CASE 13
A 60-year-old man with hypertension and type II diabetes comes in for a follow-up visit. Along with making appropriate diet and lifestyle changes, he is taking an ACE inhibitor-thiazide diuretic combination for his hypertension and metformin for his diabetes. His blood pressure and diabetes are under acceptable control. Routine blood work revealed normal electrolytes, renal function, and liver enzymes. He is noted to have elevated total cholesterol and low-density lipoprotein (LDL) levels, which have remained high in spite of his lifestyle changes. In an effort to reduce his risk of developing coronary artery disease, you start him on a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor.
 What is the mechanism of action of HMG-CoA reductase inhibitors?
What is the mechanism of action of HMG-CoA reductase inhibitors?
 What effect do they have on total and LDL cholesterol levels?
What effect do they have on total and LDL cholesterol levels?
 What are the common adverse effects of HMG-CoA reductase inhibitors?
What are the common adverse effects of HMG-CoA reductase inhibitors?
ANSWERS TO CASE 13:
Lipid-Lowering Agents
Summary: A 60-year-old man has hypertension, diabetes, and hyperlipidemia and is started on an HMG-CoA reductase inhibitor.
 Mechanism of action of HMG-CoA reductase inhibitors: Competitive inhibition of the rate-limiting enzyme in cholesterol biosynthesis results in compensatory increase in plasma cholesterol uptake in the liver mediated by an increase in the number of LDL receptors.
Mechanism of action of HMG-CoA reductase inhibitors: Competitive inhibition of the rate-limiting enzyme in cholesterol biosynthesis results in compensatory increase in plasma cholesterol uptake in the liver mediated by an increase in the number of LDL receptors.
 Effect on total cholesterol: Up to 30 percent reduction.
Effect on total cholesterol: Up to 30 percent reduction.
 Effect on LDL cholesterol: Up to 50 percent reduction.
Effect on LDL cholesterol: Up to 50 percent reduction.
 Common adverse events: Elevated liver enzymes and hepatotoxicity, myalgia and myositis, irritability, sleep disturbance, anxiety.
Common adverse events: Elevated liver enzymes and hepatotoxicity, myalgia and myositis, irritability, sleep disturbance, anxiety.
CLINICAL CORRELATION
HMG-CoA reductase inhibitors are in wide clinical use with proven benefit in lowering cholesterol levels and reducing the risk of coronary artery disease in susceptible individuals. They competitively antagonize the rate-limiting enzyme in cholesterol biosynthesis. Reduced cholesterol synthesis spurs a compensatory increase in hepatic uptake of plasma cholesterol mediated by an increase in the number of LDL receptors. The net effect of this is to lower the plasma levels of lipoproteins, especially LDL cholesterol. The effect on high-density lipoprotein (HDL) cholesterol is less pronounced. Although there are rare cases of severe hepatotoxicity reported with statins, they are generally well tolerated and routine monitoring of liver function studies (LFTs) is no longer required. Myalgia is a common side effect, but rarely severe, myositis and rhabdomyolysis have occurred. Hepatotoxicity and myositis can occur while using an HMG-CoA reductase inhibitor alone, but they become more likely when combinations of medications are used.
APPROACH TO:
Pharmacology of Lipid-Lowering Drugs
OBJECTIVES
1. Know the drugs used to treat hyperlipoproteinemias.
2. Know the adverse effects and toxicities of the drugs.
3. Know the therapeutic uses of each of the lipid-lowering agents.
DEFINITIONS
Hyperlipidemia: An elevation in either plasma cholesterol or plasma triglycerides or both.
Myopathy: General term for any disease of muscle.
Myositis: Muscle pain with increased creatinine kinase levels.
Rhabdomyolysis: Muscle pain accompanied by a greater than tenfold increase in creatinine kinase above upper limits of normal, indicating serious muscle damage.
LDL cholesterol: Low-density lipoprotein. Atherogenic lipoprotein particle. Several subfractions have been identified, and the smallest are the most atherogenic. It contains apolipoprotein B100 (Apo B100; interacts with LDL receptor), Apo E (interacts with LDL receptors and Apo E receptors), and Apo C (activates lipoprotein lipase).
HDL cholesterol: High-density lipoprotein particle involved in transporting cholesterol from the periphery back to the liver. Has antiatherosclerotic activity. Contains Apo A, C, and D.
VLDL: Very-low-density lipoprotein, a triglyceride-rich lipoprotein particle synthesized in the liver.
DISCUSSION
Class
Drugs that decrease plasma lipids are among the most commonly prescribed today. Some of these affect primarily cholesterol (eg, the statins) and are useful in the treatment of hypercholesterolemia while other agents affect primarily triglycerides (eg, gemfibrozil).
The National Cholesterol Education Program (NCEP) has classified levels of plasma cholesterol (Table 13–1). The LDL cholesterol treatment goal is determined by assessing the risk of cardiovascular disease of individual patients. The major risk factors that modify LDL goals are listed in Table 13–2.
Table 13–1 • NATIONAL CHOLESTEROL EDUCATION PROGRAM (NCEP) LEVELS OF PLASMA CHOLESTEROL
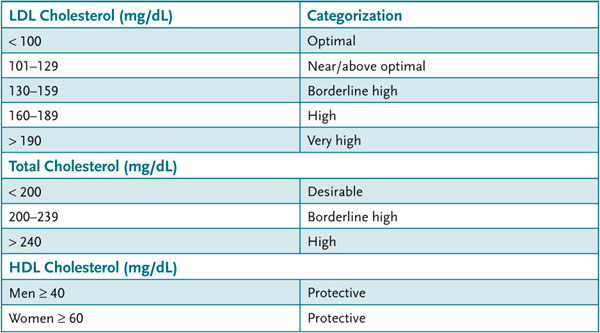
Table 13–2 • RISK FACTORS FOR CARDIOVASCULAR DISEASE
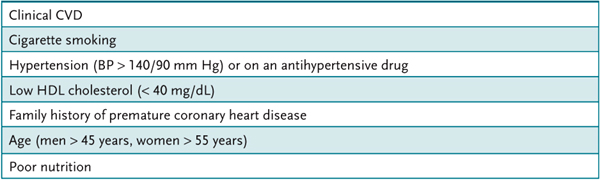
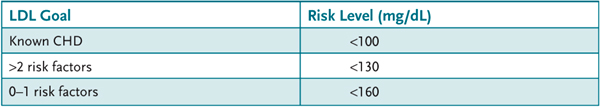
Known coronary heart diseases (CHD) include patients who have had an infarction or angina or a surgical procedure for cardiovascular disease. In addition, patients with peripheral arterial disease, abdominal aortic aneurism, or symptomatic carotid artery disease or diabetes are considered to have known CHD or a high risk for CHD. The NCEP classification and the risk assessment are combined and used to modify the LDL cholesterol goals as illustrated in Table 13–3.
Table 13–3 • CARDIOVASCULAR RISK AND LDL GOAL
AGENTS USED FOR HYPERCHOLESTEROLEMIA
Statins
Of the drugs that decrease plasma cholesterol, the statins have gained the widest use. The statins are structural analogs of the substrate HMG-CoA that inhibit the activity of the enzyme HMG-CoA reductase at nanomolar concentrations. This enzyme is required for the synthesis of isoprenoids and cholesterol. By inhibiting de novo biosynthesis of cholesterol, cellular uptake of cholesterol from plasma via the LDL receptor is increased, reducing plasma cholesterol levels. Because statins have additional actions to inhibit the production of the triglyceride-rich VLDL, this makes them useful in the management of patients with hypertriglyceridemia; atorvastatin and rosuvastatin are particularly effective in this regard. There is evidence that statins also have anti-inflammatory activity, and this may contribute to their reduction in cardiovascular events. Statins may also reduce the rate of bone resorption and thereby lessen osteoporosis. This effect is thought to be caused by the inhibition of isoprenoid biosynthesis in osteoclast precursors, which inhibits their differentiation into mature osteoclasts. A growing body of evidence suggests that statins decrease the risk of stroke, especially in the elderly. Six statins are approved in the United States: lovastatin, rosuvastatin, fluvastatin, atorvastatin, pravastatin, and simvastatin. They differ in efficacy: rosuvastatin has been reported to reduce LDL cholesterol by more than 60 percent; atorvastatin and simvastatin, approximately 50 percent; and pravastatin and fluvastatin, approximately 35 percent. Statins typically increase protective HDL-C, with pravastatin, simvastatin, and atorvastatin reported to cause an 8–10 percent increase. All of the statins are active orally. Lovastatin and simvastatin are prodrugs that are converted to their active metabolite by the liver. Lovastatin, simvastatin, and fluvastatin have relatively short half-lives (1–5 hours) and are most effective if taken at bedtime since the circadian peak of cholesterol synthesis is at night. Rosuvastatin, pravastatin, and atorvastatin have longer half-lives (14–22 hours) and their actions are independent of the time of administration.
The two major adverse effects associated with statin use are hepatotoxicity and myopathy. Hepatotoxicity was initially thought to be as high as 1 percent with elevations in hepatic transaminases as high as 3 times the upper limits. Subsequent clinical trials indicate that the actual incidence of hepatotoxicity is much lower. Muscle pain may occur in as high as 10 percent of patients and is dose dependent. Severe rhabdomyolysis has occurred rarely; however, one statin, cerivastatin, was removed from the market after several rhabdomyolysis-associated deaths.
Bile-Acid-Binding Resins
The bile acid sequestrants are also useful in reducing plasma cholesterol. Cholestyramine, colestipol, and colesevelam are ion-exchange resins that nonspecifically bind bile acids within the intestine and thereby reduce their enterohepatic circulation. This increases de novo hepatic bile acid synthesis and the cholesterol for this synthesis comes, in part, from the plasma via the LDL receptor. Bile acid sequestrants typically reduce plasma cholesterol by 15–20 percent with no effect on triglycerides. Because they are not absorbed, the bile acid sequestrants are quite safe, and adverse effects are typically gastrointestinal and include bloating and constipation. In the intestine, these agents bind many molecules other than bile acids and they impair the absorption of lipid-soluble vitamins and many drugs including digoxin, furosemide, thiazides, coumarin, and some statins. Patient adherence with these drugs is poor.
Inhibitors of Cholesterol Absorption
Ezetimibe is a different class of cholesterol-lowering drug that acts within the intestine to reduce cholesterol absorption. Cholesterol is absorbed from the small intestine by a process that includes specific transporters that include the Niemann-Pick C1-Like 1 (NPC1L1) protein, which is important for sterol absorption in the gut. Ezetimibe binds to and inhibits the function of NPC1L1, thereby reducing cholesterol absorption. Ezetimibe used alone produces a reduction in plasma cholesterol of approximately 19 percent and an approximate 10 percent decline in triglyceride levels. When combined with a statin, reductions in plasma cholesterol as high as 72 percent have been reported in clinical trials. The combination of ezetimibe and low-dose statin can be as effective in lowering LDL-C as maximal doses of a statin—with fewer adverse effects. The complementary mechanisms—inhibition of cholesterol biosynthesis by statins and inhibition of cholesterol absorption by ezetimibe—may be useful in treating patients with refractory hypercholesterolemia. Few adverse effects have been reported with ezetimibe. The most frequently reported adverse effects are diarrhea (4.1%), arthralgia (3.0%), sinusitis (2.8%), and pain in extremity (2.7%).
Nicotinic Acid
Niacin, at doses well beyond those used as a vitamin, has effects on all plasma lipids. It reduces LDL cholesterol by 20 to 30 percent and reduces triglycerides by 35–45 percent. It is the best agent available for increasing HDL. Niacin inhibits VLDL production in the liver by inhibiting both the synthesis and esterification of fatty acids. LDL levels are reduced as a consequence of the decline in VLDL synthesis. Niacin inhibits lipolysis in adipose tissue, which reduces the supply of fatty acids to the liver, further decreasing VLDL synthesis. HDL levels are increased because niacin decreases the catabolism of Apo A1. Niacin is useful in treating hypertriglyceridemia as well as hypercholesterolemia especially in the presence of low HDL. The limiting adverse effect of niacin is cutaneous flushing and itching, and dyspepsia is common at the doses (1 g/day) necessary to affect lipids. These adverse effects can be diminished by taking an aspirin 45 minutes prior the niacin. More medically serious adverse effects include hepatotoxicity and hyperglycemia. Niacin can induce an insulin-resistant state causing hyperglycemia. For this reason, niacin should not be used in diabetic patients.
Agents Used for Hypertriglyceridemia—Fibrates
The fibrates include clofibrate, fenofibrate, ciprofibrate, bezafibrate, and gemfibrozil. These agents predominantly cause a reduction of plasma triglycerides and a small decrease in LDL cholesterol. HDL levels are increased. The fibrates bind to a nuclear receptor peroxisomal proliferator-activator receptor γ (PPAR-γ) mostly in liver and skeletal muscle. Agonist-bound PPAR-γ induces lipoprotein lipase (LPL), which increases the lipolysis of triglyceride-rich VLDL and chylomicrons. Fibrates reduce triglycerides by 35 to 50 percent and LDL cholesterol by 10–20 percent. HDL levels are increased by 10 to 15 percent. All of the fibrates are orally active, but their absorption is decreased by food. The major adverse effect is gastrointestinal upset, cutaneous rash, and itching. Fibrates should not be used in patients with compromised renal function.
COMPREHENSION QUESTIONS
13.1 A 54-year-old man is noted to have hyperlipidemia, and is prescribed lovastatin. Lovastatin reduces plasma cholesterol by which of the following processes?
A. Inhibiting Apo B100 biosynthesis
B. Inhibiting cholesterol absorption
C. Inhibiting cholesterol biosynthesis
D. Interfering with bile acid reabsorption
13.2 Which of the following is a usual effect of niacin?
A. Increases HDL
B. Increases LDL
C. Increases total cholesterol
D. Increases triglycerides
13.3 A 33-year-old man has been prescribed medication for hyperlipidemia. He has been noted to have bleeding from his gums and easy bruisability. His prothrombin time is elevated. Which of the following agents is most likely to be involved?
A. Atorvastatin
B. Cholestyramine
C. Gemfibrozil
D. Niacin
ANSWERS
13.1 C. The statins are competitive inhibitors of HMG-CoA reductase and thereby inhibit de novo cholesterol biosynthesis.
13.2 A. Niacin increases HDL, decreases total and LDL cholesterol, and decreases triglycerides.
13.3 B. Cholestyramine interferes with the absorption of lipid-soluble vitamins such as vitamin K, leading to decreased levels of vitamin K-dependent coagulation factors.
PHARMACOLOGY PEARLS
 The HMG-CoA reductase inhibitors, the statins, are the initial choice of drug for the treatment of hypercholesterolemia.
The HMG-CoA reductase inhibitors, the statins, are the initial choice of drug for the treatment of hypercholesterolemia.
 The statins are structural analogs of the substrate HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) that inhibit the activity of the enzyme HMG-CoA reductase.
The statins are structural analogs of the substrate HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) that inhibit the activity of the enzyme HMG-CoA reductase.
 The two major adverse effects associated with statin use are myopathy and hepatotoxicity.
The two major adverse effects associated with statin use are myopathy and hepatotoxicity.
 Bile acid sequestrants impair the absorption of lipid-soluble vitamins and many drugs including digoxin, furosemide, thiazides, coumarin, and some statins.
Bile acid sequestrants impair the absorption of lipid-soluble vitamins and many drugs including digoxin, furosemide, thiazides, coumarin, and some statins.
 The fibrates including clofibrate, fenofibrate, ciprofibrate, bezafibrate, and gemfibrozil predominantly cause a decline in plasma triglycerides.
The fibrates including clofibrate, fenofibrate, ciprofibrate, bezafibrate, and gemfibrozil predominantly cause a decline in plasma triglycerides.
 Niacin has effects on all plasma lipids and has side effects of flushing and itching.
Niacin has effects on all plasma lipids and has side effects of flushing and itching.
REFERENCES
NCEP Report. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39.
Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, Yeo W, Payne N. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007 Apr;11(14):1–160, iii–iv.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
 What is the mechanism of action of HMG-CoA reductase inhibitors?
What is the mechanism of action of HMG-CoA reductase inhibitors? What effect do they have on total and LDL cholesterol levels?
What effect do they have on total and LDL cholesterol levels? What are the common adverse effects of HMG-CoA reductase inhibitors?
What are the common adverse effects of HMG-CoA reductase inhibitors? Mechanism of action of HMG-CoA reductase inhibitors: Competitive inhibition of the rate-limiting enzyme in cholesterol biosynthesis results in compensatory increase in plasma cholesterol uptake in the liver mediated by an increase in the number of LDL receptors.
Mechanism of action of HMG-CoA reductase inhibitors: Competitive inhibition of the rate-limiting enzyme in cholesterol biosynthesis results in compensatory increase in plasma cholesterol uptake in the liver mediated by an increase in the number of LDL receptors.

