CASE 44
A 66-year-old woman presents for an annual health maintenance visit. She is generally feeling well and has no specific complaints. She takes hydrochlorothiazide for hypertension, levothyroxine sodium for hypothyroidism, and a multivitamin. She went through menopause at age 48 and never took hormone replacement therapy. She is a former cigarette smoker, having a 30 pack-year history and having quit 20 years ago. She occasionally has a glass of wine with dinner and walks three or four times a week for exercise. On examination you note that her height is 1 inch less than it was 3 years ago. Her vital signs are normal. She has a prominent kyphoscoliosis of the spine. Her examination is otherwise unremarkable. Blood work reveals normal electrolytes, renal function, blood count, calcium, and thyroid-stimulating hormone (TSH) levels. You order a bone density test, which shows a significant reduction of density in the spine and hips. You diagnose her with osteoporosis and start her on alendronate sodium.
 What is the mechanism of action of parathyroid hormone (PTH) on the bone and in the kidney?
What is the mechanism of action of parathyroid hormone (PTH) on the bone and in the kidney?
 What is the mechanism of action of alendronate sodium?
What is the mechanism of action of alendronate sodium?
ANSWERS TO CASE 44:
Agents Affecting Calcium Homeostasis
Summary: A 66-year-old woman with osteoporosis is prescribed alendronate.
 Mechanism of action of PTH on the bone: Pulsatile administration, the normal physiologic mode, enhances bone formation. Continuous delivery, for example, as a consequence of a parathyroid tumor, results in bone resorption.
Mechanism of action of PTH on the bone: Pulsatile administration, the normal physiologic mode, enhances bone formation. Continuous delivery, for example, as a consequence of a parathyroid tumor, results in bone resorption.
 Mechanism of action of PTH in the kidney: Increases reabsorption of Ca2+ and Mg2+ and increases production of vitamin D and the active metabolite calcitriol and decreases reabsorption of phosphate, bicarbonate, amino acids, sulfate, sodium, and chloride.
Mechanism of action of PTH in the kidney: Increases reabsorption of Ca2+ and Mg2+ and increases production of vitamin D and the active metabolite calcitriol and decreases reabsorption of phosphate, bicarbonate, amino acids, sulfate, sodium, and chloride.
 Mechanism of action of alendronate sodium: Inhibition of osteoclastic activity in bone, which reduces bone reabsorption.
Mechanism of action of alendronate sodium: Inhibition of osteoclastic activity in bone, which reduces bone reabsorption.
CLINICAL CORRELATION
PTH has multiple actions on bone. Chronic elevations in PTH, for example, from a tumor, stimulate the resorption of bone via its stimulation of the number and activity of osteoclasts. This is mediated by specific PTH receptors in the bone, coupled to an increase in cyclic adenosine monophosphate (cAMP). Intermittent administration of PTH stimulates bone growth. Estrogen is an indirect inhibitor of PTH activity in the bone. This effect allows premenopausal women to maintain higher levels of bone density. Following menopause, with the resultant decrease in circulating estrogen levels, there is a relative increase in osteoclastic activity and resorption of bone, with a net loss of bone mineral density. Alendronate sodium is an analog of pyrophosphate that directly binds to bone. It inhibits osteoclastic activity, reducing the resorption of bone. This retards the progression of bone density loss and may allow for increases in density, because osteoblastic activity is not affected. It is administered orally, and its most common adverse effects are gastrointestinal (GI). It may produce esophagitis, and even esophageal perforation, if the pill were to get caught in the esophagus while swallowing. For that reason, patients taking alendronate are instructed to take it on an empty stomach with a full glass of water and to remain upright for at least 30 minutes after ingesting the medication.
APPROACH TO:
Pharmacology of Agents Regulating Calcium Homeostatis
OBJECTIVES
1. Know the structure, actions, and uses of PTH.
2. Describe the structure, actions, and uses of calcitonin (CT).
3. Describe the structure, synthesis, actions, and uses of vitamin D and its metabolites.
4. Know the secondary agents that affect calcium homeostasis and their characteristics.
DEFINITIONS
Osteocyte: A bone-maintaining cell, an embedded osteoblast.
Osteoblast: Bone-forming cells derived from the stroma of bone.
Osteoclast: Bone-resorptive cell derived from myeloid lineages.
OPG (osteoprotegerin): A member of the OPG, OPGL (osteoprotegerin ligand), RANK (receptor activator of nuclear factor-κB) signal transduction cascade that is central to bone metabolism.
DISCUSSION
Class
Calcium is the major extracellular divalent ion. It has diverse roles—including enzyme activation, secretion, excitation-contraction coupling in all muscle types, and neuronal function—and is a critical structural element in bone and teeth.
Approximately 40–50 percent of serum calcium exists as free, ionized Ca2+. This is the biologically active fraction, and it is maintained at approximately 2.5 mM in the serum. An additional 40 percent is bound to serum proteins and the remainder is complexed to ions such as phosphate, citrate, and bicarbonate. The serum concentration of Ca2+ is tightly regulated by several endocrine systems and three major tissues: the gut, kidney, and bone.
Bone is the storage depot for over 99 percent of calcium in the body, and most of the calcium is in the form of hydroxyapatite: [Ca10(PO4)6(OH)2]. Bone is a remarkably dynamic tissue and bone remodeling is a continuous process. Normal bone is continuously reabsorbed by the action of osteoclasts, and new bone is formed by the action of osteoblasts; if these two processes are not equal in magnitude, excess bone can be lost, as in osteoporosis, or too much bone can be formed. Coupling of the actions of osteoblasts and osteoclasts is largely under the control of the OPG/RANKL signaling system (Figures 44–1A and 44–1B).
Osteoblasts produce RANKL, a polypeptide that binds to receptors on osteoclasts termed RANK-R, a member of the TNF receptor family. Stimulation of RANK-R leads to increased proliferation, maturation, and activation of osteoclasts. Osteoblasts also elaborate osteoprotegerin, which is a molecular antagonist of RANKL. OPG can bind RANKL before it can activate the RANK-R on osteoclasts. Osteoclast activation is thus controlled by the ratio of OPG/RANKL that is secreted by osteoblasts. Most drugs that act to alter calcium homeostasis in bone do so by altering OPG/RANKL. Many cytokines and hormones such as estradiol and PTH alter bone metabolism by changing OPG/RANKL.
PTH is an 84-amino acid peptide synthesized in the parathyroid glands and is secreted in response to low serum-ionized Ca2+. PTH 1–34, the N-terminal portion of PTH, has full biologic activity. In the kidney it acts to increase Ca2+ reabsorption and promotes phosphate (PO42-) excretion. It has indirect effects on the GI system to increase Ca2+ absorption by stimulating vitamin D production. The effects of PTH on bone are complex and dependent on the temporal nature of its release or administration.
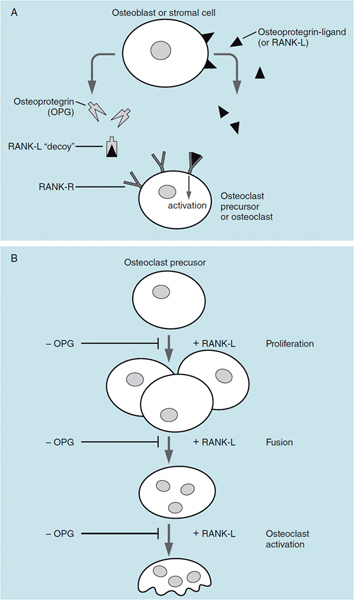
Figure 44–1. Osteoblast (A) and osteoclast (B). The OPG/RANKL-signaling system controls the number and activity of osteoclasts. Osteoblasts secrete both OPG and RANKL. Activation of RANK-R by RANKL stimulates proliferation and activation of osteoclasts. OPG is a “decoy” that binds RANKL in the interstitial space and prevents its association with its receptor (RANK-R).
Continuously elevated PTH, as in hyper-parathyroidism, increases osteoclast activity via increased RANKL and results in increased bone resorption. Pulsatile release of PTH activates osteoblasts and increases bone formation.
CT is a 32-amino acid polypeptide produced in the parafollicular cells of the thyroid. It is secreted in response to elevated serum Ca2+ levels. CT increases OPG and decreases RANKL release from osteoblasts, and its action on bone is to reduce bone turnover. In response to CT, osteoclasts withdraw reabsorptive processes, shrink in size, and retract the ruffled border from the surface of bone; CT effectively prevents all stages of osteoclastic bone resorption. It increases renal excretion of Ca2+, PO42-, Mg2+, Cl-, and K+ by decreasing reabsorption of these ions.
The third endocrine system that has effects on bone is vitamin D3 and its metabolites. Vitamin D3 (not a true vitamin in a nutritional sense) is a prehormone that undergoes a series of metabolic alterations to the final agonist in the pathway, 1,25-(OH)2. vitamin D3. Vitamin D3 is synthesized from cholesterol in the skin in a two-step photo-dependent reaction. Vitamin D3 is converted by the liver enzyme 25-hydroxylase to 25-(OH) D3; in the kidney the enzyme 1-hydroxylase metabolizes 25-(OH) D3 to 1,25-(OH) 2 D3.1,25-(OH)2 D3 acts on the intestine to increase intestinal absorption of Ca 2+. In the kidney, 1,25-(OH)2 D3 acts to increase the absorption of both Ca2+ and PO42-. 1,25-(OH)2 D3 stimulates Ca2+ mobilization from bone and enhances the resorptive action of PTH on bone. However, 1,25-(OH)2 D3 also induces osteocalcin and osteopontin, two matrix proteins important in bone formation.
Treatment of Hypocalcemia
Calcium Salts A wide variety of preparations are available for both IV and oral administration for treating acute hypocalcemic tetany. These include calcium gluconate, calcium lactate, calcium carbonate, and calcium citrate. They vary in the percentage of calcium by weight from a low of 9 percent for calcium gluconate to a high of 40 percent for calcium carbonate.
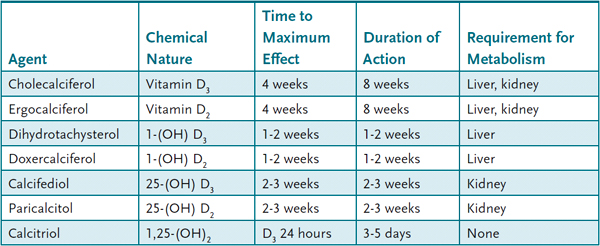
Vitamin D Several vitamin D or vitamin D-related agents are available for use for hypocalcemia and osteoporosis (Table 44–1). Selection of which agent to use depends on the desired onset of action, duration of effect, and the presence of underlying liver or kidney disease. Thiazide diuretics act on the kidney to increase Ca2+ reabsorption in the distal convoluted tubule and can be used in the treatment of hypocalcemia.
Table 44–1 • VITAMIN D-RELATED AGENTS
Treatment of Hypercalcemia Hypercalcemia has a number of pathophysiologic causes including hyperparathyroidism, Paget disease, and hypercalcemia of malignancy. CT is useful for short-term treatment of hypercalcemia. Salmon CT is more potent and has a longer half-life than human CT and is the form used therapeutically. CT has few side effects but refractoriness frequently develops. CT is available for parenteral and nasal administration. Peak plasma concentration after an inhaled dose is approximately 30 minutes after administration, but normalization of the rate of bone turnover as in Paget disease may take several months.
Bisphosphonates are analogs of pyrophosphate in which the phosphodiester (P-O-P) bond is replaced by a nonhydrolyzable bisphosphonate (P-C-P) bond. First-generation bisphosphonates included sodium etidronate. Second-generation aminobisphosphonates include risedronate, alendronate, pamidronate, tiludronate, clodronate, zoledronate, and ibandronate. The two classes of bisphosphonates have different mechanisms of action and different potencies. For example, risedronate is 1000 times more potent as an inhibitor of bone resorption than etidronate. All bisphosphonates bind to and accumulate in bone, and this provides a measure of tissue specificity. The first-generation nonnitrogenous bisphosphonates are converted into an adenosine triphosphate (ATP) analog that cannot be internalized. This metabolite impairs osteoclast function and triggers osteoclast apoptosis. The aminobisphosphonates are not converted into an ATP analog; rather they interfere with mevalonate and ubiquitin metabolism (similar to statins). This leads to impaired posttranslational modification of a number of proteins that are critical to osteoclast function. Ultimately, the aminobisphosphonates lead to osteoclast hypofunction. Etidronate is available for oral use; the aminobisphosphonates may be administered orally or by infusion. Administered orally, all the bisphosphonates have very poor (approximately 5%) bioavailability, but sufficient drug is absorbed to achieve therapeutic concentrations in bone. All bisphosphonates are approved for treatment of Paget disease; alendronate, risendronate, zoledronate, and ibandronate are also approved for prevention and treatment of osteoporosis (pamidronate is approved for treatment of osteoporosis). The remaining aminobisphosphonates are used to treat hypercalcemia of malignancy. Adverse effects of bisphosphonates include GI upset, diarrhea, and nausea. Bisphosphonates are associated with lower esophageal erosion, and the recommendation with alendronate and risedronate is to avoid lying down for 30 minutes after oral administration to avoid reflux. There have been reports of bisphosphonate use associated with the osteonecrosis of the jaw.
Loop diuretics increase the amount of Ca2+ excreted and can be used in the acute management of hypercalcemia.
Treatment of Osteoporosis Osteoporosis, loss of bone mass, affects nearly 30 percent of women aged 65 years and older and a smaller but significant percentage of men. Historically, osteoporosis has been divided into postmenopausal osteoporosis, which occurs in women and is related to the loss of ovarian hormones after menopause, and senile osteoporosis, which is age related and affects both sexes. Histologically and biochemically, they seem indistinguishable disorders of bone metabolism caused by excessive bone reabsorption or inadequate bone formation. Adequate dietary Ca2+ and vitamin D (to facilitate Ca2+ absorption) is critical in patients at risk for osteoporosis. The recommended daily allowance (RDA) for Ca2+ in patients at risk is 1200 to 1500 mg/day.
Teriparatide (PTH 1–34) has been approved for the treatment of osteoporosis. Administered intermittently, once a day by injection, teriparatide increases bone formation in excess of resorption. This treatment has been shown to increase bone mass and decrease the incidence of fractures. Studies in rats receiving very high doses of teriparatide for 2 years demonstrated an increased frequency of osteosarcoma. It is contraindicated in patients with bone malignancy or in pediatric patients. Major adverse effects are hypotension, hypocalcemia, dizziness, and nausea.
Estrogens (see Case 40) have been shown to reduce the rate of bone loss in the postmenopausal period when the rate of loss can be as high as 10 percent per year. Estrogens increase bone mineral density and decrease the incidence of vertebral and nonvertebral fractures. However, estrogens do not increase net bone formation.
Selective estrogen receptor modifiers (SERMs) are compounds whose estrogenic activities are tissue selective. Three SERMs are currently approved for use: tamoxifen, raloxifene, and toremifene. Raloxifene is approved for the prevention and treatment of osteoporosis; tamoxifen and toremifene are used to treat breast cancer. Raloxifene is a polyhydroxylated nonsteroidal compound that binds to the estrogen receptor, but it has estrogen-agonist activity only in bone and the liver; it has no effect on the uterus, and it is an estrogen antagonist in breast tissue and in the brain. It has antiresorptive activity in bone. It increases bone mineral density and has been shown to decrease the incidence of vertebral and nonvertebral fractures. Adverse effects include hot flashes and leg cramps. More serious adverse effects include an approximate threefold increase in deep vein thrombosis and pulmonary embolism.
Denosumab is a monoclonal antibody against RANKL. It is approved for use in postmenopausal women at high risk of fracture. It is also approved as a treatment to increase bone mass in patients who are at high risk of fracture from receiving androgen deprivation therapy for nonmetastatic prostate cancer or aromatase inhibitor (AI) therapy for breast cancer. In men with nonmetastatic prostate cancer, denosumab reduced the incidence of vertebral fracture.
Sodium fluoride has been examined in a number of clinical trials for the treatment of osteoporosis. Early studies using relatively high doses reported an increase in bone mineral density but no decrease in the incidence of fractures, probably because of the formation of abnormal hydroxylapatite crystals in bone. More recent studies using slow-release monofluoride have suggested a decrease in fracture rates, but fluoride is not yet approved for the treatment of osteoporosis.
COMPREHENSION QUESTIONS
44.1 Which of the following vitamin D preparations would be the most appropriate in a patient with poor renal function?
A. Calcifediol
B. Calcitriol
C. Cholecalciferol
D. Ergocalciferol
44.2 Intermittent administration of PTH produces which of the following?
A. Impaired Ca2+ absorption in the gut
B. Inhibition of 1-hydroxylase
C. Net increase in bone formation
D. Net increase in bone resorption
44.3 A 53-year-old woman who is being treated for metastatic breast cancer is noted to have some lethargy, fatigue, and an elevated serum calcium level. She is brought into the emergency department for near comatose state, thought to be caused by the hypercalcemia. After addressing the ABCs (airway, breathing, circulation), which of the following is the best therapy for this patient?
A. Bisphosphonates
B. CT
C. IV estrogen therapy
D. Saline infusion and furosemide
44.4 A postmenopausal women with a family history of osteoporosis completes a bone mineral density work-up and you find her T-score is – 2.6. She tried a short course of teriparitide a year ago but complained of serious depression and mood changes. You elect to try an antibody-based therapy and schedule a time for an injection. Which of the following is the drug you have selected?
A. Calcitonin
B. Dihydrotachysterol
C. Denusomab
D. Infliximab
ANSWERS
44.1 B. 1-Hydroxylase activity must be adequate to produce 1,25(OH)2 D3. Calcitriol is the only choice that is already 1-hydroxylated.
44.2 C. Intermittent administration of PTH on its analogs will result in bone formation. Continuous dosing or a PTH-secreting tumor will cause bone resorption.
44.3 D. Loop diuretics, given with IV normal saline, are the best choice in a patient with acute-onset hypercalcemia.
44.4 C. Denosumab is an antibody against RANLK. It blocks proliferation and activation of osteoclasts and is administered every 6 months by SQ injection. Calcitonin is not very effective in osteoporosis, DHT is a vitamin D analog. Inflizimab is an anti-TNF antibody used for RA and IBS.
PHARMACOLOGY PEARLS
 Teriparatide (PTH 1–34) is the only agent on the market that promotes new bone formation.
Teriparatide (PTH 1–34) is the only agent on the market that promotes new bone formation.
 Estrogens slow the rate of resorption but do not increase bone formation.
Estrogens slow the rate of resorption but do not increase bone formation.
 Thiazide diuretics promote renal reabsorption of Ca2+; loop diuretics have the opposite effect.
Thiazide diuretics promote renal reabsorption of Ca2+; loop diuretics have the opposite effect.
 Bisphosphonates can lead to severe esophageal erosions; patients are advised to not lie down for 30 minutes after taking them.
Bisphosphonates can lead to severe esophageal erosions; patients are advised to not lie down for 30 minutes after taking them.
REFERENCES
Cooper C, Reginster JY, Cortet B, et al. Long-term treatment of osteoporosis in postmenopausal women: a review from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the International Osteoporosis Foundation (IOF). Curr Med Res Opin. 2012;28:475–91.
Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–19.
Lyritis GP, Georgoulas T, Zafeiris CP. Bone anabolic versus bone anticatabolic treatment of postmenopausal osteoporosis. Ann N Y Acad Sci. 2010 Sep;1205:277–83.
 What is the mechanism of action of parathyroid hormone (PTH) on the bone and in the kidney?
What is the mechanism of action of parathyroid hormone (PTH) on the bone and in the kidney? What is the mechanism of action of alendronate sodium?
What is the mechanism of action of alendronate sodium? Mechanism of action of PTH on the bone: Pulsatile administration, the normal physiologic mode, enhances bone formation. Continuous delivery, for example, as a consequence of a parathyroid tumor, results in bone resorption.
Mechanism of action of PTH on the bone: Pulsatile administration, the normal physiologic mode, enhances bone formation. Continuous delivery, for example, as a consequence of a parathyroid tumor, results in bone resorption.
