CASE 39
A 67-year-old man complains of pain in his right hip for the past few weeks. He has had no injury to the area and describes the pain as a “bone ache” that does not radiate. Review of systems is positive only for some weakness of urinary stream and having to get up twice a night to go to the bathroom. His general physical examination is normal. His hip examination is normal with a full range of motion and no tenderness. Examination of his prostate reveals it to be firm, enlarged, and nodular. Blood tests show a markedly elevated prostate-specific antigen (PSA), and biopsy of the prostate shows carcinoma. A bone scan confirms the presence of metastatic disease in the right hip. Along with other adjuvant therapies, a decision is made to start depot leuprolide acetate.
 Leuprolide acetate is an analog of which hypothalamic hormone?
Leuprolide acetate is an analog of which hypothalamic hormone?
 What is the mechanism of action of leuprolide acetate?
What is the mechanism of action of leuprolide acetate?
 Which pituitary hormones are affected by leuprolide acetate, and how are they affected?
Which pituitary hormones are affected by leuprolide acetate, and how are they affected?
ANSWERS TO CASE 39:
Drugs Active on the Hypothalamus and Pituitary Gland
Summary: A67-year-old man with metastatic prostate cancer is to receive depot leuprolide acetate.
 Leuprolide acetate is an analog of which hypothalamic hormone: Gonadotropin-releasing hormone (GnRH).
Leuprolide acetate is an analog of which hypothalamic hormone: Gonadotropin-releasing hormone (GnRH).
 Mechanism of action of leuprolide acetate: Chronic administration of GnRH analog results in the reduction of the number of GnRH receptors in the pituitary (downregulation), with resultant decreases in pituitary gonadotropin production.
Mechanism of action of leuprolide acetate: Chronic administration of GnRH analog results in the reduction of the number of GnRH receptors in the pituitary (downregulation), with resultant decreases in pituitary gonadotropin production.
 Pituitary hormones affected: Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) production is reduced.
Pituitary hormones affected: Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) production is reduced.
CLINICAL CORRELATION
The hypothalamic-pituitary-gonadal axis is a classic example of a hormonal stimulation-negative feedback system. The hypothalamus produces GnRH, which binds to specific receptors on pituitary gonadotropic cells. These cells then produce LH and FSH, which act on the gonads. LH and FSH regulate the female menstrual cycle by their effects on the ovarian follicles and the ovarian production of estrogen and progesterone. In males, LH and FSH regulate spermatogenesis and the production of testosterone in the testes. Estrogen, progesterone, and testosterone then function as feedback signals for the hypothalamic production of GnRH. Leuprolide acetate is a synthetic 9-amino acid analog of GnRH. When initially administered, leuprolide acetate results in increases in LH, FSH, and gonadal steroid production because of its action as a GnRH agonist. However, with chronic administration, there is a reduction in the number of GnRH receptors in the pituitary gonadotropic cells. This causes a reduction in FSH or LH production and a resultant reduction in gonadal hormone production. In women this effect may be beneficial in conditions such as endometriosis, where estrogen stimulates the growth and activity of the ectopic endometrial tissue, which causes symptoms. The effect in men is to lower the production of testosterone to near castrate levels. Because prostate cancer is often testosterone dependent, leuprolide acetate can be used as a treatment for prostate cancer in those who are not surgical candidates, do not desire surgery, or have metastatic disease. Leuprolide acetate must be administered parenterally, and it has a depot form which is active for up to 3 months. It commonly causes “menopausal” side effects, such as hot flashes, as a result of the reduction in gonadal hormone production. Other antiandrogenic drugs such as abiraterone, which blocks the conversion of pregnenolone to androgens by inhibiting CYP17, can be used in combination with leuprolide or as sole therapeutic agents.
APPROACH TO:
Pharmacology of Neuroendocrine Drugs
OBJECTIVES
1. Understand the receptors and second messengers involved in the endocrine system.
2. Understand the hypothalamic-pituitary axis and its feedback system.
3. Know the drugs used as agonists and antagonists on the hypothalamic-pituitary axis, their therapeutic uses, mechanisms of action, and adverse effects.
DEFINITIONS
Prostate cancer: Common malignancy in men that may be confined to the prostate gland or metastasize to pelvic lymph nodes or bone.
Hormonal therapy: Various malignancies are sensitive to hormones, and thus medications that act as agonists or antagonists are used for therapy.
DISCUSSION
Class of Agents
The hypothalamic-hypophyseal-end organ system is a classic negative feedback pathway (Figure 39–1). The multiple steps in this regulatory pathway, and both positive and negative regulation, provide several targets for pharmacologic intervention. The hypothalamus secretes a number of releasing factors, including GnRH, corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), and growth hormone-releasing hormone (GHRH), that are of clinical significance.
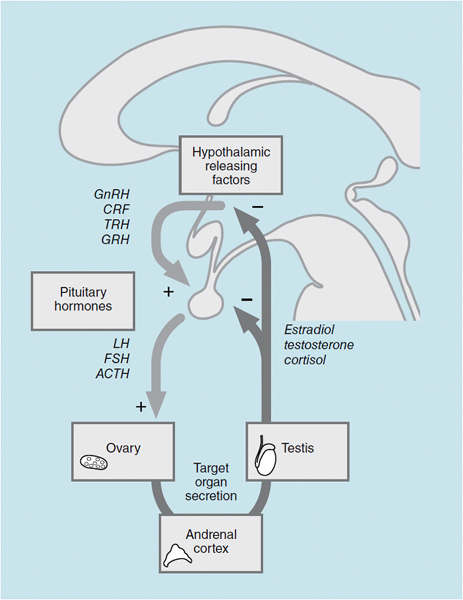
Figure 39-1. Interaction among the hypothalamus, pituitary, and gonad. GnRH = gonadotropin-releasing hormone; CRH = corticotropin-releasing hormone; TRH = thyrotropin-releasing hormone; GHRH = growth hormone-releasing hormone; LH = luteinizing hormone; FSH = follicle-stimulating hormone; ACTH = adrenocorticotropic hormone.
These neuroendocrine factors are secreted by the hypothalamus into the hypothalamic-hypophyseal portal circulation, and they act on cognate cell types within the pituitary and cause an increase in the secretion of specific pituitary hormones. For example, GnRH produces an increase in the synthesis and release of both gonadotropins, LH and FSH. This action is mediated by a specific seven-transmembrane G-protein-coupled receptor that binds GnRH in cells called gonadotrophs.
FSH acts on the ovary to cause follicular development and maturation; LH causes an increase in the production of estradiol and is required for maintenance of the corpus luteum. The LH surge at midmenstrual cycle triggers ovulation. In men, FSH is required for spermatogenesis, and LH causes an increase in testosterone production. The actions of the two gonadotropins are also mediated by specific G-protein-coupled receptors in the ovary and testis.
17β-Estradiol and testosterone are released into the circulation, and these sex hormones have effects on many tissues. Predominantly estradiol in women, and testosterone and estradiol in men (produced by peripheral conversion of testosterone to estradiol), act on the hypothalamus and pituitary to decrease the production of the releasing hormone and the gonadotropins, respectively. This closes the negative feedback loop. As in all target tissues, the estrogen and testosterone receptors in the pituitary and hypothalamus are nuclear receptors that modulate the transcription of target genes.
The adrenal cortex is regulated in a similar manner. Corticotropin-releasing factor (CRF) is released from the hypothalamus, and it elicits the synthesis and release of adrenocorticotropic hormone (ACTH) from the pituitary. ACTH acts on the adrenal cortex and causes an increase in the synthesis of cortisol from the zona fasciculata and adrenal androgens from the zona reticularis.
The secretion of growth hormone by the pituitary is regulated in a different manner. Growth hormone secretion is stimulated by the hypothalamic hormone GHRH and is inhibited by somatostatin. Somatostatin acts in a number of tissues besides the pituitary; it inhibits the release of glucagon and insulin from the pancreas and inhibits the secretion of a number of gut peptides. Prolactin secretion from the pituitary is also controlled by positive and negative regulatory factors. The most important pharmacologically is the prolactin-inhibitory factor (PIF) activity of dopamine agonists.
APPROACH TO:
Pharmacologic Uses of Hypothalamic Peptides and Analogs
Leuprolide acetate and gonadorelin acetate are synthetic peptide GnRH analogs that are administered either by subcutaneous injection, as a long-acting implant, or by IV infusion. Nafarelin acetate is a comparable peptide analog that can be administered by nasal spray. The frequency of administration is critical to the therapeutic goal. Acute or pulsatile administration of GnRH analogs increases production of LH and FSH by the pituitary. Used in this manner, GnRH analogs are useful to stimulate spermatogenesis and testosterone production in men, and to induce ovulation or treat primary hypothalamic amenorrhea in women. Chronic administration, for example, daily injections or use of depot preparations, decreases the production of FSH and LH by the pituitary. This is caused by a depression in the number of GnRH receptors on gonadotrophs. Chronic leuprolide administration can be used to achieve maximum androgen blockade (MAB), which reduces testosterone production by the testis that is therapeutically equivalent to orchiectomy. In men, this is useful to control androgen-dependent hyperproliferation as in advanced prostate cancer and prostatic hyperplasia. In women, chronic leuprolide leads to markedly diminished estrogen production, which is useful in treating a number of estrogen-dependent hyperproliferative diseases. These include endometriosis, polycystic ovary disease, and uterine leiomyomas. Chronic leuprolide has also been used to treat hirsutism in women. The major adverse effect in women is a chemical menopause with vasomotor symptoms and the potential for osteoporosis. In men, leuprolide has been associated with the flare phenomenon, increased cancer growth as a result of transient increase in testosterone production on initiation of therapy. Other adverse effects in men include hot flashes, gynecomastia, and testicular atrophy. A new class of pure GnRH antagonists, including cetrorelix and ganirelix, have been approved for treatment of infertility. These agents do not cause the initial agonist activity seen with leuprolide. Their main advantage is a reduction in the required days of fertility drug therapy per cycle from several weeks (ie, 3 weeks) to several days. These agents are not yet approved for use in men.
Somatostatin is unique among the hypothalamic peptides because of its widespread inhibitory activity on secretion and cellular proliferation. Octreotide is an 8-amino acid cyclopeptide with potent somatostatin agonist activity. Its action to decrease secretion of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide makes it useful to treat hypersecretory states such as VIPomas, chronic pancreatitis, and watery diarrhea from a number of causes including AIDS. Its antiproliferative uses include colorectal cancer and leukemia, and diabetic retinopathy. It is in clinical trials for additional malignancies. It has also been used to treat acute portal hypertension. It is approved for use in the treatment of acromegaly. Adverse effects include nausea, cramps, and increased gallstone formation.
Other hypothalamic peptides are used primarily as diagnostic agents. GHRH is a 40-amino acid peptide that can be administered IV for the diagnostic evaluation of idiopathic growth hormone deficiency. Similarly, IV administration of TRH is useful in the differential diagnosis of thyroid diseases. CRH is a 41-amino acid polypeptide found in the hypothalamus and the gut. CRH is used in cases of ACTH deficiency to distinguish between hypothalamic-pituitary or primary adrenal disease.
The PIF activity of bromocriptine or levodopa can be used to treat states of prolactin excess as in some cases of amenorrhea, galactorrhea, and prolactin-secreting tumors.
COMPREHENSION QUESTIONS
39.1 Which of the following best describes the action of somatostatin?
A. Inhibition of growth hormone release
B. Inhibition of prolactin release
C. Stimulation of insulin release
D. Stimulation of LH release
39.2 In the first 2 weeks following a single injection of leuprolide in a man, one would expect which of the following?
A. Decreased LH production
B. Decreased testosterone production
C. Increased LH receptors
D. Increased testosterone production
39.3 A 22-year-old woman has severe endometriosis with dysmenorrhea. She is treated with depot leuprolide acetate. One week after her first injection, she notes a marked increase in the dysmenorrhea. What is the explanation?
A. Direct effect of leuprolide on the endometrial implants
B. Likely flare with increased gonadotropin effect prior to downregulation of receptors
C. Probable placebo effect
D. Resistance of her endometriosis to the leuprolide and probable need for another agent
ANSWERS
39.1 A. Somatostatin is a major regulator of growth hormone, and its effect is inhibitory of growth hormone release.
39.2 D. Acute leuprolide will increase FSH/LH and sex steroid production and have little effect on receptor numbers.
39.3 B. The initial response to GnRH analog is an increase in FSH and estrogen, leading to an exacerbation of the endometriosis. Thereafter, there is a downregulation of GnRH receptors of the pituitary, leading to a decrease in FSH and estrogen.
PHARMACOLOGY PEARLS
 The frequency of administration of leuprolide determines its effect:
The frequency of administration of leuprolide determines its effect:
 Acute administration will increase FSH/LH and sex steroids, and chronic administration will decrease FSH/LH and sex steroids.
Acute administration will increase FSH/LH and sex steroids, and chronic administration will decrease FSH/LH and sex steroids.
 Chronic leuprolide administration leads to androgen blockade in men, which is useful in treating hormone-dependent cancers such as prostatic carcinoma.
Chronic leuprolide administration leads to androgen blockade in men, which is useful in treating hormone-dependent cancers such as prostatic carcinoma.
REFERENCES
Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol. 2011;29(6 Suppl):S1-8.
Bousquet C, Lasfargues C, Chalabi M, Billah SM, Susini C, Vezzosi D, Caron P, Pyronnet S. Clinical review: current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J Clin Endocrinol Metab. 2012;97:727–37.
 Leuprolide acetate is an analog of which hypothalamic hormone?
Leuprolide acetate is an analog of which hypothalamic hormone? What is the mechanism of action of leuprolide acetate?
What is the mechanism of action of leuprolide acetate? Which pituitary hormones are affected by leuprolide acetate, and how are they affected?
Which pituitary hormones are affected by leuprolide acetate, and how are they affected? Leuprolide acetate is an analog of which hypothalamic hormone: Gonadotropin-releasing hormone (GnRH).
Leuprolide acetate is an analog of which hypothalamic hormone: Gonadotropin-releasing hormone (GnRH).