene-environment interaction (GxE) occurs when the effect of exposure to a given environmental factor is conditional on the individual’s specific genotype or, conversely, when the genotype’s effect is moderated by the environment. There has been high interest in this type of interplay over the past decade, and in fact, GxE has been suggested to constitute the norm in relating genetic polymorphisms to specific behavior (Canli, 2004; Canli & Lesch, 2007). Numerous studies have shown variants of specific genes (e.g., MAO-A, SLC6A4, COMT, 5HTTLPR) to be associated with specific behavioral expressions (e.g., antisocial behavior, depression, psychotic symptoms) if exposed to given life circumstances such as maltreatment, adverse life stressors, and drugs (Caspi et al., 2002; Caspi et al., 2005; Caspi et al., 2003). The GxE approach is likely to be productive, but so far few have explored the GxE interaction for well-being.
Relevant for GxE interaction is also the notion of vantage sensitivity (Pluess & Belsky, 2013). Whereas the traditional diathesis-stress framework focuses on the combination of vulnerabilities and stressors in generating mental disorders, the concept of vantage sensitivity focuses on variation in responses to positive experiences as a function of endogenous characteristics. Some individuals—with a given genetic disposition—might have a heightened sensitivity to positive exposures, resulting in an added benefit from specific experiences.
Epigenetic mechanisms reflect long-lasting alterations in gene expression that are not associated with changes in the DNA sequence (Tsankova, Renthal, Kumar, & Nestler, 2007) and thus constitute biological processes in which environmental effects are mediated through gene expression or through altered chromosomal structure. Epigenetic modification is likely to be important for our understanding of the molecular basis of complex and multifactorial characteristics such as happiness and well-being and may account for some of the variation that has previously been attributed to heritability and environmental factors (Petronis, 2010). In fact, the epigenetic perspective has been suggested as a unifying principle in the etiology of complex traits. However, the epigenetic pathways that link specific genes to behavioral expressions are likely to be utterly complex and are to date mainly unexplored.
What Heritability Is (Not) About
Heritability is a population statistic that refers to the proportion of total variance in a given characteristic (i.e., phenotype) accounted for by genetic variance (Plomin et al., 2003; Turkheimer, 2000). As such, it is parallel to any other effect represented as R2—for example, the variance in well-being accounted for by marital status, negative life events, or happiness interventions. Thus, heritability is merely a sample-based estimate of a source of variance in a given population. Note also that the estimate will depend on the relative amount of environmental variance. Therefore, heritability may vary across different populations, age groups, genders, nations, and cohorts. Body height may serve as an illustrating example. In the industrialized world, studies have shown heritabilities of around 80%–90% for body height (Bergin et al., 2012; Dubois et al., 2012). Two hundred years ago the heritability was assumedly substantially lower—not because of changes in the genetic variance but because of higher variability in nutrition during childhood. Thus, with better nutrition for all, there are fewer environmental differences left to explain the total variation. Similarly, in countries with greater environmental variation (e.g., socioeconomic differences), the heritability of well-being may be lower than the heritability estimated in countries with fewer social inequalities.
In its basic form, heritability can be defined by the formula
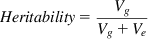 (2.1)
(2.1)
where Vg equals genetic variance and Ve equals environmental variance. Vg and Ve contribute to the observed phenotypic variance, Vph, thus:
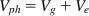 (2.2)
(2.2)
Note that in this simple formulation, additive and nonadditive genetic factors are combined into a total genetic effect. Likewise, common and nonshared environments are combined into total environmental variance. One key reminder is that of heritability as relative to the environmental variance. That is, increased environmental variance by necessity implies reduced heritability.
Another key point is that the estimated Ve typically includes naturally occurring environmental factors only, as in life-as-usual, and does not capture the effects of potential interventions. That is, in population-based twin studies, the environmental variance includes factors such as life events and social influences, but unless a substantial proportion of the sample has been exposed to an intervention, we are unlikely to detect any such effects. Adding a new intervention variance (Vi) to the existing Ve could, in theory, reduce heritability and also increase well-being. For example, laser surgery for vision correction represents an intervention that counteracts the partly genetic disposition to near- or farsightedness. The first introduction of laser operations in a population would represent an added environmental variance that could result in a reduced proportion of genetic variance in the total phenotypic variance.
To grasp the nature of the heritability construct, ponder the following question: What is the heritability of number of fingers on people’s hands in the adult population? Actually, although the fetal development of fingers obviously is genetically driven, the heritability is probably very low. First, there is very little genetic variance. With the exception of some mutations, we all have 10-finger genes. Second, most of the (little) phenotypic variance (i.e., people having anything but 10 fingers) is due to accidents and other environmental exposures. Thus, the observed variance, if any, is mostly accounted by environmental factors, which implies negligible heritability.
The notion of heritability as a population statistic also implies that it is not meaningful to index the heritability for a single individual. If well-being is found to have a heritability of 40%, this does not mean that 40% of a person’s well-being is inherited. To use body height again as an example, despite a heritability of around 85%, it would not make sense to claim that a person who is 170 cm tall has received 145 cm (170×0.85) from her or his genes and another 25 cm from the environment.
If heritability is not a relevant construct at the individual level, how can we represent the happiness range and change potential of individuals given a certain genetic disposition? We propose a way of translating heritability estimates into a conception of individual change potentials. Figure 2.1a illustrates Equation 2.1 (shown previously) and also represents part of the univariate latent factor model typically used in analyses of twin data. In this example, the genetic and environmental factors have equally strong effects on well-being, implying a heritability of 50% (i.e., 0.71 squared). Both latent factors, G and E, and the phenotype, well-being, are standardized.
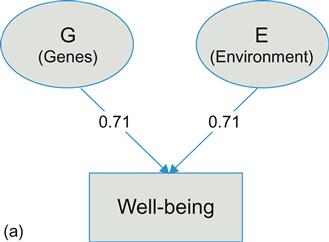
In Figure 2.1b, the phenotypic variance is shown by the upper curve, with SD=1. The lower curve represents the environmental variance in phenotypic well-being and has a standard deviation of 0.71 (hence variance of 0.5). For both curves, the vertical dotted lines designate the area under the curve containing 95% of the distribution.
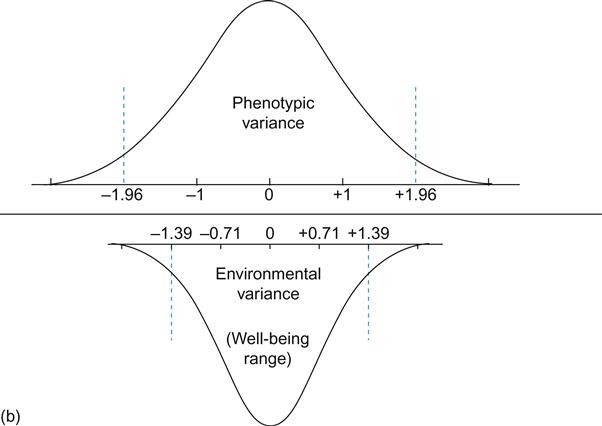
Consider a thought experiment. Imagine a person who has not only one co-twin, but 99 “co-twins,” all together 100 genetically identical individuals. Imagine also that these 100 people have an average genetic propensity for well-being (a potential set point), thus having a zero score on the latent G-factor in Figure 2.1a. All differences among the 100 “twins” will be due to environmental differences. Disregarding the 5% extreme scorers, 95% of the “twins” will have a well-being range within +/–1.39 SD (1.96×0.71). Thus, the range between the dotted lines of the lower curve (labeled “environmental variance” in Figure 2.1b) can be seen as the natural well-being range for any individual with a given genetic disposition. With a lower or higher genetic disposition, the curve would move left or right, respectively. Yet the happiness potential of most individuals is substantial.
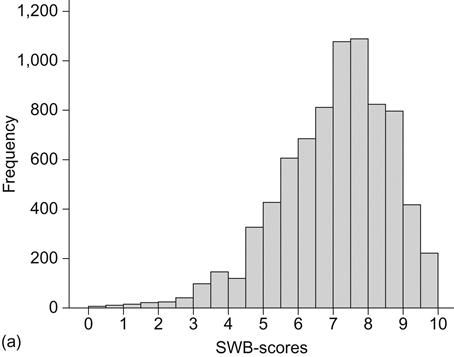
We move next to empirical data to further illustrate the point of individual happiness ranges. The data are based on the Norwegian Twin Registry (Harris, Magnus, & Tambs, 2006; Nilsen et al., 2013). A total of 7,947 twins responded to a questionnaire including an index of SWB. Several papers have been published on these data, showing a heritability of 0.44 (Nes et al., 2005; Røysamb et al., 2003). Figure 2.2a shows the distribution of well-being scores for the whole sample transformed into a 0–10 scale (mean=7.11, SD=1.59).
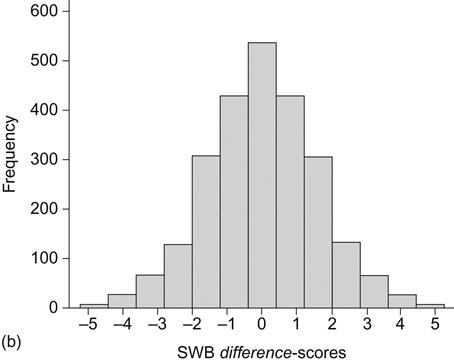
Focusing now on the MZ twins (n=2,554), we calculated the difference score within each pair of twins. Figure 2.2b shows the distribution of difference scores. Roughly half the sample (51.4%) had a difference score of at least +/–1.00, and the remaining half of pairs had an absolute difference of less than 1 point on the 0–10 scale. The mean absolute difference was 1.28 (median=1.00). Thus, in this sample of twins, with a fairly typical mean level and distribution of well-being, and a substantial heritability, the intra-pair differences among identical twins were still substantial. The difference distribution is normal, with most pairs showing moderate differences. Nevertheless, the level of variability within pairs points to substantial influences from environmental factors and a considerable range of change.
Thus far, our examples have focused on cross-sectional measures, where moderate heritability estimates are found. However, as reviewed, longitudinal twin studies indicate that around 80% of the stable variance in well-being is accounted for by genetic factors (Nes et al., 2006). Does this imply that environmental factors, such as happiness interventions, cannot have lasting effects? We believe the answer is no—for several reasons. First, again the extant estimates are based on equations that do not include potential interventions, only the naturally occurring life events and activities. Second, even without specific interventions, there are important within-pair differences across time. To illustrate this, we used our longitudinal data including well-being measures at two time points with 6 years apart (N=1,577 MZ twins). Sample mean scores were 7.24 (T1) and 7.28 (T2). We selected twins who were lastingly very happy (n=194), that is, who scored high (above 8.5) at both Time1 and Time2. The mean scores of this subsample were 9.25 and 9.22 at the two time points, respectively. If these stable high scores were due to a genetic disposition solely, we would expect their identical co-twins to show the same level of well-being. However, the co-twins of the very happy twins scored 8.4 (T1) and 8.34 (T2). Thus, the lastingly very happy twins have co-twins with higher than average scores as well, in accordance with the heritability estimates. Nevertheless, despite the same genetic make-up, at some point the two twins in each pair have split into somewhat different well-being paths. Because the twins are genetically similar, the differences are generally due to environmental factors. It remains to explore whether these include activities, life choices, life events, or other factors, but certain (unknown) factors lead some twins into lastingly more well-being than their co-twins. As such, these data support the notion of environmental effects as not only temporary, but possibly also long term.
Caveats and Criticism
Critics of twin studies and behavior genetic findings often refer to violations of the Equal Environment Assumption (EEA) as causing inflated heritability estimates (Richardson & Norgate, 2005). The EEA is a basic premise of the twin model and involves the assumption that MZ and DZ co-twins are exposed to the same amount of common environment. If MZ twins are treated more similarly—for example, more often being dressed alike—than DZ twins, this treatment or environmental exposure could lead to higher MZ similarity and thus artificially inflated heritability estimates.
However, there are some requirements for EEA violations to represent a problem. First, there has to be evidence of more similar treatment of MZ than DZ twins. Second, the treatment must causally affect the phenotype in focus—for example, well-being. If MZ twins are indeed dressed more alike than DZ twins, but variation in dressing is unrelated to happiness, then the EEA holds. To our knowledge, there is no empirical evidence of EEA violations that have caused inflated heritabilities in the well-being literature. Support for the robustness of findings is also implied by the convergence of results from classic twin studies and studies of twins reared apart. Although the majority of studies have used samples of twins reared together, evidence from twin- and sibling-adoption studies in general support findings from the former (Lykken & Tellegen, 1996; Matteson, McGue, & Iacono, 2013; Tellegen et al., 1988).
Noteworthy is also the notion of evocative gene-environment correlation (McGue, 2010; Rutter et al., 2006). Genetic dispositions may activate certain responses from the environment. Friendly and smiling people activate different responses than do hostile and criticizing people. If MZ twins, with identical genetic dispositions, tend to activate similar social responses, then the similarity in environmental exposure is genetic in its origin. Thus, MZ twins may be treated similarly because of their genetic similarity evoking similar responses in others. The mechanisms involved in gene-environment correlations are important for our understanding of the etiological processes of well-being but should not be seen as a violation of the EEA.
Sibling interaction effects are related to, yet different from, the equal environment assumption. Given that our happiness in general partly depends on the happiness of close family members, and that MZ twins tend to have a particularly close relationship with their co-twins, there might be stronger mutual influences (sibling interaction) among MZ than DZ twins. The notion of happiness as contagious (Fowler & Christakis, 2008) refers primarily to an environmental influence. If happiness is more contagious among MZ than DZ twins, this could contribute to higher MZ than DZ correlations. However, a recent study found adopted adolescents to resemble their biological family, but not their social family, on happiness (Matteson et al., 2013), thus indicating limited happiness contagion between family members.
The basic twin model is also based on the assumption of random mating. Nonrandom mating implies that people tend to fall in love with, and have children with, partners who resemble themselves. There is evidence of nonrandom, or assortative, mating for physical traits such as height and weight, health behaviors, and psychological characteristics such as personality traits, intelligence, and mental health (Ask, Idstad, Engdahl, & Tambs, 2013; Ask, Rognmo, Torvik, Røysamb, & Tambs, 2012; Dufort, Kovess, & Boivin, 1994; Merikangas, 1982). If parents of twins have somewhat similar genetic dispositions for well-being, this could have implications for the parameters estimated. MZ twins share 100% of their genes, regardless of parental resemblance. In contrast, DZ twins share 50% of their segregating genes under the random mating model, but could share a higher proportion of genes if their parents have more than randomly shared genes. This would imply an increased DZ correlation, compared to that of the random model, which in effect would lead to artificially decreased heritability estimates (given h2=2×(rMZ – rDZ)) and increased estimates of common environmental effects. Nonrandom mating may partly be controlled for in extended study designs using data from both twins and parents, and there is no evidence to date that nonrandom mating substantially influences behavior genetic findings on well-being.
Toward an Integrated Model of Genes, Environment, and Change
There is overwhelming evidence of substantial genetic influences on human happiness (Bartels & Boomsma, 2009; Nes, 2010). We believe the well-being field can benefit strongly from not disregarding or dismissing this evidence. Rather, by incorporating current knowledge about the role of genetic and environmental factors, the field is set to move forward to a deeper understanding of processes generating both stability and change in happiness. Recognizing the influence of genetic factors in well-being does not imply a pessimistic outlook on the prospect of change. On the contrary, genes provide us with a potential for change, and by taking into account the role of genes, we may be able to obtain an integrated understanding of change and thereby also develop more effective interventions.
Genes, Set Points, and Change
The finding of genetic factors accounting for a high proportion of the stable variance in well-being (Lykken & Tellegen, 1996; Nes et al., 2006) accords with the theoretical notion of a genetically based set point or set range (Fujita & Diener, 2005; Lucas, 2007; Lykken, 1999). Set point theory has been disputed (Diener et al., 2006; Headey, 2013), and there is a growing need for a more nuanced understanding of set points and change processes. Given that we accept the set point notion as a theoretical construct, several important questions may be raised.
First, how much time do we (have to) spend at the set point? As human lives unfold, happiness naturally fluctuates—depending on external events and our own making of activities, social relations, and life choices. Recall that the twin data (see earlier) indicated that roughly half the sample of identical twins reported well-being scores of more than +/–1 point apart from their co-twin (0–10 scale). Additionally, the theoretical example of 100 identical “twins” showed a substantial phenotypic variation around a genetic mean. Thus, we are probably rarely at our exact set point. On average, people would spend roughly half of the time below and half of the time above their set points. However, if there was a fixed set point, theoretically there is no reason why we should be unable to increase the time spent above this point. Happiness interventions (see, e.g., Armenta, Bao, Lyubomirsky, & Sheldon, Chapter 4 in this volume) are partly about nurturing the factors that contribute to moving up and remaining above a theoretical set point, and growing evidence testifies to their effectiveness (Bolier et al., 2013; Sin & Lyubomirsky, 2009). Of note, if set points are simply operationalized as the mean value of lifetime well-being (Headey, 2013), we will necessarily spend roughly half of the time above or below this point. However, if set points are conceived as an intrinsic tendency (analogous to a disposition to body weight, cholesterol level, or musical talent), there is certainly potential for moving to and remaining on the desired side of this point.
Yet the issue of set points might be more complex. A second important question concerns how set is the set point? Although our genes remain the same throughout life, gene expression may be turned on or off, and different genes may contribute to happiness under different circumstances, activities, and life phases (Nes et al., 2010; Nes et al., 2006; Røysamb, Harris, Magnus, Vittersø, & Tambs, 2002). The genetic dispositions that contribute to the well-being of a 20-year-old college student might be partly different from those contributing to the same person’s well-being at age 50. As well-being flows from a continuous interplay between genes and environment, theoretically it would be possible to alter the genetically based set point, not by altering the genes, but by altering activities and circumstances to allow for flourishing of genetically based potentials. For example, a person with a highly creative disposition (or musical talent, sports talent, intelligence, sociability) might alter her set point by creating a life conducive to the expression of this potential. In this sense, we have not one but several possible set points—depending on the life situations and activities we choose, create, or are exposed to. This way of conceptualizing set points accords with recent findings suggesting that life changes such as unemployment or marriage may alter individual set points (Lucas, 2007; Lucas, Clark, Georgellis, & Diener, 2004). The notion of various possible set points also accords with findings of national differences in well-being. Nations or states with high well-being scores may be seen as doing well at creating environments that allow for optimal set points. If governments build dance floors, people will be happy to do the dancing.
Positive Gene-Environment Interplay
We have reviewed some of the ways genes and environments can operate, interact, and correlate. Here, we argue that these mechanisms may be used actively to generate trajectories of increased well-being. We suggest positive gene-environment interplay as a general concept that includes collaboration and matchmaking between genes and environments. Theoretically, well-being may be enhanced through seeking and creation of environments—including activities, exposures, and relationships—that promote expression and flourishing of genetic potentials (and possibly protect against vulnerabilities).
Although the term positive gene-environment interplay is new, we believe that the processes involved are implicitly present in several extant happiness-enhancing strategies. For example, the intervention activity “using signature strengths in a new way” (Seligman et al., 2005) includes identification of individual top character strengths and exploration of new ways to use these strengths. Given that character strengths are partly heritable (Steger, Hicks, Kashdan, Krueger, & Bouchard, 2007), this activity may be seen as creating environments that allow for increased use of genetic potentials and thereby building of positive experiences and competencies.
At a more general level, person-activity fit (Lyubomirsky & Layous, 2013; Sheldon & Lyubomirsky, 2007) has been proposed as a key moderator of the effects of positive activities. Because there is individual heterogeneity in the types of activities that are optimal for enhancing well-being, identification of activities that fit an individual’s personality is seen as crucial. The notion of person-activity fit is congruent with a model of positive gene-environment interplay—or cooperation between genetic dispositions and choice of activities. Optimal matchmaking could include both amplifying and compensatory processes. By amplifying, we mean active creation of positive gene-environment correlations in which activities provide opportunities to express talents and potentials. Compensatory processes might include identification and acceptance of vulnerabilities (e.g., anxieties, addictions, lack of impulse control) followed by activities for building experiences and competencies that either balance these vulnerabilities or lead them into functional pathways. Generally, our key point is that gene-environment interplay occurs naturally, that some variants of such interplay are highly beneficial for well-being, and that happiness intervention studies could benefit from explicitly addressing the notion of genetic and environmental matchmaking.
Conclusions
Our aim has been to review some recent findings from genetically informative studies and discuss their implications for the prospect of change in well-being. Based on the current knowledge, we believe it is fair to conclude the following:
• Well-being is both heritable and changeable.
• Behavior genetic findings also imply robust evidence of environmental causes of well-being.
• Genetic factors contribute primarily to stability in well-being; environmental factors predominantly contribute to short- and long-term change.
• Heritability estimates depend on environmental variation.
• Genetic factors appear to contribute to individual set points or set ranges. Yet, because genetic influences are conditional on the environment, set points might be changed by altering activities, exposures, life choices, and relations.
• Happiness results from a continuous interplay between genes and environments. Change theories and interventions might benefit from the notion of positive gene-environment interplay. Gene-environment matchmaking implies creating environments and activities that allow for flourishing of genetic potentials—and buffer against vulnerabilities.
Finally, modern well-being research has grown into a large scientific field including several subfields and specialties. Exciting progress is continuously reported. Whereas focus on the core issues within each subfield is necessary to continued progress, we also believe in the importance of building integrative frameworks. Our aim has been to try to show that findings of changeability and heritability are not incongruent. On the contrary, models of change and models of heritability might jointly contribute to a new understanding of how happiness operates and evolves and to the development of optimal strategies for enhanced and sustained human well-being.