CASE 19
An 18-year-old man is brought into the emergency department after being found on the street unresponsive. He is lethargic and does not answer questions. He has been given 1 ampule of Dextrose intravenously without result. On examination, his heart rate is 60 beats per minute, and respiratory rate is 8 per minute and shallow. His pupils are pinpoint and not reactive. There are multiple intravenous track marks on his arms bilaterally. The emergency physician concludes that the patient has had a drug overdose.
 What is the most likely diagnosis?
What is the most likely diagnosis?
 What is the most appropriate medication for this condition?
What is the most appropriate medication for this condition?
 In addition to its therapeutic actions, what other effects might this medication produce?
In addition to its therapeutic actions, what other effects might this medication produce?
ANSWERS TO CASE 19:
Opioid Overdose
Summary: An 18-year-old unresponsive man presents with pinpoint pupils, shallow respirations, and multiple intravenous track marks on his arms bilaterally.
 Most likely diagnosis: Opioid overdose, likely heroin.
Most likely diagnosis: Opioid overdose, likely heroin.
 Most appropriate medication for this condition: Naloxone.
Most appropriate medication for this condition: Naloxone.
 Additional effects this medication might produce: Symptoms of precipitated withdrawal that may include lacrimation, rhinorrhea, sweating, dilated pupils, diarrhea, abdominal cramping, and tremor.
Additional effects this medication might produce: Symptoms of precipitated withdrawal that may include lacrimation, rhinorrhea, sweating, dilated pupils, diarrhea, abdominal cramping, and tremor.
CLINICAL CORRELATION
Opioids are drugs with morphine-like activity that reduce pain and induce tolerance and physical dependence. Certain individuals seek the euphoria obtained from the intravenous injection of opioids such as heroin. There are three different cell receptors specific for opioids: mu, kappa, and delta (μ, κ, δ), all of which exist as multiple subtypes. This patient has the classic signs of opioid overdose: somnolence, respiratory depression, and miosis.
Stimulation of the mu receptor results in analgesia (supraspinal and spinal), respiratory depression, euphoria, and physical dependence. Continuous, heavy use of opioids can result in tolerance, where more drug is required to obtain the same euphoric “high,” and also to physical dependence. Naloxone, a competitive antagonist of opioids, is used to treat opioid overdose. Its intravenous administration leads to an almost immediate reversal of all effects of the opioids.
In individuals who are physically dependent, administration of naloxone will immediately precipitate opioid withdrawal, which consists of a constellation of signs and symptoms that include nausea and vomiting, muscle aches, lacrimation or rhinorrhea, diarrhea, fever, and dilated pupils. Likewise, when someone physically dependent on opioids ceases its administration, there is a more slowly developing (hours or days) constellation of symptoms of opioid withdrawal that includes sensitivity to touch and light, goose flesh, autonomic hyperactivity, GI distress, joint and muscle aches, yawning, salivation, lacrimation, urination, defecation, and a depressed or anxious mood. In general, physical dependence induced by opioids with a short half-life tends to result in a rapid severe withdrawal, while physical dependence induced by opioids with a long half-life tends to be associated with a less severe and more gradual course of withdrawal. Although very uncomfortable, opioid withdrawal is generally not life-threatening.
The opioid methadone may be administered in a daily dose to individuals physically dependent on opioids, most notably heroin, as a “maintenance therapy” or to ameliorate the symptoms of opioid withdrawal. Only specialists licensed in addition can prescribe methadone for this purpose.
APPROACH TO:
Pharmacology of the Opioids
OBJECTIVES
1. Describe the mechanism of action of opioids as analgesics.
2. Explain how opioids reduce pain.
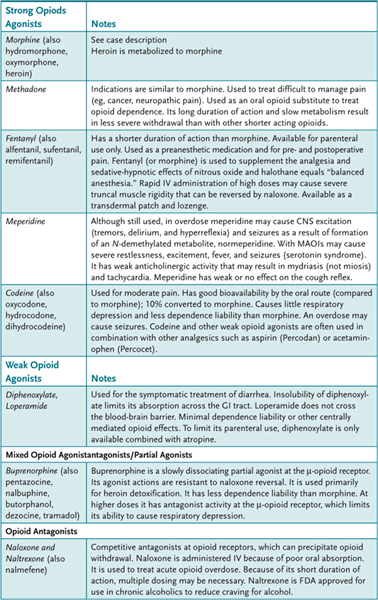
3. List the major opioid agonists and antagonists, their therapeutic uses, and their important pharmacokinetic properties.
4. Describe the adverse effects of opioids.
DEFINITIONS
Addiction: The persistent, compulsive continuation of a behavior or use of a chemical substance despite its adverse physiological, psychological, or social effects.
Drug tolerance: Decreased response to a drug with its continued administration that can be overcome by increasing the dose. A cellular tolerance develops to certain drugs of abuse that act on the CNS because of a poorly understood biochemical or homeostatic adaptation of neurons to the continued presence of the drug. Also, in addition to a cellular tolerance, a metabolic tolerance can develop to the effects of some drugs because they increase the synthesis of enzymes responsible for their own metabolism (alcohol, barbiturates).
Drug dependence: Continued need of the user to take a drug. Psychologic dependence is the compulsive behavior of a user to continue to use a drug no matter the personal or medical consequences. Inability to obtain the drug activates a “craving” that is very discomforting. Physical or physiologic dependence is a consequence of drug abstinence after chronic drug use that results in a constellation of signs and symptoms that are often opposite to the initial effects of the drug and to those sought by the user. Psychologic dependence generally precedes physical dependence but, depending on the drug, does not necessarily lead to it. The development of physical dependence, the degree of which varies considerably for different drugs of abuse, is always associated with the development of tolerance, although the exact relationship is unclear.
Endogenous opioid peptides: Class of natural endogenous peptides that bind to human mu, delta, and kappa opioid receptors. Four classes of such peptides have been described: (1) the pentapeptide enkephalins (met and leu), (2) the endorphins (β-endorphin), (3) the dynorphins (A, B, C), all of which are proteolytically released from larger precursor molecules, and (4) the endomorphins. Together, they may modulate a number of important functions of the body (eg, pain, reactions to stress and anxiety).
Fasciculation: Muscular twitching of contiguous groups of muscle fibers.
Lacrimation: Secretion of tears from the eyes.
Rhinorrhea: Mucous-like material that comes out of the nose.
DISCUSSION
Class
Morphine, the prototype opioid, is derived from opium, a crude material obtained from the seed pod of the opium poppy plant. The chemical structure of morphine is shown in Figure 19–1. Many other derivatives of the opium plant (opiates) and other drugs with similar effects (opioids) have been discovered or synthesized. Chemical modifications of the morphine structure results in significant alterations in potency and in the ratio of agonist to antagonist effects (Table 19–1). However, no major improvement in the analgesic effect of this class of opioids has been achieved; morphine is still one of the most widely used opioids. The opioids are classified in several ways: (1) strength of analgesic effect (strong and weak agents); (2) ratio of agonist to antagonist effects (pure agonists, mixed agonist-antagonists, and antagonists), and (3) actions (analgesic, antitussive, and antidiarrheal drugs). The major therapeutic application for morphine and other strong opioids (eg, fentanyl, hydromorphone, methadone) is the management of moderate to severe pain (eg, pain associated with trauma, burns, cancer, acute myocardial infarction, and renal or biliary colic). Weaker opioids such as codeine and pentazocine are used to manage mild to moderate pain. Other important therapeutic uses include the management of diarrhea (eg, codeine, diphenoxylate, loperamide), dyspnea associated with pulmonary edema secondary to acute left ventricular failure, suppression of the cough reflex (codeine), and maintenance and withdrawal therapy for opioid dependence (methadone, buprenorphine). The antitussive (cough suppressant) action and antidiarrheal action of the opioids are at least partially separable from their analgesic action. Separate drugs have been developed to exploit these effects.
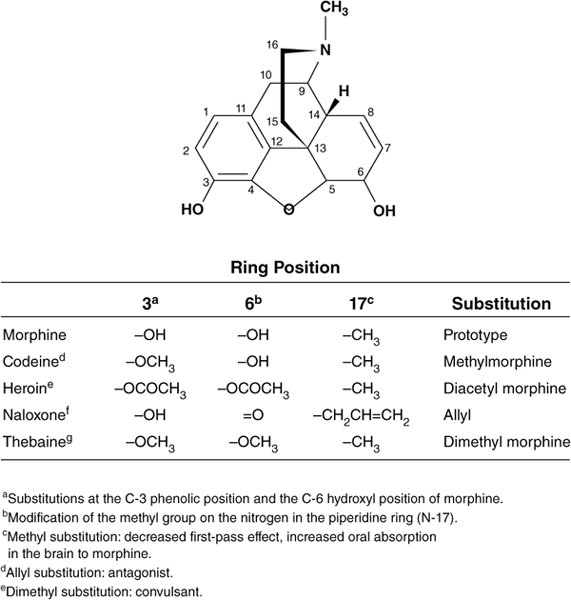
Figure 19–1. Structure-activity relationships of opioids.
Table 19–1 • SELECTED OPIOIDS
The sites of opioid action include areas in the central nervous system (CNS) where they raise the threshold to pain (ie, decrease the sensation of pain) including (1) the spinal cord, where opioids act directly on receptors on the terminals of primary afferent sensory neurons in the dorsal horn of the spinal cord to inhibit release of excitatory transmitters like substance P, (2) the thalamus, where opioids act on ascending pathways to directly inhibit pain transmission from the spinal cord to higher centers of the brain (via the spinothalamic tract and spinoreticular tract), and (3) the midbrain periaqueductal gray area and rostral ventral medulla (nucleus raphe magnus), where opioids activate descending inhibitory neurons to the spinal cord, thus preventing pain transmission. Opioids also act on the cerebral cortex, amygdala, and hippocampus to decrease the emotional reactivity to pain (ie, decrease the perception of pain). There is also a direct inhibitory effect of opioids on sensory nerve endings. In addition to the CNS, opioids also act on other organs including, notably, the GI tract and kidney.
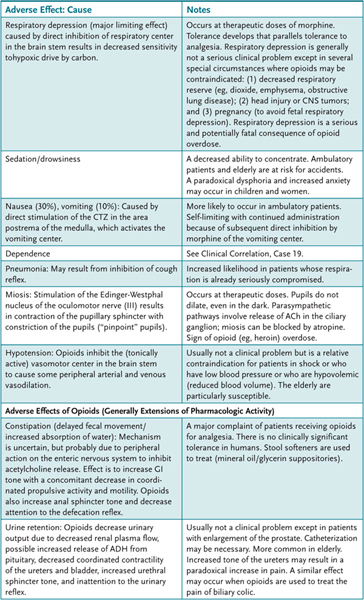
The most commonly observed effects when the opioids are used for the relief of pain are sedation, nausea and vomiting, and constipation. Large doses regularly induce respiratory depression and euphoria or mental clouding. The major adverse effects of selected opioids are presented in Table 19–2. Methadone can cause potentially fatal QTc prolongation.
Table 19–2 • ADVERSE EFFECTS OF OPIOIDS
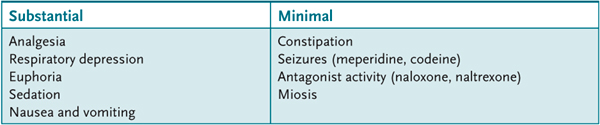
Tolerance to some effects of the opioids (Table 19–3) occurs gradually (days) with repeated administration such that a larger dose is necessary to produce the same initial effect. The tolerance is because of a direct action of opioids on neurons (ie, cellular tolerance) rather than to an increase in their metabolism (metabolic tolerance). Tolerance does not occur to all the effects of the opioid agonists or to the action of antagonists (see Table 19–3). Tolerance to one opioid agonist can confer tolerance to other opioid agonists, that is, cross-tolerance. However, there is no cross-tolerance between opioid agonists and other nonopioid drugs that act on the CNS, such as the benzodiazepines, barbiturates, ethanol, and stimulants.
Table 19–3 • ADVERSE EFFECTS BASED ON RELATIVE TOLERANCE TO OPIOIDS
Opioid-induced respiratory depression may be potentiated in the presence of sedative-hypnotic agents, antipsychotic agents, or antidepressant agents. Opioids, particularly meperidine, may interact with MAOIs (tranylcypromine, phenelzine) to cause serotonin syndrome.
Structure
Opioids may be full agonists (eg, morphine, heroin) or partial agonists (eg, buprenorphine, pentazocine). Morphine is a phenanthrene alkaloid with a phenylmethyl-piperidine ring structure. Simple chemical substitutions can markedly alter its pharmacologic properties (see Figure 19–1).
Mechanism of Action
Opioid agonists bind to G-protein-coupled neural receptors (mu, delta, kappa) to reduce adenylyl cyclase activity, to reduce presynaptic calcium conductance, which causes a decrease in neurotransmitter release, and to enhance postsynaptic potassium conductance, which causes a decrease in cell responsiveness to excitatory neurotransmitters.
Administration
Opioids are usually administered orally, but some like morphine can also be administered rectally or parenterally.
Specialized administration
Patient-controlled analgesia (PCA): By infusion (morphine/meperidine/hydromorphone).
Regional analgesia: Epidural route is favored because it produces fewer adverse effects. They may also be administered into subarachnoid or intrathecal spaces. There may be delayed respiratory depression, nausea, and vomiting that can be reversed with naloxone.
Transdermal fentanyl patch: Used for chronic pain
Buccal fentanyl lozenge/lollipop
Butorphanol nasal spray
Narcotic combinations with acetaminophen and NSAIDs
Pharmacokinetics
Most opioids are absorbed well. Morphine, given orally, shows variable but significant first-pass metabolism (glucuronide conjugation) with a low oral to parenteral potency ratios (25%). It is usually given parenterally. Codeine and methadone are well absorbed after oral administration (approximately 60%) because of limited first-pass metabolism.
All opioids are metabolized by the liver. Metabolism usually results in more polar metabolites and frequently involves conjugation of the phenolic hydroxyl with glucuronic acid. Excretion is primarily by way of the kidneys. In addition to inactive metabolites, morphine is conjugated in the liver to morphine-3-glucuronide, which has neuroexcitatory properties. Morphine is also metabolized (10%) to morphine-6-glucuronide, which at high levels has analgesic potency greater than morphine itself. Codeine and heroin are metabolized to morphine. Heroin is also metabolized to morphine. Meperidine is metabolized to normeperidine that causes seizures in patients where it accumulates. For this reason, its use is discouraged.
The fetal blood-brain barrier is readily crossed by the opioids, and infants born to mothers given (or self-administering) large doses of opioids may have severe respiratory depression.
COMPREHENSION QUESTIONS
19.1 A 25-year-old man underwent surgery for an inguinal hernia. Postoperatively, he receives intravenous morphine sulfate for his pain. Morphine produces analgesia through which of the following actions?
A. Activation of neuronal adenylyl cyclase
B. Increased presynaptic neurotransmitter release
C. Reduction of postsynaptic neuronal potassium conductance
D. Reduction of presynaptic neuronal calcium conductance
19.2 Which of the following opioid agonists is not metabolized to an active agent with analgesic activity?
A. Morphine
B. Codeine
C. Heroin
D. Meperidine
19.3 QTc prolongation is a potentially fatal adverse effect associated with which opioid?
A. Methadone
B. Hydrocone
C. Fentanyl
D. Morphine
ANSWERS
19.1 D. Opioid agonists bind to G-protein-coupled receptors to reduce adenylyl cyclase activity, to reduce presynaptic calcium conductance, which results in a decrease in neurotransmitter release, and to enhance postsynaptic potassium conductance, which results in decreased responsiveness to excitatory neurotransmitters.
19.2 D. Meperidine is metabolized to normeperidine that may result in seizures. Morphine is metabolized to morphine-6 glucuronide. Codeine and heroin are metabolized, in part, to morphine.
19.3 A. Potentially fatal QTc prolongation is a side effect unique to methadone. ECG monitoring is important during its therapeutic use.
PHARMACOLOGY PEARLS
 Seeking the relief of pain is one of the most common reasons for patient visit.
Seeking the relief of pain is one of the most common reasons for patient visit.
 Prescription drug abuse is a growing problem, and accidental deaths from use of oxycodone, hydrocodone, and morphine, among others, are increasing.
Prescription drug abuse is a growing problem, and accidental deaths from use of oxycodone, hydrocodone, and morphine, among others, are increasing.
 Seizures may occur in patients with renal failure because of the action of the morphine metabolite morphine-3-glucuronide.
Seizures may occur in patients with renal failure because of the action of the morphine metabolite morphine-3-glucuronide.
 Treatment of chronic, noncancer pain using opioids remains controversial. However, treatment of acute pain, or pain in patients with a terminal illness, is generally medically necessary.
Treatment of chronic, noncancer pain using opioids remains controversial. However, treatment of acute pain, or pain in patients with a terminal illness, is generally medically necessary.
REFERENCES
Ferrante FM. Principles of opioid pharmacotherapy: practical implications of basic mechanisms. J Pain Symptom Manage. 1996;11(5):265–73.
Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10.
Loeser JD, ed. Bonica’s Management of Pain. Philadelphia: Lippincott Williams & Wilkins, 2001.
McCleane G, Smith HS. Opioids for persistent noncancer pain. Med Clin North Am. 2007;91(2):177–97.
Turk DC, Wilson HD, Cahana A. Treatment of chronic noncancer pain. Lancet. 2011;377:2226.
Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155:325.
 What is the most likely diagnosis?
What is the most likely diagnosis? What is the most appropriate medication for this condition?
What is the most appropriate medication for this condition? In addition to its therapeutic actions, what other effects might this medication produce?
In addition to its therapeutic actions, what other effects might this medication produce? Most likely diagnosis: Opioid overdose, likely heroin.
Most likely diagnosis: Opioid overdose, likely heroin.


