CASE 52
A 22-year-old man is brought to the emergency room unresponsive and in respiratory distress. He was found unconscious at home next to a suicide note and an empty bottle of methanol. A brief history from an accompanying family member is significant for the patient having depression, but he is not currently on any medications. On examination he is not responsive to verbal stimuli but has pupillary and pain responses, and he is tachypneic and tachycardic (rapid respiratory and heart rates). His lungs are clear. You quickly institute supportive measures, intubate the patient, and send blood tests that confirm a profound anion-gap metabolic acidosis. No other drugs are found in his system. You diagnose him with an acute methanol overdose, start him on IV fluids, sodium bicarbonate, and an IV infusion of ethanol.
 What enzyme metabolizes methanol?
What enzyme metabolizes methanol?
 What is the rationale for using ethanol to treat methanol toxicity?
What is the rationale for using ethanol to treat methanol toxicity?
ANSWERS TO CASE 52:
Solvent Toxicities
Summary: A 22-year-old man with methanol poisoning is being treated with IV ethanol.
 Enzyme that metabolizes methanol: Alcohol dehydrogenase.
Enzyme that metabolizes methanol: Alcohol dehydrogenase.
 Rationale for ethanol in methanol poisoning: Competes for metabolism by alcohol dehydrogenase to reduce the production of toxic metabolites of methanol.
Rationale for ethanol in methanol poisoning: Competes for metabolism by alcohol dehydrogenase to reduce the production of toxic metabolites of methanol.
CLINICAL CORRELATION
The toxicity of methanol is primarily mediated by its metabolites. Methanol is metabolized by alcohol dehydrogenase to formaldehyde and subsequently to formic acid, the most likely cause of major organ toxicity. Formic acid inhibits cytochrome oxidase activity, resulting in tissue hypoxia and lactic acid production. The metabolic acidosis that occurs in methanol overdose is a result of the combination of formic acid and lactic acid that is produced. The most characteristic symptom in methanol poisoning is visual disturbances with blurred vision and a sense of “being in a snowstorm.” Isopropyl alcohol and ethylene glycol similarly are metabolized by alcohol dehydrogenase to toxic metabolites. Unfortunately, the toxic metabolites of all of these solvents can cause permanent neurologic damage, blindness, coma, and death. In these clinical settings ethanol can be used therapeutically. It is given by continuous IV infusion to compete for metabolism by alcohol dehydrogenase. With hemodialysis, this can help to reduce the ongoing production of toxins. Sodium bicarbonate can be given to help correct the metabolic acidosis. Fomepizole, another available, very costly (∼$4000 per patient) inhibitor of alcohol dehydrogenase is also available.
APPROACH TO:
Pharmacology of Solvent Toxicity
OBJECTIVES
1. Outline the basic principles of toxicology, including the dose-response relationship and risk and duration of exposure to toxins.
2. List the classes of solvent toxins and describe how exposure occurs and the effects of exposure.
DEFINITIONS
Toxicology: Study of the deleterious effects of chemical, biological, and physical substances, including their deleterious effects on the human body.
Xenobiotics: Deleterious foreign substances.
Toxicokinetics: Study of the absorption, distribution, metabolism, and elimination of xenobiotics.
DISCUSSION
Class
In considering the human toxicity of xenobiotics, it is important to keep in mind the following general principles:
The toxicokinetics of xenobiotics is equivalent to the pharmacokinetics described for drugs used as therapeutic agents.
Exposure to toxic substances is generally either occupational or environmental (air, soil, water, etc).
Certain xenobiotics (eg, acids, alkali, strong reducing and oxidizing agents, detergents) cause nonspecific damage to tissues by altering proteins, nucleic acids, lipids, and other macromolecules that are integral to cell structure and integrity.
Biotransformation of chemical toxicants may result in formation of reactive metabolites or the production of free radicals and reactive oxygen that form covalent bonds with proteins, nucleic acids, and lipids to disrupt cell function.
For many xenobiotics, a dose-response relationship for toxicity cannot be directly determined from human data but rather must be based on data derived strictly from animal studies.
In addition to decreasing or eliminating exposure, management of the poisoned patient is supportive and depends on the specific tissue or organ or tissue involved (Table 52–1).
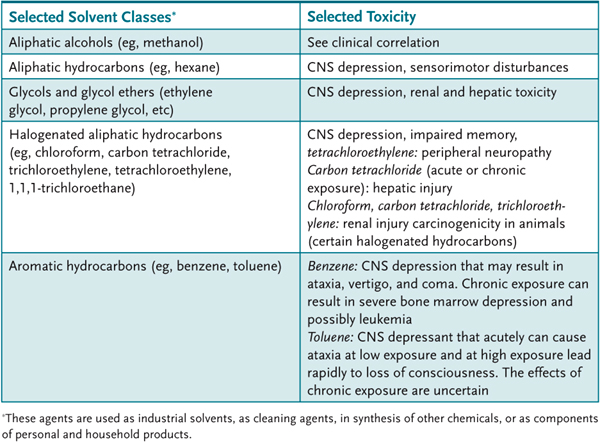
Table 52–1 • SOLVENT CLASSIFICATION AND TOXICITY
COMPREHENSION QUESTIONS
52.1 Which of the following is the most characteristic symptom in methanol poisoning?
A. Carcinogenicity
B. Hepatic injury
C. Renal injury
D. Visual disturbances
52.2 A 45-year-old alcoholic male is brought into the emergency department due to ingestion of wood alcohol (methanol). Which of the following compounds is the most likely cause of organ toxicity from methanol?
A. Formaldehyde
B. Formic acid
C. Lactic acid
D. Methanol
52.3 A 34-year-old lab technician has been noted to have anemia, low white blood cell count, and thrombocytopenia. Severe bone marrow depression is most likely to result from exposure to which of the following solvents?
A. Benzene
B. Ethylene glycol
C. Hexane
D. Toluene
ANSWERS
52.1 D. The most characteristic symptom in methanol poisoning is visual disturbances. Hepatic injury, renal injury, and potential carcinogenicity are more characteristic of the halogenated aliphatic hydrocarbons overdose.
52.2 B. Methanol is metabolized by alcohol dehydrogenase to formaldehyde, which is then metabolized to formic acid, the most likely cause of methanol’s organ toxicity. Formic acid inhibition of cytochrome oxidase activity results in tissue hypoxia with the production of lactic acid, which with formic acid, can result in metabolic acidosis.
52.3 A. Chronic exposure to benzene can result in bone marrow depression. Exposure to toluene results in CNS depression. The effects of chronic exposure to toluene are uncertain. Hexane is more likely to cause CNS depression and sensorimotor disturbances. Ethylene glycol is likely to cause CNS disturbances and renal disturbances.
PHARMACOLOGY PEARLS
 Toxicity from the solvents commonly affect the CNS, causing sedation or CNS depression.
Toxicity from the solvents commonly affect the CNS, causing sedation or CNS depression.
 Exposure to carbon tetrachloride can lead to hepatic toxicity.
Exposure to carbon tetrachloride can lead to hepatic toxicity.
 Chronic exposure to benzene may lead to bone marrow depression and possibly leukemia.
Chronic exposure to benzene may lead to bone marrow depression and possibly leukemia.
 Regional poison control centers are available 24 hours a day (1-800-222-1222) to assist with treatment of the poisoned patient.
Regional poison control centers are available 24 hours a day (1-800-222-1222) to assist with treatment of the poisoned patient.
REFERENCES
Klassen CD, ed. Casarett and Doull’s Toxicology, the Basic Science of Poisons, 8th ed. New York: McGraw-Hill, 2010.
Rom WM, Markopwitz S, ed. Environmental and Occupational Medicine, 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins, 2007.
Sivilotti ML. Ethanol: tastes great! Fomepizole: less filling! Ann Emerg Med 2009;53:451.
U.S. National Library of Medicine. Toxnet: http://toxnet.nlm.nih.gov, 2007.
 What enzyme metabolizes methanol?
What enzyme metabolizes methanol? What is the rationale for using ethanol to treat methanol toxicity?
What is the rationale for using ethanol to treat methanol toxicity? Enzyme that metabolizes methanol: Alcohol dehydrogenase.
Enzyme that metabolizes methanol: Alcohol dehydrogenase.