Metals
Abstract
Metals and metalloids are naturally occurring elements comprising about two-thirds of the periodic table of elements. They are primarily found in soil but also may be dissolved in water, especially at low pH. Several metals are essential for living organisms, such as copper, iron, and zinc. Others, such as arsenic, lead, and mercury, are highly toxic to most organisms. Exposure to metals usually comes through ingestion of food, water, or soil, but actual assimilation is complex and depends on the presence of other metals and whether the metal of concern is in its elemental form, an ionic form, or attached to some organic molecule such as methyl. Organometals are particularly toxic because they are more readily taken into cells, where they can cause many issues, including cancer and genotoxicity.
Keywords
Heavy metals; metalloid; minimum daily requirement (MDR); competitive binding; metallothionein; minamata disease; organometals
Introduction
In Chapters 4 and 5, we studied organochlorine and current-use pesticides. These compounds are intentionally designed to be lethal towards unwanted organisms including plants and animals. In Chapters 6 and 7, we looked at polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs) that are not designed to kill, but are persistent and can be very toxic. All of these are organic molecules because they have carbon and hydrogen as principal constituents, however none are required for living organisms. Other than PAHs, which can be naturally occurring, the vast majority of the chemicals in those chapters were also synthetic. In this chapter, we discuss a very different type of contaminant that is both inorganic and, in some cases, required for life; they all are naturally occurring in their native form. Metals and metalloids are elements that occupy a large portion of the Periodic Chart of Elements (Fig. 8.1).
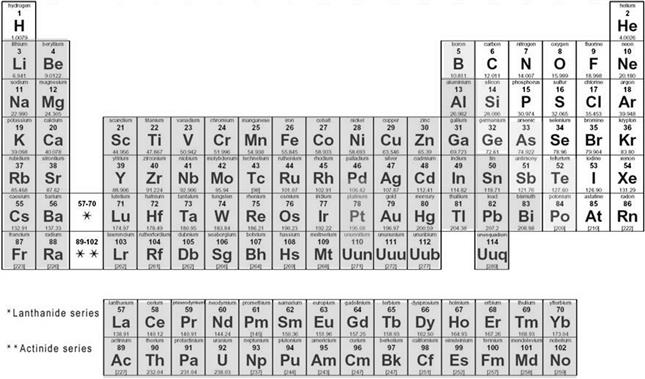
Given the breadth of elements that belong to the metals group, there is a wide diversity in their chemical behavior. In general, with the exception of mercury, metals share common properties of being malleable (can be shaped permanently); ductile (capable of being drawn into thin strands); generally good electrical and thermal conductors; fusible (capable of being melted and blended); opaque; have a metallic luster; solid at room temperature; typically contain two to three electrons in outermost valence shell; and easily ionized. Solubility of metals in water varies from those elements at the far left of the periodic chart—that need to be protected from water due to high reactivity—to those that, as we progress from left to right, are soluble especially in acidic solutions, and those that are reactive only with strong acids to those that are virtually inert. Mercury is unique in that it is a liquid at room temperature and hence is neither malleable nor ductile except at low temperatures. Metalloids share some but not all of the properties of metals and include boron (B), selenium (Se), germanium (Ge), arsenic (As), actinium (Ac), tellurium (Te), and polonium (Po).
From a toxicological perspective, the metals that have received the greatest attention are cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), copper (Cu), nickel (Ni), and zinc (Zn). These are called heavy metals because most have higher molecular weights and densities than iron (Fe). However, that source of infinite wisdom, Wikipedia, considers any metal of environmental concern a heavy metal. Thus, the term seems to be in the eye of the beholder, so to speak. Suffice to say that the technicalities of what constitutes a heavy metal or even what distinguishes a metal from a metalloid are not all that pertinent for our study. If you are really interested in these matters, many universities offer courses in inorganic chemistry, perhaps you have already taken one. For our purposes, we will focus on the metals that are of greatest ecotoxicological significance.
Important characteristics of heavy metals include their solubility in water and their concomitant lipophobicity. Metals may bioconcentrate for short durations but do not biomagnify because they have these characteristics. However, some heavy metals complex with organic molecules and these can bioconcentrate along food chains.
Sources of Metals in the Environment
The natural sources of metals are in the lithosphere or terrestrial environment. Volcanoes are a natural source of metals from deep within the earth to the crust. Sometimes they occur in their elemental or native forms, but are often combined with other metals, sulfides, carbamates, oxides, and other chemical groups. Metals with economic value are mined and smelted in various ways to produce purer forms of the raw substance. The mining and processing of metal ores are among the principal anthropogenic sources of metals in the environment. Other sources of environmental contamination occur when metals are used in industrial applications. Historically, mining and industrial processes have been far from environmentally friendly and often allowed effluents to contaminate rivers and streams. This still goes on despite regulations to the contrary, but the amount of pollution has decreased dramatically since the 1960s. Another way that some metals enter the atmosphere is through combustion of fossil fuels, especially coal. Technological advancements can remove many of these contaminants from the coal and from combustion products—the so-called smokestack scrubbers, leading to cleaner exhausts. The key to clean burning, however, is installing these scrubbers which easily cost many thousands of dollars. Prior to catalytic converters, automobile exhausts were also significant sources of lead, but the converters now require unleaded gasoline.
Slag, or the residue that is left behind after minerals are extracted from ore, can still contain high concentrations of metal mixtures, even with more efficient extraction procedures. Modern treatment of this slag usually involves depositing it into landfills. Older and poorly managed landfills can be inadequately isolated from the underlying soil, allowing the metals and other toxins to leach into groundwater.
Biological Effects of Metals
Paracelsus (1493–1541) who some call the “father of modern toxicology,” stated that the “dose makes the poison,” meaning that all things can be poisonous if given in sufficient amounts. He may well have been thinking of metals. Among animals and plants some metals are necessary in comparatively large amounts and are macronutrients. Most essential metals, however are needed in only small quantities and are called micronutrients. What distinguishes large versus small quantities? One way is to consider lipids, carbohydrates, and proteins as macronutrients. However, the amount of iron, copper, or zinc needed by animals is high compared to other metals and these can be considered as macronutrients as well. The amount of these macronutrients and micronutrients that humans require each day is called the Minimum Daily Requirements (MDRs) that are established by the US Food and Drug Administration. These MDRs may vary somewhat by sex and age. Other standards for nutrients including these essential minerals include the Recommended Daily Allowances (RDAs) that are set somewhat higher and indicate what people in certain age and sex brackets should be ingesting; these are set by the Food and Nutrition Board of the National Institute of Medicine. Tolerable Upper Levels (TUL), also defined by the Food and Nutrition Board, indicate the maximum amount of intake before toxicity occurs. Maximum allowable amounts usually exceed MDRs by at least an order of magnitude but eventually metal toxicity can occur at persistently high dietary concentrations.
There is a balance between sufficient intake of metals and toxic intake (Fig. 8.2). At very low intake, physiological problems can occur because the processes dependent on the metals cannot take place. At somewhat higher levels, minor physiological problems that might be difficult to diagnose can occur. At some range, there is an optimum amount of metal being assimilated. At higher doses than optimum, toxicity may begin to set in and become a serious problem, possibly leading to death at even higher intake concentrations.
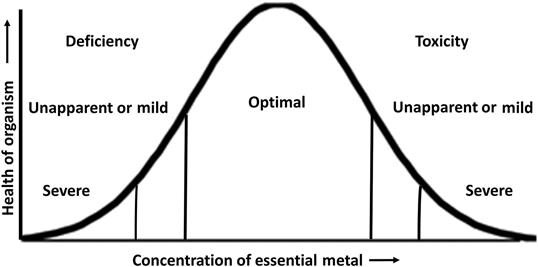
This means that even biologically essential metals can exert a wide variety of toxic effects given sufficient dosage. Elemental metals can chelate with enzymes, thereby interfering with the processes that these enzymes synergize. Depending on the metal and exposure concentration, serious neurological, metabolic, reproductive, teratogenic, and immunological damage can occur through exposure. For instance, both lead and mercury, two of the most toxic metals, can produce very serious neurological effects in humans and wildlife.
Plants tend to be more tolerant to metal exposure than animals, but they too can experience problems with photosynthesis, discoloration of leaves, reduced growth, and root death. Hyper accumulator plants can tolerate, uptake, and translocate high levels of certain heavy metals that would be toxic to most organisms. These plants can have leaves that contain >100 mg/kg of Cd, >1000 mg/kg of Ni and Cu, or >10,000 mg/kg of Zn and Mn (dry weight) when grown in metal-rich environments (Kamal et al., 2004).
Many factors affect the toxicity of metals. Some of these factors include valence state of the metal, whether the metal is combined with an organic molecule, environmental pH, concentration of organic matter, and calcium concentrations in soil or water, the relative concentration of other metals, and a class of metal-transporting proteins call metallothioneins. Many metals can assume multiple valence states and toxicity may vary with state. Chromium, for example, is mostly found in its elemental state, as a trivalent Cr3+ ion, or as a hexavalent Cr6+ ion. Of these, elemental chromium is essentially nontoxic, Cr3+ has a low toxicity, but Cr6+ has high toxicity.
Metals can occur in their inorganic forms either as ions or as nonionic elements. They can also combine with organic molecules such as ethyl or methyl groups. Organometals can be intentionally produced for a variety of industrial or agricultural purposes, but they can also occur naturally. Most often the organic portions augment the toxicity of the metal itself because they facilitate biological assimilation and ease the passage into cells. Inside the cells, the organometals can become DNA adducts and cause cancers, malformations, and other toxic effects. Some of the metals used to form organometals include mercury, boron, silicon, selenium, germanium, tin, lead, arsenic, and platinum. Serious environmental issues have been caused by methylmercury and selenomethionine, both of which form through natural processes.
A major factor in determining the valence state of a metal is the pH of the environment. Metals are oxidized at low pH, which means they move from a low ionization state to a higher ionization state (eg, elemental Zn or Zn0 to Zn2+). In a dry environment, these ionic forms may combine with oxygen to form oxides or sulfur to form sulfides. In aqueous or moist environments such as lakes, streams, or after heavy rains the ionic forms are more water soluble than the elemental metal. Soluble metallic molecules are also more readily assimilated by organisms than elemental forms.
In the 1980s, a great deal of concern was given to acid precipitation which is produced when hydrogen sulfide, nitrites, or nitrates are released through fossil fuel combustion, then transported through the atmosphere, and come down in wet (eg, rain) or dry (particulate) deposition. Upon entering lakes or streams, these pollutants reduce the pH of the water and increase the solubility of metals. Aluminum (Al) has been closely associated with the toxic effects of acid precipitation. Aluminum is the most common metal and the third most common mineral in the Earth’s crust. The valence state of Al varies from 2− to 3+ with anions occurring in alkaline waters and cations in acidic systems. Under acidic conditions, dissolved Al results in reduced reproduction among fish and amphibians. Acidification could also affect the growth and development of young waterbirds because aluminum forms insoluble complexes with phosphorus, preventing the normal development of bony tissue (Sparling, 1991). Studies determined that for much of the United States, natural buffering or acid-neutralizing capacity of soils reduced the risks of acid precipitation but regions of low buffering in the United States and Canada remain at greater threat of damage (more on this in Chapter 9).
Metals tend to bind with organic matter either in water or soil. This is a physical binding, not chemical bonding. Bound metals are less bioavailable than free, dissolved metals in the water column. Numerous studies have shown that the concentration of dissolved organic matter (DOM) in water significantly affects the availability and hence toxicity of metals in aquatic organisms. Similarly, soils and sediments with high organic content are generally less toxic than those with low content because of binding.
Calcium and other metals affect toxicity in another way. Calcium in soil or water often exists as calcium carbonate (CaCO3). Calcium carbonate buffers acidity, thus reducing the solubility of metals. In addition, the calcium ion (Ca2+) is preferentially taken up by the digestive systems of animals or by plant cells compared with metals. In this way, environments with moderate to high calcium-soluble concentrations of metals may be reduced and the solutions impeded from entering the blood or cells, thus further reducing toxicity. Metals with biological functions such as zinc may also preferentially bond to cells and inhibit the uptake of other metals. This is called competitive binding and it can ameliorate the effects of more toxic metals.
Metallthionein (MT) is a family of cysteine-rich, low molecular weight proteins that have the capacity to bind to metals, whether they are essential or not. Metallothioneins are found across the fungi, plant, and animal kingdoms and are even found in prokaryotes. In higher animals, they are produced by the liver and kidneys and are localized in the membranes of the Golgi apparatus. Metallothioneins provide protection against metal toxicity and oxidative stress and are involved in regulation of essential metals. Their production is induced by the presence of metals and other minerals in the bloodstream. Metallthionein has been documented to bind with a wide range of metals including cadmium, zinc, mercury, copper, arsenic, and silver. Metallothioneins specific for copper and zinc occur in many organisms. Thionein, the organic basis for metallothionein, picks up a metal when it enters a cell and carries the metal to another part of the cell where it is released or secreted. Cysteine residues from MTs can capture harmful oxidant radicals like the superoxide and hydroxyl radicals. In this reaction, cysteine is oxidized to cystine, and the metal ions that were bound to cysteine are released to the bloodstream and away from cells. This mechanism is important in the control of oxidative stress by metallothioneins.
Characteristics of Selected Metals
In this section, we describe several metals in greater detail, some of these are essential nutrients, some are very lethal, so as to provide a deeper understanding of the ecotoxicology of metals.
The US EPA (2014) lists several metals as priority pollutants; these are chemical pollutants that are regulated and have analytical methods. This list includes antimony, arsenic, beryllium, cadmium, chromium, copper, lead, mercury, nickel, selenium, silver, thallium, and zinc. Here we will focus on arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), mercury (Hg), and zinc (Zn). As we have previously suggested, other metals can be toxic at high concentrations and may have environmental relevance in highly contaminated sites such as around mining or certain industries. However, many of the details presented in this select list can be applied to other metals as well. Very basic characteristics of these metals can be found in Table 8.1. The US EPA’s Maximum Contaminant Level (MCL) is the maximum concentration allowable in drinking water. The Occupational Safety and Health Administration (OSHA) publishes Permissible Exposure Limits (PELs) that define the maximum concentration of a contaminant in workplace air. In contrast to RDA and TUL, these standards are for most contaminants, not just nutrients.
Table 8.1
Basic Characteristics of Selected Metals
| Metal | Symbol | Atomic Number | Atomic Mass | Melting Point | Boiling Point | Density (g/cm3) | EPA MCLa (mg/L) | OSHA PELb (mg/m3) |
| Arsenic | As | 33 | 74.9 | Undefined | 615°C, 1137°F | 74.9 | 0.010 | 0.2 |
| Cadmium | Cd | 48 | 112.4 | 321°C, 610°F | 767°C, 1413°F | 8.65 | 0.005 | 0.005 |
| Chromium | Cr | 24 | 52.0 | 1907°C, 3465°F | 2671°C, 4840°F | 7.19 | 0.1 | 0.5 (Cr6+) |
| Copper | Cu | 29 | 63.5 | 1084°C, 1984°F | 2502°C, 4643°F | 8.96 | 1.3 | 1 |
| Lead | Pb | 82 | 207.2 | 327°C, 621°F | 1749°C, 3180°F | 11.34 | 0 | 0.05 |
| Mercury | Hg | 80 | 200.6 | −38.8°C, −37.9°F | 356.7°C, 674.1°F | 3.53 | 0.002 | 0.1 |
| Zinc | Zn | 30 | 65.4 | 419.5°C, 787.1°F | 907°C, 1665°F | 7.14 | 5 | Not defined |

aMaximum concentration level in drinking water.
bPermissible environmental level in workplace air.
Arsenic
General Characteristics
Arsenic is a metalloid, which means that it has several characteristics in common with metals but some features that differ. Arsenic, unlike other metallic elements, does not have a true melting point. Rather, it sublimates (goes directly from solid to gas) at what would be its boiling point under standard conditions. It is rarely found naturally in elemental form, being combined with several other elements in the Earth’s crust, and it occurs in ionization states of 5+, 3+, 2+, 1+, and 3−. Arsenic makes up about 1.5 mg/kg (0.00015%) of the Earth’s crust, making it the 53rd most abundant element and moderately rare. In 2012, 45,000 tonnes (metric tons) of AsO3 were produced, with Chile and China as the major producers (USGS, 2014a).
Environmental Concentrations and Uses of Arsenic
Soil generally contains 1–40 mg/kg of arsenic with an average of 3–4 mg/kg (ATSDR, 2007b). Contaminated soils, of course, can have higher concentrations of As. These values were higher than the US EPA’s recommended maximum concentration of 7.2 mg/kg As, but generally lower than the Probable Effects Concentration (PEC) of 42.0 mg/kg. The PEC is the level of a contaminant in the media (ie, surface water, sediment, soil) that is likely to cause adverse effects. Arsenic in soil is predominately either arsenite (As5+) or arsenate (As3+); of these two, arsenite is more soluble, mobile, and toxic. However, arsenate affects cellular phosphorylation more strongly than arsenite. Seawater and freshwater have only 1.6 μg/L As on average, but contaminated sites can have up to 1000 μg/L. Air can have 1 to 2000 ng As/m3 (ATSDR, 2007a).
In addition to being the literary poison of choice for little old ladies with blue hair, As was and is used in a variety of industries. In agriculture, As was used as a wood preservative between the 1950s and 2004 when concern about its toxicity led to its ban in the European Union and the United States; other nations still use As for this purpose, however. Until 2013, As was used in insecticides. Surprisingly, As has been used in poultry and swine food to increase food efficiency and weight gain—increased growth led to the idea that As may be an essential nutrient but, if it is, no specific function has ever been identified. The practice has been almost totally abandoned, but some producers still use it for turkey in a product labeled nitrasone. Most authorities discount the possibility that As may be a nutrient.
The primary industrial use of As is in alloys with other metals, especially lead and copper. An arsenic/lead alloy can extend the life of car batteries by reducing the loss of zinc from the electrode plates. Arsenic is also used in alloys as a semiconductor. Arsenic has been used as a pigment in Paris Green and, in the 1800s, was used to make candies green. Imagine passing out As-laced candies for Halloween today!
One more historical use of As was important to biologists. Taxidermists and naturalists would use As as a preservative for study skins and mounts. The taxidermist would have a small cup of As powder nearby, and every so often he would lick his finger, pick up the powder on the moistened finger, and apply it to the skin. Unfortunately, there would be residues of As on his finger the next time he licked it, so a lot of taxidermists were poisoned in that way. Old taxidermist mounts may still have As residues and should be treated with care. Hat makers who used natural furs and early human embalmers had the same occupational risk.
Biological Effects of Arsenic
Arsenic and phosphorus are similar in molecular size and reactivity and, consequently, As can compete with phosphorus in biological reactions. Arsenic interferes with ATP, RNA, and DNA production through several mechanisms. It can interfere with the citric acid cycle of respiration by inhibiting cofactors for pyruvate dehydrogenase. It can uncouple oxidative phosphorylation, thus inhibiting the energy-linked reduction of NAD+ and mitochondrial respiration, and it increases hydrogen peroxide production in cells which can increase oxidative stress. These metabolic disturbances can lead to death from multisystem organ failure.
Arsenic can function as an endocrine disruptor by affecting gene regulation through receptors on thyroid cells. The metal binds with the receptor, gains access to a cell, and disrupts gene activity associated with those receptors. As a result, As has been implicated for interfering with thyroid activity in rats, fish, and perhaps humans (ATSDR, 2007a).
Indian cricket frogs (Rana limnocharis) were exposed to sodium arsenite at concentrations ranging from 0 to 400 μg/L through metamorphosis (Singha et al., 2014). Neither increased lethality nor reduced body mass was noted. However, sodium arsenite accelerated the rate of metamorphosis at 100 and 400 μg/L, reduced body size, and induced developmental deformities such as loss of limbs. Significant genotoxicity occurred at both concentrations of sodium arsenite/L. Naïve earthworms also showed genotoxicity that was positively related to the concentration of As in soil. However, there was evidence of adaptation in that worms that had been collected from As-contaminated soil did not show any adverse DNA effects (Button et al., 2010).
Arsenic may be toxic to plants, but there are species-specific differences. White clover (Trifolium repens) was exposed to soil concentrations of 5−20 mg/kg As, 20–60 mg/kg Cd, or a combination of both metals (Ghiani et al., 2014). The As-only treated plants assimilated As proportionally to the amount in soil. However when As and Cd were mixed, As reduced the uptake of Cd while Cd facilitated the uptake of As. All As and Cd treatments resulted in increased genotoxicity and As was more effective in inducing genotoxicity than Cd. The fern Pteris vittata is an As hyper accumulator and is used to remove As during remediation or clean-up operations. Arsenic can actually increase plant growth in hyper accumulators (Tu and Ma, 2002).
Cadmium
General Characteristics
Cadmium is insoluble in water, has a high sheen, and is resistant to corrosion. The average concentration of Cd in the Earth’s crust is between 0.1 and 0.5 mg/kg (0.0001 to 0.0005%) although, it can be much higher in contaminated sites. This means that Cd is moderately rare compared with many other metals. As a salt, Cd binds with sulfates and chlorides and is considerably more soluble than elemental Cd. Typical concentrations in fresh or salt water are around 0.05 μg/kg and 0.003 pg/m3in air (Eisler, 2000). Most of the world’s Cd is in soil but because of the vast amount of water, its other principal location is oceans. Cadmium is usually found with other minerals such as zinc and is extracted during the mining of those metals. Global production of Cd is approaching 23,000 tonnes per year with most from eastern Asia.
Environmental Concentrations and Uses of Cadmium
Approximately 86% of mined Cd is used in the production of Ni-Cad for use in batteries. Another 6% of available Cd is used in electroplating, particularly in aircraft due to the metal’s resistance to corrosion. Other industrial uses include a protectant in nuclear fission studies; yellow, orange, or red pigments in paint; solders; or in polyvinyl chloride (PVC) pipe as a stabilizer. Many instruments use Cd in semiconductors.
Some Cd may enter the environment through natural weathering of soils but most comes from human-related activities associated with mining, industrial effluents, smelting, fuel combustion, improper disposal of metal containing materials, through the application of phosphate fertilizers, or in sewage sludge. Wet and dry deposition of Cd from the atmosphere may also contribute sizable amounts of Cd to soil in the areas surrounding sources of atmospheric emissions. The implementation of the Resource Conservation and Recovery Act (RCRA) in 1976 affected disposal of Cd and many other materials through increased recycling; nearly 100% of Cd can be recycled. Nevertheless, Cd has been identified in at least 61% of the 1669 hazardous waste sites that have been proposed for inclusion on the EPA National Priorities List (ATSDR, 2012).
Stormwater detention ponds are designed to temporarily hold runoff to allow solids to settle and thereby reduce pollution in urban streams. Stephensen et al. (2014) found that concentrations of Cd in sediments of detention ponds ranged from approximately 0.08–0.9 mg/kg dosing weight (dw) compared with 0.1–3.5 mg/kg dw in nearby lakes. In fauna of these water bodies, largely consisting of mollusks and dragonfly nymphs, average Cd concentrations ranged from 0.05–0.53 mg/kg in ponds and 0.02–0.18 mg/kg in lakes. Overall the authors concluded that there really were not many differences in Cd concentrations between lakes and stormwater ponds; both sources seemed to be equally polluted.
Unless there is a strong source of Cd, the metal is often lower in concentration than many other metals. For example, in boat yards servicing pleasure boats, sediment concentrations of Cd averaged 0.52 mg/kg dw compared with 540, 440, 400, and 2.50 mg/kg for copper, lead, zinc, and mercury, respectively (Eisler, 2000).
In comparison with other metals, Cd concentrations in natural organisms also tend to be low. In essence, Cd concentrations in biota are not very interesting. With the exception of a few values in the hundreds of mg/kg, the vast majority of aquatic organisms, regardless of taxa, have concentrations from below detection level to approximately 20 mg/kg (Eisler, 2000). Most have values less than 5 mg/kg, which is not considered to be of concern. In addition, there were few to no patterns among groups of organisms with similar habits such as freshwater plants or aquatic birds.
Biological Effects of Cadmium
For humans, acute inhalation exposure to cadmium at concentrations more than approximately 5 mg/m3 may cause destruction of lung epithelial cells, resulting in pulmonary edema, tracheobronchitis (inflammation of the trachea or bronchi), and pneumonia. Target organs for Cd storage include the kidney and liver and these organs are most likely to be affected by Cd toxicity (ATSDR, 2012).
Aquatic organisms tend to be more sensitive to Cd than terrestrial animals, especially birds and mammals. The 96-h LC50 concentrations for the water flea Daphnia magna are approximately 10 μg/L (US EPA, 1980). For fish, the LC50 values are similar: for example, 1–2 μg/L in striped bass (Morone saxatilis, Hughes, 1973); and 1–6 μg/L in rainbow trout (Onchorhynchus mykiss, Chapman, 1978). In contrast, mallard (Anas platyrhynchus) drakes fed up to 200 mg/kg in their diets for 90 days experienced no mortality or any loss of body weight. Laying in mallard hens that were fed that amount of Cd decreased, but did not stop altogether (White and Finley, 1978). Similarly, it takes at least 250 mg Cd/kg body weight in rats and 150 mg Cd/kg body weight for guinea pigs (Cavia sp.) before lethality is attained (US EPA, 1980).
Sublethal effects in invertebrates ranged from decreased growth rates to population declines over a period of several days. Earthworms (Eisenia fetida) exposed to Cd concentrations greater than 100 mg/kg in soil experienced higher mortality than controls (Žatauskaitė and Sodienė, 2014). Cadmium reduced the weight of juveniles, retarded growth, and delayed sexual maturation with worms at the highest concentrations (250 and 500 mg/kg) failing to mature. In addition, there was evidence that Cd increased lipid peroxidation, a form of oxidative stress.
Cadmium has been linked to cancer in several organisms. Lerebours et al. (2014) conducted a field study on European flatfish (Limanda limanda) in the North Sea and English Channel. While these sites have many contaminants, variations in the concentration of Cd highly correlated with the occurrence of retinoblastoma, an eye cancer. Cadmium was also associated with other cancerous or precancerous tumors in flatfish. The frequency of malignant tumors ranged from 0–20% of sampled animals and precancerous tumors ranged from 19–43%. Mean Cd concentrations in fish tissues ranged from 48–406 μg/kg.
Cancer and other effects of Cd toxicity have been observed in terrestrial animals. The US Department of Health and Human Services and the International Agency for Research on Cancer both list Cd as a known human carcinogen and the US EPA lists it as a probable carcinogen. Although studies on humans have sometimes been equivocal, laboratory studies on other animals strongly support the carcinogenic activity of Cd; lung cancer from inhalation seems to be a major risk in humans (ATSDR, 2012). Cadmium combined with a methyl or ethyl group is more serious than elemental Cd. Recall from previous chapters that adducts such as methyl groups to DNA can interfere with normal gene activation which can result in cancers.
In waterfowl, sublethal effects of Cd include reduced growth, kidney damage, and testicular damage (Blus et al., 1993). These effects, however, occurred at mg/kg concentrations, many times greater than effects seen in aquatic organisms and at concentrations that are higher than most environmental circumstances. Cadmium can also affect the endocrine system, influencing hormone production. The metal is associated with reduced gonadal function, altered secretions of adrenal corticotropic hormone (ACTH), growth hormone (GH), corticosterone, and thyroid stimulating hormone (TSH) by the pituitary. Some other effects in birds include bone marrow loss, anemia, liver hypertrophy, kidney damage, and testicular damage.
Chromium
General Characteristics
Chromium (Cr) is described as a hard, brittle, lustrous metal that resists tarnishing and takes a high polish. These attributes have made Cr a highly desired metal for automobile enthusiasts for generations. The metal is the 22nd most common element in the Earth’s crust and has an average concentration in uncontaminated soil of 100 mg/kg (with a range of 1–300 mg/kg). Concentrations range from 5–800 μg/L in seawater and 26 μg to 5.2 mg/L in freshwater. Approximately 44% of the 8.7 million tons of chromium ore mined each year comes from South Africa, but Eastern Europe and Turkey are also major mining areas. In nature, Cr occurs mostly as Cr0 (elemental), Cr3+, and Cr6+, with Cr3+ being the most common ion and hexavalent or Cr6+ the most toxic.
Environmental Concentrations and Uses of Chromium
Chromium adds considerable strength to metal alloys and is used with iron in high-speed tool steels to reduce wear. Nickel-based alloys often contain Cr because of its strength and to enhance resistance to high temperatures. Nickel/chromium alloys are especially useful in jet engines and gas turbines. Chromates are also used as protective oxide layers on metals like aluminum, zinc, and cadmium.
Crocoite or lead chromate (PbCrO4) was used as a yellow pigment in paint, resulting in the color Chrome Yellow (as on school buses in the United States). Due to the toxicity of both the Pb and Cr, the pigment was discontinued. By varying the associated elements and pH, Cr can also produce red and green pigments. Trace amounts of Cr3+ give natural and synthetic rubies their red color. The first laser, built in 1960, used a synthetic ruby with Cr3+. Cr6+ is both highly toxic and magnetic. It is used in wood preservatives, especially in the chemical chromated copper arsenate which contains 35–65% Cr. Believe it or not, back in the “old days,” recordings were made on tape and the magnetic coating of the higher-quality tapes was chromium dioxide or CrO2. These tapes can still be purchased by audiophiles although other media such as compact discs and MP3 have taken over much of the popular market.
Environmental concentrations of Cr are more variable and can be much higher than cadmium. In terrestrial environments, contaminated sites can have Cr concentrations in excess of 4700 mg/kg. Pfeiffer et al. (1980) found that sediments just outside of the discharge pipe of a Brazilian electroplating plant had concentrations ranging from 1420–54,300 mg/kg. The concentrations decreased with distance from the plant. Fortunately, Cr tends to firmly bind with soil and sediment particles so the amounts that would be biologically available to fish and organisms living in the water column are probably far less than the measured concentrations (Eisler, 2000). Exposure would be greater for organisms that live in sediments such as worms and various insects because sediment metals can mix with pore water; the water that fills the pores among sediment and soil particles.
Chromium, like other metals has an affinity to adhere to soil particles. The same Brazilian study discussed previously (Pfeiffer et al., 1980) found that filtered, suspended particles had 2210–61,070 mg/kg of Cr attached to them. Chromium attached to these particles can easily be transported through the body of water and eventually deposited. For instance, at 50 m from a discharge point, the Cr concentration was 15,260 mg/kg, but at 600 m, it was 22% greater. Water can have high Cr concentrations in contaminated areas. Unpolluted waters have concentrations in the low-to-medium-μg/L range. However, untreated industrial effluents can have concentrations exceeding 5000 mg/L. While background atmospheric concentrations are approximately 0.001 μg/m3, urban concentrations in North America are 10–30 times higher and up to 500 times higher in cities that have steel mills (ATSDR, 2012).
Biological Effects of Chromium
Chromium is readily taken up by organisms and some bioconcentration in plants and in aquatic animals occurs because it is an essential element. However, the element is also depurated or expelled fairly rapidly and even in highly contaminated sites does not appear to biomagnify through the food chain. In general, Cr concentrations range from below detection level to 100 mg/kg in marine algae, 25 mg/kg in marine or freshwater mollusks, and 3 mg/kg in crustaceans (Eisler, 2000). Of course, animals living in the immediate vicinity of industrial effluents can have elevated concentrations of the metal. For example, snails living approximately 9 km below a tannery had 450 mg Cr/kg dw body weight in their tissues (Eisler, 2000). Mammals generally have Cd concentrations below 20 mg/kg dw (Eisler, 2000).
The value of Cr as a nutrient in mammals, including humans, has been debated for many years. Currently, the National Institutes of Health cites Cr as “a mineral that humans require in trace amounts, although its mechanisms of action in the body and the amounts needed for optimal health are not well defined.” The institute describes Cr as having a role in counteracting diabetes, especially diabetes type II; regulating the metabolism of fats, carbohydrates, and proteins; and enhancing body weight and condition. No specific RDA has been set, but less stringent “Adequate Intake” levels are 35 μg/day for adult men; 24 to 45 μg/day for women, depending on pregnancy status; and 0.2 to 15 μg/day for children, depending on age. The typical normal intake for adult women and men is 23 to 29 μg/day and 39 to 54 μg/day, respectively, so the recommended amount is easily obtained from diets for all except pregnant or lactating women.
Based on an extensive review on the effects of Cr to aquatic organisms, Eisler (2000) concluded the following: (1) Cr6+ is more toxic than Cr3+ to freshwater organisms in soft or acidic waters; (2) organisms at younger life stages are more sensitive than those at older life stages; (3) the 96-h LC50 assays are inadequate to explain the effects of Cr on population mortality patterns; (4) in saltwater environments, algae tend to be more resistant to Cr toxicity as salinity increases; and (5) pH seriously affects the toxicity of Cr6+. Item 3 is a very common concern when trying to estimate risk. Long-term exposures to most contaminants induce harm that short-term, acute exposures often do not reveal and, under natural conditions, other factors that we have mentioned can ameliorate or intensify toxicity. Van der Putte et al. (1981) supported the findings of many other studies in showing that the toxicity of Cr was inversely related to pH in water (Fig. 8.3). Chromium in soft waters is more toxic than in hard water. In bluegill (Lepomis macrochirus), for instance, the 96-h LC50 was 118 mg/L in soft water and 213 mg/L in hard water (US EPA, 1980).
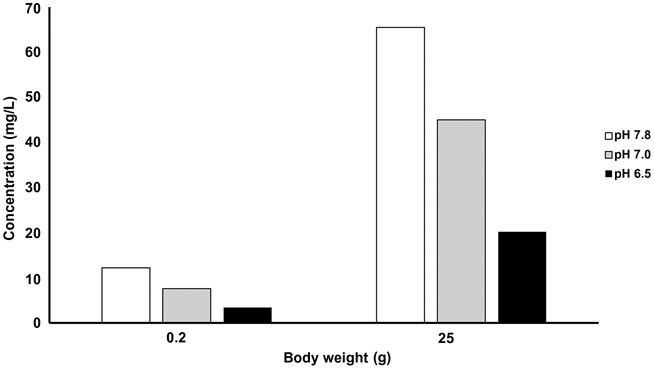
With regard to sublethal effects, growth of several freshwater algae species is inhibited by Cr6+ concentrations between 10 and 45 μg/L and effects were most observable at low pHs. The US EPA (1980) reported that 1900 μg Cr6+/L significantly inhibited root growth in water milfoil Myriophyllum spicatum, but that it took five times that amount of Cr3+ to have the same effect. Chromium can also reduce growth of terrestrial plant shoots and roots. For instance, Cr6+ was 10 times more powerful in stunting growth of barley than Cr3+.
Among freshwater fish, hexavalent Cr decreased growth in rainbow trout and Chinook salmon (Onchorhynchus tshwytscha) fingerlings at 21 μg/L (US EPA, 1980). Coho salmon (O. kisutch) that were migrating towards the sea experienced problems in osmoregulation when subjected to 230 μg/L and they showed decreased immune functions at 500 μg Cr6+/L (Sugatt, 1980a).
Birds can accumulate Cr in their tissues including feathers and eggshells which offer noninvasive means of evaluating exposure and uptake of this and other metals. However, as for lethal toxicity, few studies have examined the effects of Cr in wild birds or mammals. Dietary supplements with 2 mg/kg chromium chloride (CrCl3) increased weight gain, food conversion efficiency, and immune responses while they decreased respiration rate and body temperature in broiler chicks (Norain et al., 2013). Most current research seems to be focused on how Cr supplements can increase growth and immune functions rather than on adverse effects of Cr on birds, especially domestic fowl. Concentrations of CrCl3 of approximately 1.5 mg/kg in the diet apparently have many beneficial results. However, teratogenicity or improper embryonic development of broiler chicks was observed when Cr6+ was injected into eggs (Gilani and Marano, 1979). These abnormalities included twisted and shortened limbs, small eyes, everted viscera, exposed brains, parrot-like beaks, and stunted growth.
According to Eisler (2000), under appropriate conditions hexavalent Cr is a human and animal carcinogen and mutagen. Trivalent Cr can also produce tumors if it is in solution at concentrations far exceeding those that would be environmentally relevant. Hexavalent Cr can also cause skin ulcerations, dermatitis, mucous membrane ulcerations, and bronchial cancer in humans. Hexavalent Cr is also a spermicide, causes birth defects, and causes spontaneous abortions in rodents.
Copper
General Characteristics
Next to iron, copper is arguably the most important metal to living organisms. All living organisms require it for normal growth and physiology. Copper has a high electroconductivity and high thermoconductivity and is easily malleable. Copper will oxidize, but unlike rust on iron, the oxidized coating protects the copper from further oxidation. If exposed to the atmosphere, a green patina or verdigris (copper carbonate) forms. The Statue of Liberty is a good example of this (Fig. 8.4). Copper occurs naturally as elemental, Cu1+ (cuprous), or Cu2+ (cupric) but it can also be found as Cu3+ and Cu4+ ionic states. Typical concentrations of Cu in the earth’s crust range from 2–250 mg/kg. Freshwater and marine concentrations range from 0.5–1000 μg/L with an average of approximately 10 μg/L. The amount of Cu in uncontaminated air varies from a few nanograms per cubic meter to 200 ng/m3 (ATSDR, 2005). Copper is a valuable metal with many different purposes and, for that reason, some 197,000,000 tonnes of Cu are extracted per year (USGS, 2014b). Chile, followed by the United States, Indonesia, and Peru are the top producers of Cu globally. Copper recycling is extensive and, excluding Cu wire which requires new metal, nearly 75% of copper use in the United States comes from recycling.
Environmental Concentrations and Uses of Copper
Copper is often blended into brass (copper mixed with zinc) or bronze (typically copper with tin, but other metals can also be used with copper). Cupronickel is a blend of copper and nickel and used in low-valued coins such as—you guessed it—nickels. Today, the “copper” penny is actually only 2.5 % copper and 97.5% plated zinc. The major applications of Cu are in electrical wires (60%), roofing and plumbing (20%), and industrial machinery (15%). Other uses include cookware, architecture and building supplies, and antifouling paint on boats. Copper sulfate is used in agriculture as fungicides, insecticides, repellents, or algicides; medicines for humans and livestock; and in nutritional supplements. Unfortunately, the amount of Cu sulfate needed to adequately control algae blooms in lakes and ponds can also be toxic to other organisms.
The largest source of Cu pollution through human activities is to land. Major sources of this contamination include mining and milling operations. Other sources include agriculture, sludge from publicly owned treatment works, and municipal and industrial solid wastes. Fertilizers made from livestock feces often contain high concentrations of heavy metals from animal feed that is poorly digested. Spread over a long period of time, heavy metal concentrations can build up until they become toxic to crops.
Copper is released into water as a result of natural weathering of soil and discharges from industries and sewage treatment plants. According to the ATSDR (2005), domestic wastewater is the major anthropogenic source of Cu in waterways with concentrations of Cu discharged into wastewater treatment plants, averaging approximately 0.5 mg/L but can be several times higher. Acid mine drainages usually have low (<4.0) pH, which leads to high solubility and bioavailability of metals. The US EPA found copper in 96% of 86 samples of runoff from 19 cities throughout the United States with concentrations ranging from 1–100 μg/L and a mean of 18.7 μg/L (Cole et al., 1984). Copper enters the atmosphere naturally from windblown dust and volcanoes, but the principal anthropogenic atmospheric sources of Cu are smelters where concentrations can range from 7–138 ng/m3 (ATSDR, 2005).
Concentrations of Copper in Organisms
Copper and other metals can be taken up by plants following at least one of three pathways: (1) metals may not be taken up at all; (2) the metal is picked up by the roots, but the plant may have ways of inhibiting the ability of metals to move from roots to shoots; or (3) metals may move more or less freely through the plant and may accumulate in certain plant parts. Since Cu is required by living organisms, it is more readily taken up than some other metals. Some plants are hyper accumulators of Cu. Kamal et al. (2004) tested the ability of three aquatic plant species; water mint (Mentha aquatic), parrot feather (Myriophyllum aquaticum), and creeping primrose (Ludwigia palustris); to accumulate Cu in a laboratory situation. Following a starting concentration of 5.56 mg Cu/L in solution, all three species partially depleted Cu in the water column over the course of 21 days. At this time, the final Cu concentrations in water were reduced by approximately 40% for all three plant species. The final plant concentrations ranged from 304–840 mg/kg dw. In contrast, controls had 11–25 mg/kg dw at the end of the study. This study shows that bioconcentration of Cu does occur and it varies among species. In a review of several terrestrial species of plants, Eisler (2000) determined that such species usually have concentrations below 50 mg/kg dw. However, those near contaminated sites can have concentrations that exceed 10,000 mg/kg dw.
De Jonge et al. (2014) conducted a study in which they measured water chemistry and body burdens of Cu and other metals in four groups of insects: two stoneflies (Lectura sp. and Perlodidae), black flies (Simulidae), and mayflies (Rhithrogena sp). Water concentrations of Cu ranged from 0.19–9.52 μg/L. In the insects, the concentrations ranged from 11.4–876 μg/L so bioconcentration was evident in all four groups with biological concentration factors of 60–92. Several water chemistry factors influenced body concentrations including concentrations of free-ion Cu, pH, dissolved organic matter, and water hardness. Body burdens of Cu increased with Cu water concentrations, and as pH, dissolved organic matter, and hardness decreased. Terrestrial invertebrates such as insects have Cu concentrations in the range from less than 5–140 mg/kg dw (Eisler, 2000).
Typical concentrations of Cu in fish range from 1.5 to 25 mg/kg dw (Eisler, 2000). In studies that examined organ-specific concentrations, kidneys and livers tended to have higher concentrations than other organs (Fig. 8.5). Among the few amphibians examined, concentrations were similar to those found in fish.
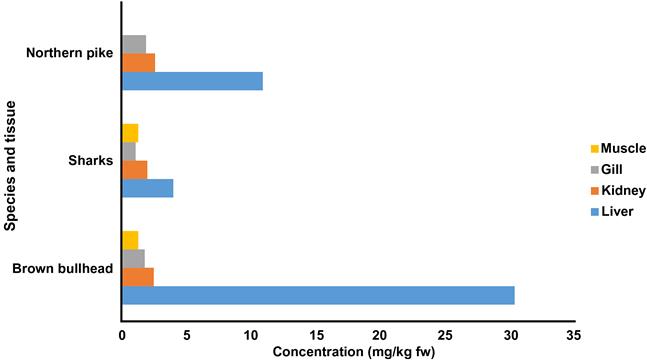
In birds, low-background whole body concentrations are approximately 5 mg/kg dw and highs are near 50 mg/kg (Eisler, 2000). Concentrations in mammals are in the same region as birds.
Biological Effects of Copper
Too much or too little Cu can be harmful to any organism including plants where deficiency of Cu is characterized by reduced growth, dark coloration of roots, and chlorotic (blotchy or yellowish) leaves, reduced fertility, and withering. Most plants have the ability to efficiently eliminate excess Cu. It is only when levels of the metal get very high that serious harm can occur. “Very high,” of course, depends on the species. For instance, in cucumbers soil concentrations <2 mg/kg dw cause Cu deficiency, cucumbers grown with concentrations between 2 and 10 mg/kg dw are healthy, and when concentrations exceed 10 mg/kg dw the plants show signs of Cu toxicity (Eisler, 2000). In soybeans, the concentrations for deficiency, optimal, and toxicity are <4, between 10 and 30, and >50 mg/kg, respectively, with some margin of leeway between 4 and 10 and between 30 and 50, depending on other soil factors. Copper toxicity inhibits root elongation and branching, which reduces the ability of the plant to absorb water and nutrients from the soil.
In vertebrates, Cu is involved with many enzyme functions including cytochrome C oxidase which is part of the electron transport system in mitochondria; Cu/Zn superoxide dismutase; and in many enzymes involved with protein synthesis, oxidative phosphorylation, iron transport, and synthesis of neurotransmitters. Daily dietary ingestion in humans may provide 1–5 mg/day (ATSDR, 2005), of which only 20–50% is absorbed. Cu deficiency is very rare unless there is some genetic or physiological issue that inhibits Cu uptake because it is generally available in most foods. In addition, Cu deficiency has been tied to neurological conditions, including sensory ataxia (loss of coordination), spasticity, muscle weakness, and loss of vision, or damage to the peripheral nerves or spine.
Copper deficiencies have not been reported in wild birds or mammals, but have occurred in domestic livestock and laboratory animals. Sudden death can occur in chickens, swine, and cattle that are deprived of Cu. Other effects center on the multitude of enzyme reactions that Cu is involved in and result in increased EROD activity, anemia, acute inflammation, lesions in the central nervous system, and reduced phospholipid synthesis. In other words, having too little Cu is not a good thing.
At high Cu concentrations, acute (96-h) lethal toxicity concentrations of Cu were 260 μg/L in nematodes, 1700 μg/L in snails, 560 μg/L in oysters, 26 μg/L in a species of clam, and 50 μg/L in black abalone (Haliotis cracherodil, reviewed by Eisler, 2000). Among fish, the US EPA (1980) reported that low LC50s ranged from 13.8 μg/L for rainbow trout, and for goldfish (Crassius auratus) it was 36 μg/L when calcium carbonate was low and 300 μg/L at higher levels of CaCO3/L. For a few less sensitive species, the LC50s included 8000 μg/L for mummichog (Fundulus heteroclitus), 937 μg/L in green sunfish (Lepomis cyanellus), and 1100 μg/L for bluegill larvae (summarized by Eisler, 2000).
In amphibians, a few LC50 values included 2696 μg/L for the tolerant Fowler’s toad (Bufo [Anaxyrus] fowleri) embryos, 1120 μg/L in two-lined salamander (Eurycea bislineata) juveniles over 48 h, and 50 μg/L in the more sensitive northern leopard frog (Rana [Lithobates] pipens) embryos over eight days (Eisler, 2000). Marbled salamander embryos (Ambystoma opacum) did not die from Cu concentrations as high as 1000 μg/L, but they did hatch earlier than controls and those exposed to lower concentrations of Cu (Soteropoulos et al., 2014). Larvae experienced high mortality at both 500 and 1000 μg/L. The lack of mortality among embryos is most likely due to protection provided by the jelly coating around eggs.
In general, mammals and birds are 100–1000 times more resistant to Cu than aquatic animals, but some ruminant animals (ie, sheep, goats, and cattle) are significantly more sensitive to Cu toxicity than nonruminants (Eisler, 2000). Among ruminants, sheep seem to be particularly sensitive. Copper deficiency can be one cause of swayback or lordosis in ungulates (Jaiser and Winston, 2010).
The liver is a major storage organ for copper and often has the highest concentrations of all organs. Effects in mammals include kidney damage, increased mortality, gastric ulcers, and liver pathology. Toxic reference values, which are concentrations that might produce some effects, for nine species of mammals including six wildlife species and three domesticated animals ranged from 0.9 to 4.5 mg/kg (Ford and Beyer, 2014). Herbivores ingest considerable amounts of soil with their foods, so accounting for food consumption, soil concentrations of copper that are considered safe ranged from 109 mg/kg in bighorn sheep (Ovis candadensis) to 2013 mg/kg in horses.
Sublethal effects in birds include Cu accumulation in the liver and kidneys, heart and skeletal lesions, anorexia, and listlessness. It does not appear that mortality would occur at environmentally realistic concentrations except perhaps in highly contaminated sites. Ford and Beyer (2014) estimated that mourning doves (Zenidura macroura), mallards, and Canada geese (Branta canadensis) could ingest 3.3 mg Cu/kg/day safely. They further calculated that soils could have from 689 mg/kg for mourning doves to 1008 mg/kg for the waterfowl species and still be considered safe.
When it became apparent that waterfowl and some endangered species were dying due to lead shot exposure (see the FOCUS section), several studies evaluated the suitability of other metals as substitutes. In one study, copper shotgun pellets were fed to American kestrels (Falco sparverius). Birds were fed 5 mg Cu/g body weight nine times during a 38-day exposure trial (Franson et al., 2012). Essentially all birds retained the pellets for at least 1 h, but most regurgitated them within 12 h. Copper concentrations were higher in the livers of dosed birds compared to controls, but there was no apparent difference in blood concentrations between the two groups. Copper exposure elicited metallothionein in dosed male birds, but not females. No clinical signs were observed, and there was no treatment effect on body mass; hemoglobin or methemoglobin (a form of hemoglobin, in which the iron in the heme group is in the Fe3+ (ferric) state, not the Fe2+ (ferrous) of normal hemoglobin) in the blood; or on Cu concentrations in kidney, plasma biochemistries, or hematocrit. The authors concluded that the copper pellets posed little threat to the kestrels or (presumably) related species although longer exposures might produce negative effects.
Lead
General Characteristics
Finally, we get to a metal that has high risk of environmental toxicity. Along with mercury, lead is a very toxic metal whose distribution has increased tremendously due to human activities. Lead is soft, malleable, and heavy. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air. Lead has a shiny chrome-silver luster when it is melted into a liquid. It is also the heaviest nonradioactive element. Its most common valence states are either Pb0 or Pb2+. Lead is ranked 37th in abundance among elements with an average concentration in soil and sediments of 10 mg/kg (0.001%); actual concentrations of course will vary from site to site. Lead seldom occurs as a pure metal, rather it is found in various oxides and sulfides, such as the ores galena, cerussite, and anglesite. Other metals often found with Pb include zinc, silver, and copper. Annual global extraction of lead ore is around 8.8 million tons with Australia, China, and the United States as the principal mining countries.
Uses of Lead
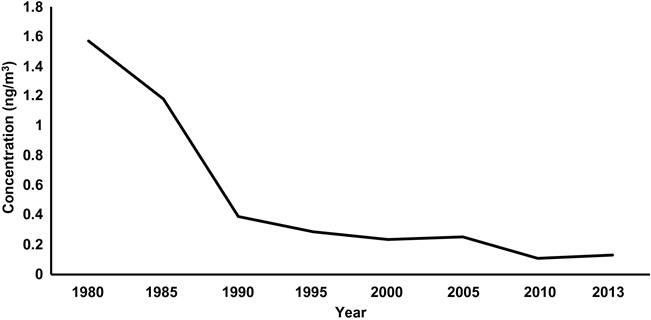
Contrary to public opinion, lead is not found in pencils—it is actually a form of carbon called graphite. Approximately half the amount of Pb mined goes to the automobile industry as leaded batteries. The other major uses of lead include small arms ammunition, shotgun pellets, fishing sinkers and weights for fishing nets, and tire balancing. Less common uses include electrodes, radiation shielding, building industry, sculptures, leaded glass, ceramic glazes, and semiconductors. Lead is still used in pigments in industrial paints but its use in home paints was banned in the United States in 1978 due to its high toxicity. We still find lead paint in slums and houses that have not been well maintained. Somewhat appalling, lead carbonate was used as a white pigment on the faces of Geisha girls (Fig. 8.6) and for other “white face” uses. The lead chromate was indeed toxic to those who used it. Lead found a use in plumbing joints until 1998 when the EPA banned its use for that purpose. Organic lead, especially tetraethyl lead, was used in gasoline for automobiles from the 1920s until the early 1970s as an octane booster. However, after the organic portion was combusted, the lead was emitted through tail pipes into the atmosphere or onto road surfaces where it could be part of runoff into rivers and streams. Following the ban, the output of Pb from vehicles declined by 95% and atmospheric Pb has declined by 94% (US EPA, 2014, Fig. 8.7). The EPA promulgated a maximum atmospheric discharge of Pb from any single source at 1.5 μg/m3 in 1977, which still remains the standard (US EPA, 2009).

Environmental Concentrations of Lead
In 1978, Nriagu (1978) estimated that only the lithosphere—soil and sediments—retained an appreciable amount of lead (approximately 99.9% of the total amount in the world). This estimate has probably not changed very much. If anything, as stricter controls were placed on Pb emissions, the atmosphere has even less lead than at that time.
Eisler (2000) reported that air typically has a Pb concentration of around 0.1 μg/m3 to maybe 100 times that in metropolitan areas. In the smelting and refining of lead, mean concentrations of lead in air can reach 4400 μg/m3; in the manufacture of storage batteries, mean airborne concentrations of lead ranging from 50–5400 μg/m3 have been recorded; and in the breathing zone of welders of structural steel, an average lead concentration of 1200 μg/m3has been found (ATSDR, 2007b).
The amount of lead released by industry to water sources is less than that in the atmosphere or on land. The EPA Toxic Release Inventory in 2006 reported that 118,700 kg (118.7 tonnes) of Pb had been dumped into bodies of water, mostly streams and rivers. Of the known aquatic releases of lead, the largest ones are from the steel and iron industries and Pb production and processing operations (US EPA, 1982). Urban runoff and atmospheric deposition are significant, indirect sources of lead found in the aquatic environment (ATSDR, 2007b). For example, in a brief review of urban stormwater ponds in the Netherlands, Langeveld et al. (2012) reported that Pb concentrations ranged from 2–239 μg/L.
Water pH and dissolved salt concentrations heavily influence the amount of soluble Pb in surface waters (ATSDR, 2007b). At pH >5.4, the total solubility of lead is approximately 30 μg/L in hard water (high calcium or magnesium concentrations) and approximately 500 μg/L in soft water. Sulfate ions, if present in soft water, limit the lead concentration in solution through the formation of lead sulfate. In general, relatively clean bodies of water in the United States have up to 50 μg/L of dissolved lead. However, most of the lead in the water column is bound to particulates, either finely suspended particles or coarse particles that eventually precipitate to the bottom. Demayo et al. (1982) found that Tennessee streams had concentrations of dissolved Pb that ranged from 0.01–0.02 μg/L, the amount bound to dissolved organic carbon ranged from 30–84 μg/L, colloidal or suspended lead on fine particles from 62–2820 μg/L, and that coarse particle Pb from 124–653 μg/L.
The Toxic Release Inventory (US EPA, 2015) reported that in 2004, 6221 tonnes of lead were released to the land, both onsite and offsite, despite that, approximately 80% of current lead usage is in batteries and 95% of that is recycled (International Lead Association, 2015). In addition, 83 and 3977 tons of lead and lead compounds, respectively, were injected underground (ATSDR, 2007b). As the ATSDR pointed out, however, while the majority of lead releases are to land, they constitute much lower exposure risks to humans or wildlife than releases to air and water. Terrestrial releases are required by law to be isolated from environmental areas such as aquifers and water bodies. Of course, these landfills can leak so they need to be monitored regularly. Risk is further reduced because most of the lead released to land is tightly bound to organic material and becomes immobile (ATSDR, 2007b). Concentrations in soil or sediment can range from 10 to 11,000 mg/kg (Eisler, 2000). In the United States, sediments along a 195-mile (295-km) stretch of the Mississippi River in Missouri had 8.06–13.25 mg Pb/kg dw, but one location near the effluent of a smelter had 1710 mg/kg (Missouri DNR, 2010). Baseline measurements of sediment Pb in the Coeur d’ Alene River Valley, an historically important mining area, ranged from 1900 to more than 5000 mg/kg dw; waterfowl sampled from that area had blood Pb levels that indicated clinical or severely critical clinical toxicity concentrations (Spears et al., 2007).
Concentrations of Lead in Organisms
Lead that is present on the surface of plants reflects deposition levels but not what is actually assimilated; for that, one needs Pb concentrations to be incorporated into tissues. Although the bioavailability of lead to plants is limited because of the strong adsorption of lead to soil organic matter, it increases as pH and organic matter content of the soil drop. Most plants, if they assimilate Pb to any degree, seem to sequester it in their roots, allowing little to enter shoots or leaves. While there are hundreds of plant species that are known hyper accumulators of metals, there are only a few that have been identified as hyper accumulators of Pb, meaning that they can have more than 1000 mg Pb/kg in their tissues (Auguy et al., 2013). Among these include the grasses Agrostis tenuis and Festuca ovina; some penny cresses; a sorrel Rumex acetosa; and the aptly named leadworts, family Plumbaginaceae. Auguy et al. (2013) investigated the mustard Hirschfeldia incana, another suspected hyper accumulator. They found the plant growing wild on lead-mined land in Morocco with soils having 26–9479 mg Pb/kg dw. Leaves from these plants had 0.53–1.43 g Pb/kg dw (yes, grams), with an average of 0.79 g/kg. The authors cultivated seeds from these plants in both hydroponic media and soil. In soil spiked with 7531 mg Pb/kg dw, leaves contained 3.58 g/kg after a few weeks. In hydroponic solutions of 62.1 mg/L, roots had 121 g Pb/kg dw, but shoots had only 3% (3.6 g/kg dw) of that.
In uncontaminated sites, plants and invertebrates tend to have higher concentrations of Pb than birds or mammals. Aquatic invertebrates had 5–58 mg Pb/kg dw in their tissues unless they lived near a contaminated site where they could have >11,000 mg/kg dw (Eisler, 2000). High values for invertebrates included 14,233 mg/kg dw in black fly larvae (Simulium sp.) near a Missouri tailings, 981 mg/kg in worms (Eisenia rosea) in an Illinois area receiving lead-laden sludge, and 931 mg/kg dw in the limpet (Acmaea digitalis) near bridges in California.
Fish tend to have relatively low concentrations of Pb in their tissues. In a couple of recent papers a food fish common to Pakistan had 0.12–1.74 mg/kg Pb in its muscle which was the lowest concentration among six heavy metals (Ahmed et al., 2015). In India, Pb concentrations in the muscles of five food fish ranged from 0.073–0.386 mg/kg fresh weight; Pb concentrations ranked second lowest among 10 metals (Giri and Singh, 2015). Aquatic organisms will pick up lead through their gills, ingestion of food and sediment, or through their skin.
There are not enough data on Pb levels in amphibians to make meaningful generalizations. Sparling and Lowe (1996) found that northern cricket frogs (Acris crepitans) collected from experimental ponds had whole body concentrations of Pb ranging from 6.7–19.7 mg/kg dw. These concentrations were significantly and positively correlated with sediment concentrations. More recently, Ilizaliturri-Hernández et al. (2012) found that blood lead levels in giant toads (Rhinella marina) of Mexico ranged from 10.8–70.6 μg/dL (deciliters—multiply those values by 10 to determine concentrations per liter) with significant differences existing among giant toads that came from rural areas (mean 4.7 μg/dL), urban/industrial areas, (8.46 μg/dL), and industrial areas (22.0 μg/dL).
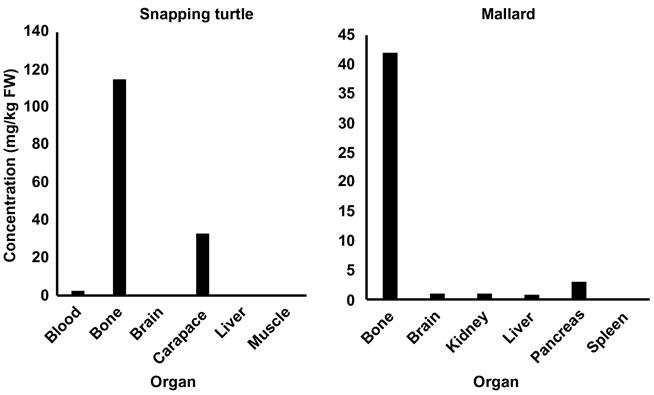
Grillitsch and Schiesari (2010) produced an extensive list of metal concentrations in reptiles. Concentrations in unpolluted sites were similar or lower than those observed in birds and mammals while contaminated sites had higher concentrations. Among hundreds of data points some high concentrations included 115 mg/kg ww in bones of snapping turtles (Chelydra serpentine) located within the lead-mining area of Missouri, 136 mg/kg dw in the femur of box turtles (Terrepene carolina) collected from an area near a lead smelter in Missouri, and 105–386 mg/kg ww in captive alligators (Alligator mississippensis) in Louisiana.
Terrestrial vertebrates will assimilate Pb through inhalation or ingestion, but ingestion is by far the more common way. Inhaled Pb will enter the lungs and almost immediately enter the bloodstream. Ingested lead may be reduced by the pH of the gut and absorbed more slowly into the blood; much of the ingested lead might be depurated with feces. The amount of lead actually absorbed by the digestive system depends on the pH of the gut and certain dietary factors. Anything that facilitates elimination, such as fiber, will facilitate depuration of lead. Diets high in calcium and iron will inhibit lead absorption. Elemental Pb seems to be absorbed less readily than organically bound lead, but elemental Pb is more readily absorbed than some inorganic lead complexes.
We focused on the liver, which was the most consistently used organ in Eisler’s (2000) summary and an organ for Pb storage in animals, because lead concentrations can vary significantly among tissue types (Table 8.2). Actually, bone tends to have the highest tissue concentrations of inorganic Pb among organs (Fig. 8.8), but Pb is accumulated and stored in bone with little bioavailability to the rest of the organism while concentrations in liver can become freely available. Organic forms of Pb are usually highest in kidney. In birds, blood, liver, and kidney were the most frequently analyzed tissues, but samples also included gut contents, various bones, brain, pancreas, spleen, feathers, carcass, eggs, lungs, and muscle. Tissues analyzed in mammals were similarly variable but also included hair, antlers, feces, blubber, skin, and teeth. Basically, over all these studies, if an organ could be analyzed it was. Concentrations in birds and mammals, even in contaminated sites, did not reach the highs observed in plants or invertebrates but were many times greater than those seen in supposedly clean sites.
Table 8.2
Summary of Lead Concentrations Found as Either Dry Weight (dw) or Fresh Weight (fw) in Various Biological Groups
| Group | Conditions | N | Mean Concentration +SE (mg/kg) | Maximum (mg/kg) |
| Plants | Noncontaminated | 41 | 14.3 ±3.86 dw | 123 dw |
| Plants | Contaminated sites | 30 | 1228 ±557 dw | 11300 dw |
| Terrestrial invertebrates | Noncontaminated Sites | 23 | 15.1 ±3.7 dw | 52 dw |
| Aquatic invertebrates | Noncontaminated sites | 15 | 10.6 ±3.6 dw | 58 dw |
| All invertebrates | Contaminated sites | 28 | 680 ±503 dw | 14233 dw |
| Fish liver | Noncontaminated sites | 7 | 0.43 ±0.06 | 0.6 |
| Fish liver | Contaminated sites | 3 | 9.1 ±3.0 | 6.5 |
| Bird livers | Noncontaminated sites | 19 | 0.69 ±0.37 fw | 6 fw |
| Bird livers | Contaminated sites | 25 | 37.5 ±12.8 fw | 315 fw |
| Mammal livers | Noncontaminated sites | 20 | 3.02 ±0.6 dw | 8.7 dw |
| Mammal livers | Noncontaminated sites | 15 | 1.05 ±0.34 fw | 5.1 fw |
| Mammal livers | Contaminated sites | 12 | 13.9 ±3.2 dw | 30 dw |
| Mammal livers | Contaminated sites | 10 | 22.9 ±11.4 dw | 120 fw |
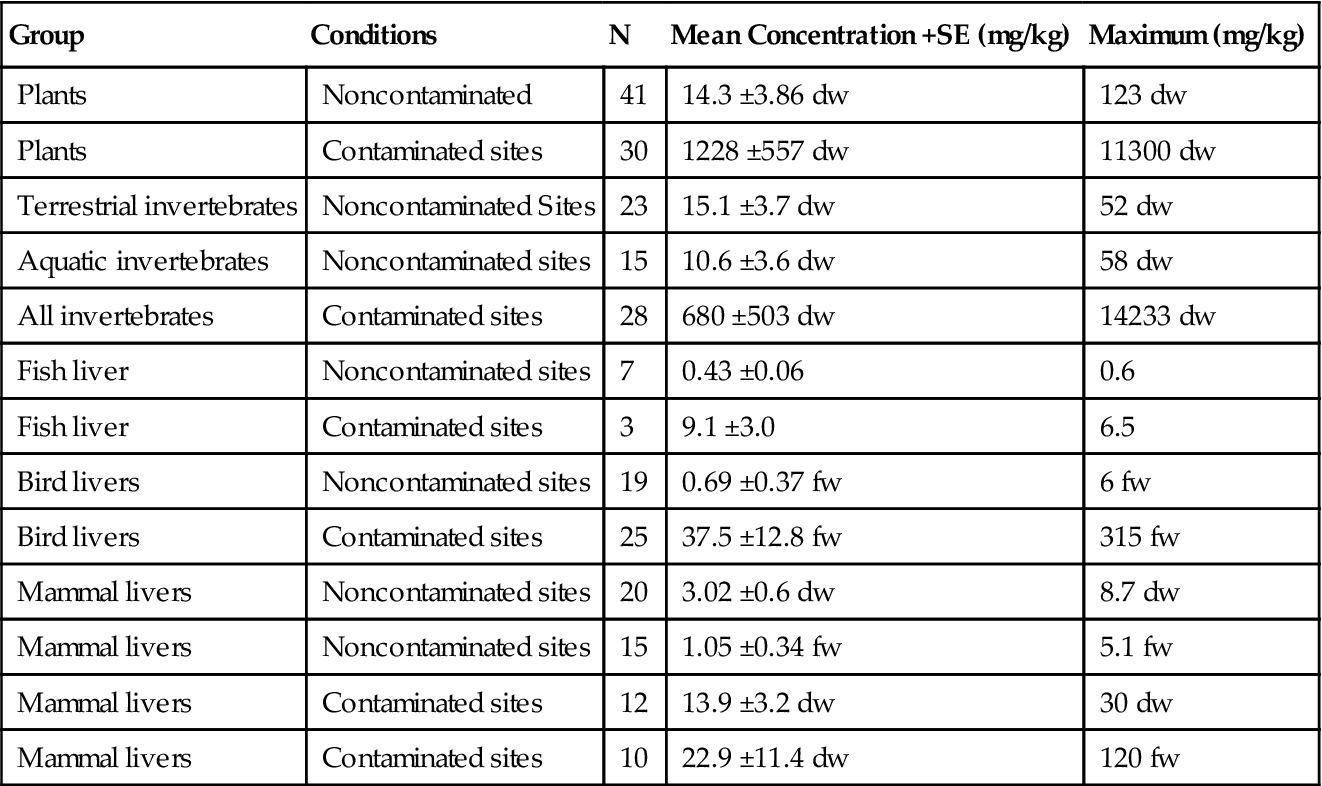
From Eisler (2000).
Biological Effects of Lead
Lead can negatively affect every organism and virtually every biological system within organisms; it is a highly toxic, cumulative, metabolic poison. According to Eisler (2000), environmental pollution from Pb is so high that its body burdens in many human populations are closer than any other contaminant to producing clinical toxicity. This can often be said about wildlife as well. Lead can be a mutagen, teratogen, carcinogen (in animals, but not conclusively in humans) or cocarcinogen (demonstrated in humans). Lead can disrupt reproduction, impair liver and thyroid functions, and attack the immune system. Its primary target is the nervous system especially in children (ATSDR, 2007b) and, by extension, young animals. A primary mechanism of Pb toxicity is that it binds or deactivates many proteins and enzymes in organisms (ATSDR, 2007b).
Plant responses to lead vary widely. The bioavailability of Pb in circumneutral to alkaline soils is very low. Regardless of pH, organic matter can bind Pb and reduce its uptake; thus, soils with low pH and low organic matter would make Pb most available. When Pb is assimilated, it reduces photosynthesis, reduces mitosis, inhibits growth, reduces pollen germination and seed viability, and impairs water absorption. Inhibition of photosynthesis occurs through blocking of sulfhydryl groups and diminishing phosphate concentrations in cells.
Acute toxicity in aquatic invertebrates can occur at ppb concentrations under laboratory conditions. In the water flea, Daphnia magna, 96-h LC50s varied with water hardness with increased softness resulting in greater toxicity (US EPA, 1985). Tolerance over multiple generations can also occur—isopods (Asellus meridianus) raised in clean conditions had a 48-h LC50 of 280 μg/L while those collected from a lead-contaminated river needed 3500 μg/L to attain their LC50 (Demayo et al., 1982).
Hariharan et al. (2014) conducted a multiphase study on the effects of environmentally relevant concentrations of Pb on the green mussel (Perna viridis). Under laboratory conditions they conducted an acute toxicity test with Pb concentrations ranging from 0–11.55 mg/L and for a chronic, 30-day test, they reduced the concentrations to range from 0–0.232 mg/L. They found that the acute 96-h LC50 was 2.62 mg/L. Mortality increased with the duration of the study, so that at the end of the 30-day trial, only 45% of the mussels survived at 0.11 mg Pb/L. Lead exposure increased oxidative stress in the animals, as determined by several bioindicators including reduced glutathione and glutathione-S-transferase. Histopathology was observed in the chronic test with gill filament and lamellar structures being damaged at 0.054 mg/L and higher concentrations; the same levels of Pb resulted in damage to the adductor muscle—the muscle that closes the shell—with 0.11 mg/kg, resulting in separation of muscle fibers.
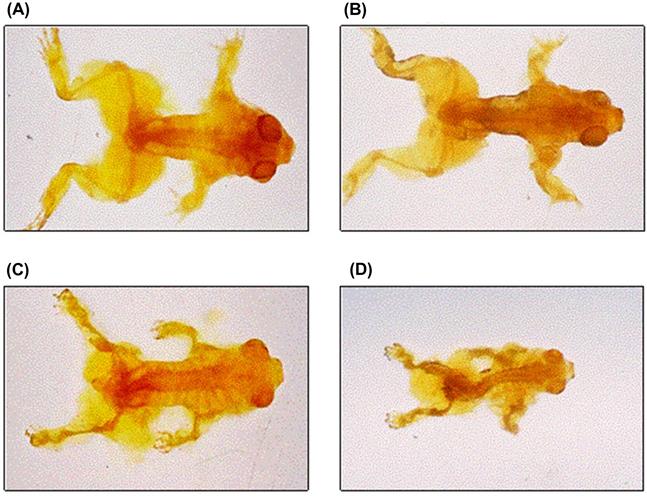
In a routine investigation of ponds at the Prime Hook National Wildlife Refuge, US Fish and Wildlife Service contaminants biologist Sherry Krest located a wetland on the refuge border that had numerous adult frogs but no evidence of any larval forms or tadpoles. Further investigation revealed that the wetland was located behind a berm used by a hunting club to stop ammunition from entering the refuge. Unfortunately, overshooting gradually deposited lead shot into the wetland over a long period of time and the sediments had accumulated a mean of 5700 mg Pb/kg dw. Sparling et al. (2006) conducted a lab study to assess the effects of these high concentrations on larval southern leopard frogs (Rana [Lithobates] sphenocephala). They exposed recently hatched larvae to sediments that were mixed with up to 7580 mg/kg dw, which equated to 24.4 mg/L in soil pore water. Although reduced growth and development were noted at 2360 mg/kg dw, the long term LC50 was 3228 mg/kg (12.5 mg/L) and all animals died within 5 days at concentrations ≥3940 mg/kg. Those that died, however, were probably the fortunate ones because severe skeletal and spinal problems, including twisted spines, shortened long bones, and deformed digits were common among survivors, reaching a 100% occurrence rate at concentrations ≥2360 mg/kg (Fig. 8.9). As a side note, those concentrations may seem high, and they are, but in this case they were environmentally relevant.
For most aquatic biota, Eisler (2000) concluded that: (1) dissolved waterborne Pb was the most toxic form; (2) organic lead compounds were more toxic than inorganic compounds; (3) adverse effects in some species were observed at 1 μg Pb/L; and (4) effects were more apparent at elevated water temperatures, reduced pH, comparatively soft waters, and in younger life stages.
A lot of research has been conducted on the effects of Pb on birds and mammals. In the case of birds, much of this interest has been generated by poisoning due to lead shotgun pellets and fishing sinkers that enter the aquatic environment and are picked up by waterfowl or result in secondary toxicity when scavengers consume contaminated carcasses. These findings will be summarized in the FOCUS section later in this chapter.
Signs of Pb toxicosis in birds include loss of appetite, lethargy, weakness, emaciation, tremors, drooped wings, green-stained feces, impaired locomotion, unsteadiness, and poor depth perception. Internally, birds will have microscopic lesions in the proventriculus (an upper portion of the digestive tract), pectoral muscles, and proximal tubules of the kidney. They may display enlarged, bile-filled gall bladder; anemia; reduced brain weight; fluid or edema around the brain; abnormal skeletal development; and esophageal impaction. Other signs include fluid-filled lungs; abnormal gizzard linings; an unusually pale, emaciated, and dehydrated carcass; and elevated Pb concentrations in liver, kidneys, and blood. Organic lead is about 10 to 100 times more toxic than inorganic lead, depending on species or sex. Altricial chicks—those that spend some time in the nest before becoming independent—seem to be more sensitive to lead than precocial chicks and more sensitive than adults of the same species.
As we discussed in Chapter 3, one of the enzymes necessary for heme or blood formation in vertebrates and many invertebrates that can be useful in diagnosing if an animal has been exposed to Pb is the enzyme delta-aminolevulinate dehydratase or ALAD. Lead inhibits the gene Aminoleuvulinic dehydratase from producing the enzyme and even small exposures to lead can reduce ALAD activity by 90% or more. In the study on giant toads reported previously (Ilizaliturri-Hernández et al., 2012), the authors found a significant drop in ALAD with increasing concentrations of blood Pb.
Several generalizations on mammalian toxicity have been developed from extensive research on the effects of lead in laboratory and wild animals: (1) lead toxicosis can occur in real environments with actual exposure concentrations; (2) organic lead is usually more toxic than inorganic lead; (3) there is considerable variation among species in sensitivity to Pb; and (4) as with assimilation of Pb, many environmental factors (including calcium, magnesium, pH, and organic matter) can affect lead toxicity; diets deficient in some basic nutrients such as calcium, minerals, and fats can contribute to lethal and sublethal expression (Eisler, 2000). Signs of Pb poisoning in mammals include those seen in birds, but also involve spontaneous abortions, blindness, peripheral nerve disease, poor performance in tests involving learning or memory, and various blood disorders.
Mercury
General Characteristics
Mercury (Hg) is a contaminant of global concern. Several international conferences, agreements, and conventions have been established to regulate and reduce Hg concentrations in the environment, particularly as they relate to human health. Mercury is unique among metals in several ways. Along with lead, Hg is extremely toxic to living organisms and has no known biological function. However, Hg is the only metal that is liquid under standard temperature and pressure conditions. It can form organic complexes such as methylmercury (MeHg), which are fat soluble and can both bioconcentrate and biomagnify through food chains. Mercury is resistant to most acids, although concentrated sulfuric acid can dissolve the metal. In contrast, Hg itself dissolves several other metals to form amalgams. Gold and silver are two commercially useful amalgams. Dental amalgams using Hg are used in dentistry although their popularity is fading. Mercury is also infamous for forming an amalgam with aluminum by dissolving the lighter metal. For this reason, the transport of Hg by aircraft is largely banned—imagine having your plane dissolve while you are flying it!
Mercury is an exceptionally rare metal in the Earth’s crust, it is the 66th most common element in the crust and has an average concentration of 0.08 mg/kg in soils and sediments. That means that 0.000008% of the earth’s soil is Hg. When found, however, Hg tends to pool and form relatively rich but widely scattered pockets. Mercury commonly occurs in one of three valence conditions: 0, 1+, and 2+; higher valence states can occur but are very rare. All forms of inorganic Hg are toxic. As we will see, Hg has a tendency to become organic by becoming methylated or ethylated under certain environmental conditions. Methylmercury, the most common form of organomercury, is considerably more toxic than any of the inorganic ionic states. The 1+ oxidation state often takes the form of Hg2+2 with two Hg atoms forming a dimer with a 2+ charge. When combined with Cl or some other anion, the Hg atoms remain united. The most common Hg-containing ore is cinnabar (HgS). Approximately 1810 tons of Hg are extracted each year with China accounting for 75% of the total production (USGS, 2014a). The last Hg mine in the United States closed in 1992 (USGS, 2014c).
Environmental Concentrations of Mercury
Mercury occurs in the lithosphere, atmosphere, hydrosphere, and biosphere. Since it occurs in both organic and inorganic forms in all of these media and because bacteria are involved in the conversion of Hg from one state to another, a mercury cycle has been recognized (Fig. 8.10). Starting with the atmosphere, either inorganic or organic Hg can be transported long distances until it eventually precipitates on land or water. The primary sources for the 5500–8900 tons of Hg emitted into the atmosphere each year are natural processes such as volcanoes and combustion in coal-fired plants. Together these account for 82% of atmospheric Hg (Pacyna et al., 2006). Other sources include gold mining and smelters.
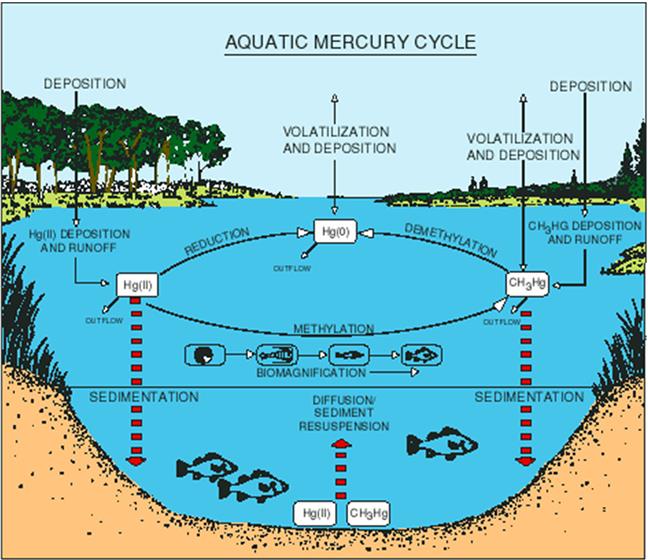
On land or water Hg can volatilize as elemental Hg back into the atmosphere, enter the food chain, or runoff into water bodies as ionic or elemental Hg. Mercury in runoff or effluents amasses to approximately 1000 tons per year globally (UNEP, 2013). In water, inorganic Hg can be combined with organic molecules, usually a methyl group, and become organomercury; similarly, microorganisms can convert organomercury into inorganic Hg. Organomercury, because it is fat soluble due the organic portion, readily bioaccumulates at low trophic levels and even bioconcentrates among predators such as fish-eating birds and mammals. Much of the Hg that enters water bodies settles into sediments and can be locked in place for millions of years until some natural event such as a volcano eruption releases it back into the atmosphere.
Over the past few decades, scientists have become increasingly concerned about Hg in the South Water District of Florida which includes the Everglades. Fish consumption advisories cover more than a million acres in the district; sale of captured alligators for food has been curtailed because of Hg contamination and records show that the concentration of Hg jumped sixfold between 1900 and 1992 due to changes in hydrology, agriculture, and urban development (USGS, 2013).
The exact proportions may have changed somewhat, but in 1994, Sundolf et al. (1994) estimated that 61% of the Hg was due to atmospheric deposition from human sources, especially solid waste combustion facilities (15%); medical waste incinerators (14%); paint manufacturing and applications (11%); electric utility industries (11%); private residences (2%); and combustion of fossil fuels, electrical apparatuses including lighting (6%). All other anthropogenic sources, including burning of sugar cane (also a major source of PAHs), open burning, sewage sludge disposal, and dental industries accounted for approximately 3% of the total emitted to the environment. Virtually all natural sources (39%) were attributed to release from the soils due to natural processes including microbiological transformations between organic and inorganic forms. In general, organomercury represents only 1% or less of total Hg, even in contaminated sites but, as we will see, this small percent accounts for considerable harm to the environment.
Mercury is persistent. While its mean retention time in the atmosphere ranges from 6–90 days, it can last in soils for 1000 years; ocean water for 2000 years and ocean sediments for more than 1 million years (Eisler, 2000). These retention times are correlated with the importance of each reservoir in regards to total Hg contained. Ocean sediments account for 330 ×109 tons, ocean water for 4.15 ×109 tons; soils for 21 ×106 tons, the atmosphere for 850 tons, and freshwater for only 4 tons. Although the atmosphere is not a primary reservoir for holding Hg, it serves as the major conduit from one reservoir to another.
Uses for Mercury
Mercury has been used in industry, medicine, and agriculture but its demand has been halved since 1980, primarily due to concern regarding its toxicity. In industry, Hg has found use in measuring devices such as thermometers, barometers, manometers, and sphygmomanometers; in float valves; in electrical purposes such as mercury switches, mercury relays, fluorescent lamps, and lighting; and in the production of some types of telescopes. Today, Hg in thermometers and other instruments has been mostly replaced with colored alcohol or digital devices. If you have a thermometer with red fluid used to indicate the temperature, it uses alcohol; if it is silver, it is likely to be Hg. In the 1880s, Hg was used as a bath in making felt hats. The metal neatly separated the fur from the skin to facilitate obtaining the felt. This was in addition to the arsenic that they also used. Unfortunately, hat manufacturers did not recognize the neurotoxicity produced by Hg, and many hat makers developed various neurological disorders. Combined with arsenic toxicity, it is no wonder that the phrase “mad as a hatter” became widespread and perhaps was the inspiration for the Mad Hatter in Lewis Carroll’s novel Alice Adventures in Wonderland (Fig. 8.11). Although hat makers were affected by both arsenic and Hg, they didn’t even have hazard pay at that time.
In agriculture, Hg has been used as a seed coating to protect against fungal and other infections. Unfortunately, some people ate seed corn and other seeds protected with the organomercury coating and became poisoned. This practice of coating seeds with Hg was stopped in the United States in 1995, but still occurs in some other nations.
In medicine, Hg has been used for hundreds of years—going all the way back to ancient Egypt and China. It is still used in Chinese medicine, but its use in western medicine has almost disappeared. As mentioned above, Hg is used with other metals in dental amalgams. It is also found in thimerosal as a preservative for vaccines and related fluids.
Concentrations of Mercury in Organisms
Organomercury and elemental Hg are very different with regards to bioaccumulation. In particular, organomercury can easily bioconcentrate. This is due to a few salient features of organomercury. Organomercury can biomagnify in aquatic food chains so that almost all Hg accumulated in tertiary levels consists of methylmercury because it can be generated within lower trophic levels and is lipid soluble; inorganic mercury has far less propensity to biomagnify. While inorganic Hg is tightly bound to metallothioneins that facilitate Hg loss, relations between organomercury and metallothioneins are weaker and organisms are less capable of regulating organomercury so there is a greater tendency to retain it. Organomercury is more completely absorbed or assimilated from foods than inorganic mercury, it is also more soluble in lipids, passes more readily through biological membranes; and is slower to be excreted (Eisler, 2000). All of these factors add up to considerably more risk to organisms from organomercury than from inorganic forms of the metal.
Among plants in clean freshwater, the average concentration of Hg was 0.11 mg/kg fw; in contaminated sites, the mean was 10.6 mg/kg fw (Eisler, 2000). Freshwater invertebrates had mean concentrations of 0.21 mg/kg in clean water and 3.66 mg/kg in contaminated sites. Marine invertebrates averaged 0.40 mg/kg dw in reference sites and 6.97 mg/kg dw in polluted sites.
In general, mean Hg concentrations in muscle or whole bodies of fish seldom exceed 0.5 mg/kg fw although maxima can exceed 1.1 mg/kg fw. Various species of tuna collected from highly polluted areas in the Indian Ocean had muscle concentrations exceeding 24 mg/kg (NAS, 1978). Older fish, as measured by age, length, or weight, tend to have higher concentrations than young ones in the same waters because organisms are quick to take up Hg and slow to lose it.
Among amphibians and reptiles, Hg concentrations generally ranged from below detection limits to less than 0.5 mg/kg fw (Eisler, 2000). Amphibians collected below an acetate fiber mill with a history of Hg use in Virginia streams had Hg concentrations that were 3.5 to 22 times those of the same species collected from reference streams; mean values were 3.45 mg/kg for adults and 2.48 mg/kg for larvae (Bergeron et al., 2010).
Considerable research has been conducted on Hg levels in birds and mammals. Many of the tissues and whole body residue levels were below 1.0 mg/kg but the frequency of higher concentrations was greater than seen in aquatic organisms, suggesting that terrestrial organisms—or perhaps homeothermic animals—are more prone to Hg assimilation than aquatic organisms. High concentrations included great blue heron (Areda herodias) livers from Lake St. Clair, Ontario (175 mg/kg fw); livers in male (187 mg/kg fw) and female (100 mg/kg fw) common loons (Gavia immer) collected as dead birds from New England; and 295 mg/kg dw in livers of several species of albatrosses collected in New Zealand (reviewed by Eisler, 2000). Carnivorous birds generally have higher concentrations of Hg than herbivores.
Feathers have been a reliable source for nondestructive Hg residue analyses. Feathers can be used to compare recently collected animals to museum specimens and get a time sequence perspective on changes in Hg concentrations. They can also be used to compare different ages, sites, or other factors among rare or endangered species of birds without fear of affecting populations. Residues in feathers can reflect differences in conditions between the two sites because molting patterns vary among species with some species molting and regrowing feathers on the breeding and on the wintering grounds. Unlike with some other metals, Hg seems to have a particular affinity with feathers and concentrations in these structures are often higher than in soft tissues. Hair offers a similar benefit for mammals.
If you recall, marine mammals were among those that had the highest concentrations of organic contaminants such as PCBs and organochlorine pesticides. Such is the case with Hg, especially organomercury, because it shares the lipophilicity of other organic molecules and can biomagnify. Mercury concentrations in marine mammals typically measure less than 15 mg/kg (Eisler, 2000). However, among the highest concentrations reported were in livers of harbor seals (Phoca vitulina) in California (81–700 mg/kg fw); livers of adult harbor porpoises from the North Sea (504 mg/kg dw); and 1026 mg/kg in the livers of female California sea lions (Zalophus californianus). In contrast, elevated concentrations in nonmarine mammals seldom exceeded 50 mg/kg, although hair from dead Florida panthers (Felis concolor coryi) had Hg concentrations of 130 mg/kg fw (Roelke et al., 1991) and domestic cats that ate fish from below a chlor-alkali plant in Ontario had up to 392 mg/kg fw in their fur (Jensen, 1980).
Effects of Mercury on Animals
The 96-h LC50 for Dapnia sp. is 5 μg/L (US EPA, 1980), and this species is a model for several other species of aquatic invertebrates. Lethal concentrations of total Hg generally range from 0.1–2.0 μg/L for sensitive fish, but the high end may be around 150 μg/L to produce a 96-h LC50. Among sensitive species of fish, adverse sublethal effects can occur at water concentrations of 0.03–0.1 μg Hg/L.
Lethal concentrations in amphibians also range widely from 1.3 μg/L in embryos and larvae of narrow-mouthed toads (Gastrophyrne carolinensis, Birge et al., 1979) to 4400 μg/L in adult female river frogs (Rana heckscheri, Punzo, 1993).
Birds and mammals are substantially more resistant to Hg toxicosis than most aquatic animals. Median lethal doses to organomercury typically range from 2.2–50 mg/kg body weight or 4–40 mg/kg dietary among sensitive birds. Toxicity of inorganic mercury is much, much lower. For instance, in Japanese quail (Coturnix japonica), the LC50 using inorganic Hg was between 2956 and 5086 mg/kg in the diet over 5 days of exposure followed by 7 days of observation. However, only 31–47 mg/kg dietary methylmercury was needed to produce 50% mortality. Given as an acute oral dose, 26–54 mg/kg inorganic Hg and 11–33.7 methylmercury produced LC50s over a 14-day observation period in the same species (Hill and Soares, 1987).
Among mammals, a 96-h LD50 of 17.9 mg/kg oral dose was obtained for mule deer (Odocoileus hemionus, Hudson et al., 1984). Mink are particularly sensitive to Hg, and it only took 1.0 mg/kg to produce 100% mortality of the test animals over a 2-month period (Sheffy and Stamant, 1982).
In birds and mammals, sublethal effects of Hg include teratogenesis, mutagenesis, and cancer. It can negatively affect reproduction and growth, behavior, and blood chemistry. It is also infamous for causing neurological disorders involving motor coordination, hearing, vision, and (in humans) thought processes. Histopathology in kidneys, livers, pancreas, and heart are common. As we have mentioned many times, organic mercury is substantially more potent in producing these effects that inorganic Hg, but both can produce significant harm at environmentally realistic concentrations. For sensitive birds, sublethal toxicity can occur at 640 μg Hg/kg body weight or 50–500 μg/kg in diet. Sensitive mammals may be affected at 250 μg Hg daily/kg body weight or 1100 μg/kg in diet.
A Major Human Catastrophe
One of the great human environmental tragedies occurred due to methylmercury. The Japanese-owned Chisso Corporation dumped untreated effluents into Minamata Bay, Japan, from 1932 to 1968; in fact, dumping occurred well past 1968, but in that year some minor treatment of effluents was installed. The operation started as a fertilizer plant but eventually moved into chemical production, making acetylaldehyde and related products with no prevention of dumping their wastes into nearby rivers and ultimately into Minamata Bay. The resulting effluents were also heavily contaminated with several metals and other pollutants. Through this period, shellfish in the bay bioaccumulated exceedingly high concentrations of methylmercury. The local populace fed extensively on these shellfish, not having any idea of the consequences. The first human victim was a young girl in April 1956. The physicians employed by the Chisso Corporation had no concept of what was causing her difficulty in walking and talking or convulsions. By May of that year, an epidemic of neurological conditions had been declared and through that summer the number of cases continued to increase. Patients with the “Minamata disease” complained of a loss of sensation and numbness in their hands and feet. The disease affected their ability to grasp small objects; hindered their ability to run or walk; altered the pitch of their voices; and compromised their ability to see, hear, or swallow. At the same time, domestic cats and fish-eating wildlife were observed having spasms and convulsions. Cats were moving erratically, crows were falling from the sky, and fish were dying along the seashore (Withrow and Vail, 2007). By November 1956, investigators began to put the pieces of the puzzle together and identified that the one common factor among humans and animals was a diet high in fish and shellfish. They concluded that a heavy metal was involved in the disease and Chisso became the chief suspect. Rather that cooperating with the investigation, Chisso refused to provide information or offer any other assistance. They even conducted some of their own experiments that led to confirmation of Hg as the toxic agent but did not release their findings to the public or to the authorities. As a result, progress in finding the actual cause and solving it was slow, and it was not until 1959 that organic mercury was identified as the toxic agent. At this point, Chisso did “help” by agreeing to dredge the contaminated sediments around its effluent areas, but whether they had a change of conscience or only wanted to reclaim the Hg in the discharge is unclear.
Between 1953 and 1957, the fishing industry in Minamata Bay declined by 91%. The fishermen demanded reimbursement and, after more stonewalling, Chisso finally set up funds for reparations amounting to an astounding sum equivalent to $55,600 directly to the fishers and another $41,700 to reestablish fishing. In 1959, the government required that Chisso provide a “sympathy fund” for those affected by the pollution. If you were officially certified as having Minamata disease, the company would compensate you the equivalent of $278 per year (the equivalent of $2455 today after inflation), $83/year if you were a child, and your family could claim a one-time benefit of $889 if it was proven that you had died due to the disease. Many other examples of corruption occurred in this situation and the full extent of reparations and responsibility have still not been met. As of March 2001, 2265 victims had been officially certified (1784 of whom have died), and more than 10,000 people have received financial compensation from Chisso, although they are not recognized as official victims. As a result of this disaster, the United Nations established the Minamata Convention on Mercury in 2013. The principal articles of this international convention include a ban on new Hg mines, the phase-out of existing ones, control measures on air emissions, and regulation of small-scale gold mining (UNEP, 2015). Wikipedia does a good job of detailing the entire situation (http://en.wikipedia.org/wiki/Minamata_disease), but an official summary may be seen at the website on the topic established by the Japan Ministry of the Environment (2002).
As a closing note on Hg, it has been known since the 1960s that selenium (Se) can reduce methylmercury toxicity in vertebrates (eg, Ralston and Raymond, 2010). Selenium, especially at high concentrations, substantially reduces the neurotoxic and lethal effects of methylmercury. Recent studies have shown that Se-enriched diets can even reverse some of the more severe symptoms of methylmercury toxicity. Methylmercury is a highly specific, irreversible inhibitor of Se-dependent enzymes (selenoenzymes) that are necessary to combat oxidative damage, particularly in the brain and neuroendocrine tissues. Inhibition of selenoenzyme activities in these vulnerable tissues appears to be a direct cause of the pathology produced by methylmercury. Mercury has a very high binding affinity for selenium so that under conditions of low dietary selenium, most or all of it is bound to Hg.
Zinc
General Characteristics
The last metal that we will discuss is one, like copper, that is required by all living organisms. As a result, we’ll often see relatively high concentrations of this metal in tissues, even from plants and animals living in reference sites. As with copper, Zn deficiencies are more common than Zn toxicity. Approximately 31% of the global human population lives at risk of Zn deficiency (ATSDR, 2005). Severe deficiencies contribute to 176,000 diarrheal deaths, 406,000 pneumonia deaths, and 207,000 deaths due to malaria each year (Caulfield and Black, 2004). On the other hand, there are natural populations of plants and animals that are at risk from Zn toxicosis near smelters and industries that utilize Zn.
Mining of Zn ore dates back to 10,000 BC when the metal, along with copper, was blended into bronze, establishing the Bronze Age. It is the 24th most abundant element and the 4th most abundant metal in the earth’s crust. If the distribution of Zn were homogeneous, it would amount to 75 mg Zn/kg of soil or sediment (0.0075%) but the actual distribution can range from 5 to 770 mg/kg with an average of 64 mg/kg in the crust and 30 μg/L in seawater. Zinc naturally occurs as Zn1+ and Zn2+, although Zn2+ is by far the most common oxidation state. India, followed by Australia and Peru are currently the largest Zn producers of the 12,250 tons of elemental Zn extracted per year (USGS, 2014a).
Environmental Concentrations and Uses of Zinc
In the atmosphere, Zn typically ranges from 0.1 to 4 μg/m3 (USGS, 2014a). The behavior of zinc and its cycling is similar to the other metals we have discussed. Zinc in the atmosphere generally adheres to particulates which precipitate onto land and water. Additional Zn comes from volcanoes, smelters, and industrial processes. Approximately 21,450 tons of Zn are released by the United States each year (ATSDR, 2005). Of that, 414 tons (1.9%) enters the air, 26 tons (0.12%) is released into water, 16,900 tons (78%) consists of contaminants on land, and 4100 tons (19%) is stored on land. Zinc is found in soils and surficial materials of the contiguous United States at concentrations between <5 and 2900 mg/kg, with a mean of 60 mg/kg. The zinc background concentrations in surface waters are usually <0.05 mg/L, but can range from 0.002–50 mg/L (ATSDR, 2005). Similar concentrations occur in seawater. Canadian sediments from reference sites ranged from 50–180 mg/kg dw. Such sediments in the United States were also 10–75 mg/kg dw.
Zinc is one of the most commonly used metals. The primary use of Zn is in galvanizing steel, which accounts for 55% of the world’s production. Other uses include mixing into different alloys for industrial purposes (21%), brass and bronze production (16%), and miscellaneous uses (8%). Zinc is used in a host of metal and other products including solder, pipe organs, machine bearings, pigments, fire retardants, and wood preservatives. Zinc is also present in virtually every multivitamin on the market and is found in many pharmaceutical products. If you’ve ever used a white cream on your nose to prevent sunburn, you’ve probably used zinc.
Biological Effects of Zinc
Red and brown algae in marine environments are very efficient accumulators of Zn and can have concentrations greater than 1 g/kg (Eisler, 2000). Zinc concentrations in terrestrial and aquatic invertebrates often run into the high mg/kg or even g/kg ranges. Earthworm concentrations run from 120 to 1600 mg/kg dw among several sampling sites. Oysters from contaminated sites in England had as much as 12.6 g/kg in their soft tissues (Bryan et al., 1987).
Fish, birds, and mammals typically have lower concentrations than seen in invertebrates. Whereas concentrations can exceed 500 mg/kg in fish, the majority of reported values ranged from less than 10 mg/kg to approximately 200 mg/kg. Birds collected from reference sites generally had concentrations that were less than 60 mg Zn/kg dw. The highest Zn concentrations in birds tend to be in liver and kidney and the lowest in muscle (Eisler, 2000). Feathers appear to have similar concentrations as other body tissues and can be used to monitor Zn levels without being invasive.
Ungulates have often been reported with elevated Zn concentrations exceeding 100 mg/kg fw in liver or kidneys. These include white-tailed deer (Odocoileus virginianus) and red deer (Cervus elaphus) living near a Zn smelter (Sileo and Beyer, 1985). Typical concentrations for small mammals, mice, voles, and shrews range in relatively clean sites ranged from 16–204 mg Zn/kg dw (Eisler, 2000). In contaminated sites, concentrations could be higher at 370 mg Zn/kg dw. Marine mammals do not have unusually high concentrations of Zn as they do with some other contaminants because it is not fat soluble and does not bioconcentrate. Among several seals and whales, concentrations ranged from 47–406 mg/kg dw in liver, 14–140 mg/kg dw in muscle, and 37–353 mg/kg in kidneys (Eisler, 2000).
From a health perspective, Zn is involved in 100–300 enzymes in plants and animals. In addition, it provides structural support for DNA transcription, has numerous neurological benefits, and is very active with metallothioneins. Zinc can bolster the immune system and there are all sorts of products that one can take to avoid or reduce colds. The USDA established the recommended daily allowance for women as 8 mg/day, during breastfeeding and pregnancy, the recommended intake goes up to 13 mg/day. For men, the dose is 11 mg/day. Vitamin supplements can provide assurance of adequate intake, but most people in developed countries obtain sufficient Zn from a well-balanced diet.
As mentioned, Zn deficiency poses a greater risk than too much Zn for humans and for wildlife. Signs of deficiencies include depression; diarrhea; lack of appetite; depressed growth; reproductive and maturation delays; teratogenesis; alopecia (hair loss); eye and skin lesions; altered behavior including cognition, reduced activity, and less play among animals; impaired immunity; and defects in carbohydrate utilization (ATSDR, 2005). Mild zinc deficiency depresses immunity, although excessive zinc does as well. Animals with a diet deficient in zinc require twice as much food in order to attain the same weight gain as animals given sufficient zinc (ATSDR, 2005).
However, Zn toxicosis can affect all groups of organisms around highly contaminated sites. Sensitive plants can die when soil concentrations exceed 100 mg/kg dw. Sublethal effects in plants include poor growth and inhibited reproduction. Among invertebrates, reduced growth may be seen at 300–1000 mg Zn/kg diet dw (Eisler, 2000) and reduced survival may be experienced in the range of 470–6400 mg/kg in soil. Sensitive invertebrates include the earthworm Eisenia foetida, a common lab animal, and the slug Arion ater. Effects on invertebrate communities including loss of species richness and lower numbers of survivors occurred at 1600 mg/kg dw in the soil (Beyer and Anderson, 1985).
For fish, a few generalizations can be made (Eisler, 2000). Freshwater species tend to be more sensitive than marine species. Embryos and larvae are more sensitive than adults. For brown trout (Salmo trutta), fry, a very sensitive species and life stage, death occurred within the range of 4.9–9.8 μg/L (Sayer et al., 1989). For other species, lethal and sublethal effects begin at 50–230 μg/L in sensitive species. Zinc toxicosis in fish is indicated by hyperactivity followed by lethargy, loss of coordination, gill hemorrhaging, and extensive production of mucus. In extreme cases, death is often a result of apoxia (loss of blood oxygen) due to gill deterioration.
Less sensitive birds demonstrated reduced growth with diets having more than 2000 mg/kg in food and reduced survival at concentrations exceeding 3000 mg/kg diet. Ducks (Anas sp.) demonstrated decreased survival with 2500–3000 mg/kg dietary Zn and, when force-fed Zn, died at 746 mg/kg body weight (Eisler, 2000). Signs of Zn poisoning in birds include ataxia, flaccid paralysis, and histopathology and necrosis of pancreas. Chickens fed 15,000 mg/kg in their food as zinc oxide showed reproductive problems but no mortality after 7 days.
Mammals tend to be quite tolerant to Zn toxicity. Lab animals such as rats suffer adverse effects at 0.8 mg Zn/m3 in air, 90 to 300 mg/kg in their diets, 300 mg/L in drinking water, and 350 mg/kg body weight in oral doses (Eisler, 2000). Zinc targets the pancreas of mammals, resulting in loss of function. It also has serious neurological, hematological, immunological, and cardiovascular effects. Zinc toxicosis adversely affects development, growth, pancreatic fibrosis, acute diarrhea, copper deficiencies, and impaired reproduction (ATSDR, 2005).
Focus—Avian Mortality Due to Lead Shot, Bullets, and Weights
This is a story of how sportsmen unwittingly contributed to the death of millions of birds over the course of several decades.
Since the invention of firearms, the material of choice for ammunition has been lead. Lead shot, musket balls, and bullets have the density to fly well and accurately and have the compressibility so that when they strike their intended target, they flatten out to some extent, increasing the likelihood of inflicting maximum damage and making a clean kill.
However, this section focuses on a problem that have little to do with the actual killing power of firearm-propelled ammunition and more to do with the leftover lead following a hunt.
There are three situations dealing with sportsmen-derived environmental lead that contribute to avian mortality in a major way. These include concentrations of shotgun pellets in aquatic and upland situations; lead bullets in the tissues of animals that were shot but escaped, later to die; and lead fishing sinkers and weights.
With regard to shotgun pellets and their effect on birds, initial attention was paid to waterfowl and Pb contamination in wetland areas. Now that regulatory action has curtailed the use of lead ammunition in wetlands (see the discussion of this topic later in this chapter), greater focus is being placed on upland birds such as doves and their habitats. As early as 1880, hunters and biologists began to be aware that lead shot inflicted an insidious mortality factor. If a gamebird, such as a mallard duck is struck by lead shot but not killed or crippled, it has a good likelihood of surviving because after the shot enters muscle tissue, a capsule of scar tissue forms around the pellets and isolates them from the rest of the body with little or no adverse effects. However, shotgun pellets span the range of size occupied by the seeds that serve as natural foods for these birds. As a result, birds may also ingest pellets when feeding in contaminated areas. Ingested lead is by far the principal mortality factor in all three scenarios mentioned above. Once in the gizzard, the grinding effect of the muscles combined with acidic secretions dissolve the lead pellets and facilitates movement of the soluble lead into the circulatory system where it can have a host of adverse effects. Waterfowl suffering from lead toxicosis, for example, are weak and often unable to fly. They may demonstrate edema in the head, atrophy of breast muscles, lack of appetite, impaction of the proventriculus, ulcers, green feces, and diarrhea. Death often occurs through gradual starvation.
Public hunting areas operated privately and by states and federal governments are highly attractive to birds and to hunters and, through the course of time, substantial concentrations of lead pellets can build up. In areas managed for dove hunting, for example, estimates of the number of shot present range from 3228–167,593/ha before hunting to 17,628 to 860,185 after hunting (Lewis and Legler, 1985). An earlier study conducted in a public area for hunting Canada geese measured only 7512 pellets/ha, but the pellets were larger than those used for doves and in one year, the authors found more than 900 dead geese in the area (Szymczak and Adrian, 1978). Canada geese are field feeders, so this concentration of lead pellets was an upland estimate. In what has to be an extreme case, Perroy et al. (2014) estimated that the concentration of pellets from a wetland on an old trap shooting range was greater than 50,000 pellets/m2 or 5 million pellets/ha (Fig. 8.12). Perhaps the highest density of pellets found in a National Wildlife Refuge was 2 million pellets/ha (Thomas et al., 2001). The authors reported that conventional tilling practices can reduce the amount of pellets available to birds by almost 50% and these practices are being used on public lands. Since it often takes one or two #4 pellets to be lethal (Puckett and Slota, 2014), the abundance of shot is very important in determining how many birds could die. In semicontrolled studies, it was estimated that 1–4% of the mourning doves that visit public hunting areas could die from lead ingestion (Plautz et al., 2011). While the percentage values seem small, they translate into many thousands of birds.

It took decades from the first scientific studies on the effects of spent shot in wetland environments to the execution of federal and state laws regulating lead shot. The first solid evidence that lead shot was a mortality factor for waterfowl occurred in 1919. Friend (1989) and others estimated that 1.8–2.4 million waterfowl were dying each year due to ingested shot and federal legislation was enacted in 1991. At that time, Congress banned the use of lead shot on National Wildlife Refuges and waterfowl production areas. Shortly thereafter, some states enacted bans on their properties and by 2011, 35 states had banned the use of lead shot on designated hunting areas. One of the barriers to legislation was finding an adequate substitute for lead, something that would have similar flight patterns and killing effectiveness. Ultimately, this led to such substitutes as steel, bismuth, and various alloys of tungsten shot.
Another method of lead toxicity that is of concern for wildlife is secondary toxicity. In this scenario, an animal is shot with bullets or shotgun pellets that lodge in its tissues and is not recovered by the hunter for whatever reason. When the carcass is scavenged by other animals, the scavenger consumes the contaminated tissues and becomes poisoned. Secondary poisoning can be serious for a variety of scavenging species but arguably, the most vulnerable of these is the California condor (Gymnogyps californianus) (Fig. 8.13) and the bald eagle (Haliaeetus leucocephalus). The trouble begins when the lead bullet or buckshot sometimes fragments into scores of small pieces in the body.

Warner et al. (2014) examined the bodies of 58 bald eagles found dead in 2012 in the Midwest. Sixty percent of the birds had detectable lead concentrations with 38% of them having lethal concentrations. Raptors show many of the same signs of lead toxicity as waterfowl (Fig. 8.14). The authors went on to identify the source of this lead on the Fish and Wildlife Service’s Upper Mississippi River National Wildlife and Fish Refuge which allows deer hunting. Thirty-six percent of offal piles contained lead fragments ranging from 1–107 particles per pile. They concluded that offal piles were likely the source of lead for bald eagles.

Overhunting large carnivores, intentional killing of condors in a mistaken belief that they preyed on lambs and calves, and habitat destruction coupled with a low reproductive rate are factors that have led to the decline and near extinction of condors, but lead ingestion has also been an important factor. In the 1980s, all wild condors were captured and brought to zoos where they encouraged to breed. Offspring of these birds were released back into the wild in areas that had been closed to hunting by federal law. Approximately 62% of 150 free-ranging condors sampled 15 years after the release program began had blood lead levels, and the percent of animals with lead increased with age and independence away from sanctuaries where clean food was provided (Kelly et al., 2014). Maximum lead concentrations in blood also remained high over those years. It does not seem that condors are free from the risk of lead at this time. However, steps continue to be taken to protect the endangered bird. In 2008, eight counties in California within the heart of Condor country were closed to all forms of lead shot and bullets.
The last topic of lead toxicity in this section is on lead weights and sinkers used in fishing. Similar to lead shot used in waterfowl hunting, lead weights can be ingested and cause toxicosis. Many of the split shot and other forms of weights are also within the size range of seeds (Fig. 8.15) and are mistakenly eaten. Concern about lead weights began in England in the 1970s and 1980s and centered on swans that were found dead or dying. An estimated 4000 mute swans (Cygnus olor) died from lead poisoning each year. In 1987, Britain banned use of leaded weights ranging in size from 0.06–28.36 g. The swan population has increased in size since then, but it is not clear how much of the increase was due to the lead ban. Kelly and Kelly (2004) reported that of 1421 swans brought into a wildlife hospital in England between 2000 and 2002, between 15% and 18% had fishing tackle issues including getting caught in lines, getting hooked, and showing signs of possible lead toxicity. Of 921 birds tested for the presence of lead in their blood, 74% proved positive with concentrations ranging from 0.2 μg/dL to 2.3 mg/dL. Lead in sediment continues to be a problem in Britain, both as lead weights and gunshot.
Study Questions
1. What are some of the common characteristics of metals?
2. How many elements are listed as metals on the periodic chart of elements? How about metalloids? Nonmetals?
3. How would you define a heavy metal (please ignore the music style)?
4. What did Paracelsus mean when he said that the dose makes the poison?
5. Describe the relationship between deficiency and toxicity for metals that are essential for diets.
6. Discuss some reasons why organometals are usually more toxic than elemental or inorganic metals.
7. What role does pH have in the solubility and bioavailability of metals?
8. True or False. Hard water with a high concentration of calcium reduces the toxicity of many metals compared with soft water.
9. What group of proteins is highly involved with metal transport and excretion?
10. True or False. Arsenic does not have a true melting point.
11. List four or five major uses of the following metals: arsenic, copper, cadmium, lead, mercury, zinc.
12. Why was the occupation of making hats in the 19th century a risky business?
13. Which of the metals that were individually described are suspected or confirmed endocrine disruptors? Which are suspected or confirmed carcinogens? Which are essential to living organisms? Include both elemental and organic forms of the metals in your answer.
14. As a general rule, is the toxicity of metals to fish more or less than to birds or mammals?
15. What is the effect of particulate matter such as dissolved organic matter in water concerning bioavailability of metals?
16. Rank the individually described metals from low to high in the amount mined each year globally.
17. Why are lead pellets that become imbedded in muscle tissue not very toxic whereas those that are consumed can be lethal?
18. What is secondary toxicity and how does it affect California condors?