CASE 53
A woman brings her 5-year-old son into your office for evaluation. He has had progressive difficulty walking in the past few months and has seemed irritable. He has also seemed quite tired. He has vomited on one or two occasions over the past week. His past medical history is unremarkable. He was born after an uncomplicated, full-term pregnancy. He has had all of his vaccines and has achieved all of his developmental milestones for his age. He lives with his parents in a home that was built in the 1920s, which they have been renovating. On examination, the child is somewhat restless but cooperative. His conjunctiva and mucous membranes are pale. Neurologic examination is significant for an ataxic gait (clumsiness). His general examination is otherwise unremarkable. A complete blood count (CBC) shows him to be anemic. A serum lead level is markedly elevated. You admit him to the hospital and start him on dimercaprol and ethylenediaminetetraacetic acid (EDTA).
 How does chronic lead exposure cause anemia?
How does chronic lead exposure cause anemia?
 What is the mechanism of action of dimercaprol and EDTA?
What is the mechanism of action of dimercaprol and EDTA?
ANSWERS TO CASE 53:
Heavy Metal Poisoning
Summary: A 5-year-old boy with lead toxicity is admitted to the hospital and started on dimercaprol and EDTA.
 Mechanism of lead-induced anemia: Inhibition of δ-aminolevulinic acid dehydratase, which blocks the conversion of δ-aminolevulinic acid to porphobilinogen, disrupting hemoglobin synthesis.
Mechanism of lead-induced anemia: Inhibition of δ-aminolevulinic acid dehydratase, which blocks the conversion of δ-aminolevulinic acid to porphobilinogen, disrupting hemoglobin synthesis.
 Mechanism of action of dimercaprol and EDTA: Dimercaprol and EDTA increase the excretion of heavy metals by chelation, which forms soluble substances that can be excreted.
Mechanism of action of dimercaprol and EDTA: Dimercaprol and EDTA increase the excretion of heavy metals by chelation, which forms soluble substances that can be excreted.
CLINICAL CORRELATION
Most lead toxicity in children is a result of GI ingestion. Children absorb a greater portion of ingested lead than adults do. The source of this lead is often from lead-based paint that was widely used before the 1970s. Inorganic lead binds to hemoglobin and distributes to soft tissues, including the brain. It later accumulates in the bone, from which it is eliminated very slowly. Lead produces anemia via the inhibition of the enzyme δ-aminolevulinic acid dehydratase, which converts δ-aminolevulinic acid to porphobilinogen. This interrupts the pathway of synthesis of hemoglobin. Lead can also cause CNS effects, especially in children. Common signs include vertigo, ataxia, headache, restlessness, and irritability. Vomiting, delirium, and seizures may occur. Lowered IQ and behavioral disturbances may be the result of childhood exposure. Peripheral neuropathy is another possible outcome of chronic lead poisoning.
Treatment of lead toxicity requires cessation of exposure and, in severe cases, chelation therapy. In children, dimercaprol and EDTA are the first-line drugs of choice frequently used. Calcium disodium edentate is administered intravenously or intramuscularly; dimercaprol is given intramuscularly. These drugs are chelators, which bind lead, forming soluble compounds that are excreted in the urine. Other drugs used for chelation include succimer, calcium disodium EDTA, and penicillamine.
Lead screening is a required component of well child exams. In addition to serum lead testing, questionnaires are also done to assess for exposure in the environment or food. Common sources include foreign candies, home remedies for abdominal pain, paint in old homes, family member’s occupational exposure, and cooking in foreign-made pots.
APPROACH TO:
Pharmacology of Heavy Metal Poisoning
OBJECTIVES
1. Discuss the general principles related to heavy metal poisoning.
2. List the common sources of heavy metal exposure and describe their mechanisms of action and their toxicities (Table 53–1).
Table 53–1 • HEAVY METALS AND TOXIC EFFECTS
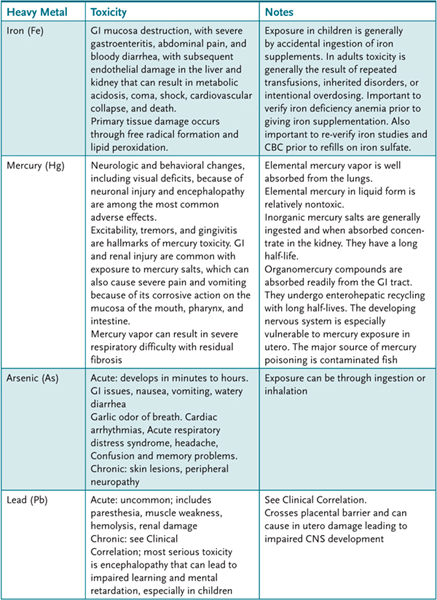
3. List agents used for the management of heavy metal poisoning and describe their mechanisms of action.
DISCUSSION
Class
The general principles related to heavy metal toxicity are the following:
Exposure to heavy metals may be acute or chronic, generally through accidental occupational or environmental exposure.
Most, if not all, heavy metals interact with sulfhydryl groups and perhaps other functional groups of cell proteins to cause their toxicity.
Multiple organ systems may be affected but particularly the CNS, liver, kidney, and respiratory and immune systems.
Management of metal poisoning involves removal of the source, decontamination, and supportive treatment for symptoms. In addition, metal chelators can be used to remove or prevent metal binding to important cell constituents (Table 53–2). The major chelators interact with the same functional groups as the heavy metals to form complexes that can then be eliminated from the body. With the exception of deferoxamine, their selectivity is relatively poor in that they also bind important endogenous divalent cations, notably Ca2+.
Table 53–2 • HEAVY METAL CHELATORS

COMPREHENSION QUESTIONS
53.1 A 4-year-old child is suspected of having lead poisoning from paint from an old building. Which of the following heavy metal chelators is used as a primary treatment for mercury poisoning?
A. Dimercaprol
B. EDTA
C. Penicillamine
D. Succimer
53.2 A 3-year-old girl is brought into the emergency department for possible heavy metal poisoning. The child has severe gastroenteritis, abdominal pain, and bloody diarrhea. Which of the following heavy metals is most likely the cause of this child’s symptoms?
A. Arsenic
B. Elemental liquid mercury
C. Iron
D. Lead
53.3 A 2-year-old toddler is noted to have a hemoglobin level of 9 g/dL (anemia), likely from pica (eating the dirt around a battery plant). A peripheral smear indicates some basophilic stippling of red blood cells. Serum lead levels confirm lead poisoning. Lead-induced anemia is a result of inhibition of which of the following enzymes?
A. Aldehyde dehydrogenase
B. Cytochrome oxidase
C. δ-Aminolevulinic acid dehydratase
D. Monoamine oxidase
ANSWERS
53.1 A. Dimercaprol is used as a primary heavy metal chelator for treatment of mercury poisoning. EDTA and succimer are used for lead poisoning. Penicillamine is used to treat copper poisoning. EDTA and penicillamine are used as adjuncts to treat mercury poisoning.
53.2 C. Iron poisoning causes characteristic GI mucosa destruction, with severe gastroenteritis, abdominal pain, and bloody diarrhea. Elemental liquid mercury is relatively nontoxic. A nonspecific GI, CNS, and cardiovascular toxicity is caused by arsenic. The most serious (chronic) toxicity from lead poisoning is encephalopathy.
53.3 C. Lead produces anemia via the inhibition of the enzyme δ-aminolevulinic acid dehydratase, which converts δ-aminolevulinic acid to porphobilinogen. This interrupts the pathway of synthesis of hemoglobin.
PHARMACOLOGY PEARLS
 Iron toxicity can lead to gastroenteritis, liver, and kidney damage and, if severe enough, death.
Iron toxicity can lead to gastroenteritis, liver, and kidney damage and, if severe enough, death.
 The most common manifestations of mercury poisoning are the neuronal toxicity such as visual deficits.
The most common manifestations of mercury poisoning are the neuronal toxicity such as visual deficits.
 Lead poisoning can lead to mental retardation in the developing fetus or children.
Lead poisoning can lead to mental retardation in the developing fetus or children.
 Regional poison centers are available to assist with treatment (1-800-222-1222).
Regional poison centers are available to assist with treatment (1-800-222-1222).
REFERENCES
American Academy of Pediatrics Committee on Environmental Health. Lead exposure in children: prevention, detection and management. Pediatrics 2005;116:1036.
Clarkson TW, Magos L, Myers GJ. The toxicity of mercury-current exposures and clinical manifestations. N Engl J Med. 2003;349:1731.
Fine JS. Iron poisoning. Curr Probl Pediatr. 200;30:71.
Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167.
Klassen CD, ed. Casarett and Doull’s Toxicology, the Basic Science of Poisons, 8th ed. New York: McGraw-Hill, 2010.
Rom WM, Markopwitz S, ed. Environmental and Occupational Medicine, 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins, 2007.
Shannon M, Woolf A, Binns H. Chelation therapy in children exposed to lead. N Engl J Med. 2001;345:1212.
U.S. National Library of Medicine. Toxnet: http://toxnet.nlm.nih.gov, 2007.
 How does chronic lead exposure cause anemia?
How does chronic lead exposure cause anemia? What is the mechanism of action of dimercaprol and EDTA?
What is the mechanism of action of dimercaprol and EDTA? Mechanism of lead-induced anemia: Inhibition of δ-aminolevulinic acid dehydratase, which blocks the conversion of δ-aminolevulinic acid to porphobilinogen, disrupting hemoglobin synthesis.
Mechanism of lead-induced anemia: Inhibition of δ-aminolevulinic acid dehydratase, which blocks the conversion of δ-aminolevulinic acid to porphobilinogen, disrupting hemoglobin synthesis.
