Community-Level and Ecosystem-Level Effects of Environmental Chemicals
Abstract
Traditionally, ecotoxicology has focused on the responses of individuals to contaminant exposure with some studies on populations. However, contaminants can affect entire communities and even ecosystems, although our understanding is limited in these areas. This chapter discusses some ways that contaminants interact with other stressors at the community-level and proposes some ways that ecosystems may be examined from an ecotoxicological perspective.
Keywords
Airshed; watershed; biological index of integrity; community; ecosystem
Introduction
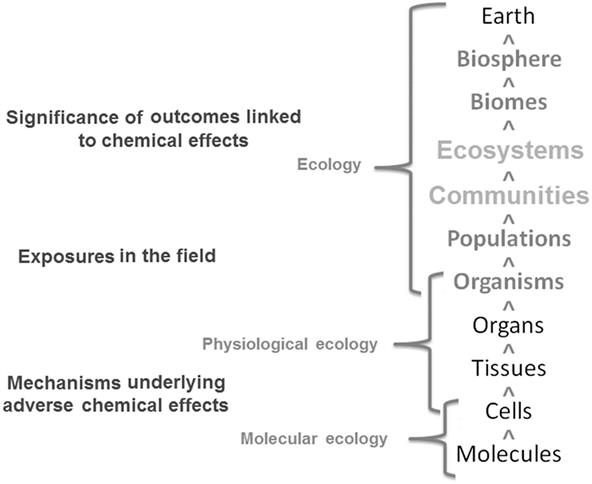
As we introduced in Chapter 10, hierarchical models—those capturing scale differences across time and space, or those representing different levels of biological organization—can reflect actual circumstances and be implemented practically to evaluate chemical effects on biological components of many ecosystems. This chapter extends our previous consideration of how chemicals affect populations by examining the populations of many species that occupy shared habitats within a given landscape; that is, we briefly consider communities, and at larger scales, ecosystems. Indeed, scale differences are encountered in many contemporary issues and are related to chemical releases into the environment that directly result in soil, water, and air pollution to lesser or greater extents. Indirectly, these chemical releases may also serve as contributing factors in exposures potentially linked to other adverse outcomes, such as biodiversity loss and climate change (Fig. 11.1).
Communities, Ecosystems, and Spatiotemporal Scales
Multiple stressors—in many cases strongly dominated by chemical stressors—impact ecological systems from local to global scales. Thus, understanding these environmental issues requires perspectives that consider interactions among physicochemical and biological processes at multiple spatial and temporal scales. In addition, as suggested in the previous chapters of this book, we encourage the following of an analytical path that employs integrated field and laboratory studies with field surveys or remote sensing to determine what occurs on the ground and laboratory toxicity studies to test which hypotheses developed in the field are valid. If there is a regulatory concern, results of these findings can then be applied to informed resource management practices, such as remediation.
Our focus in this chapter resides on large-scale interactions that are intractably linked to higher levels of biological organization. These higher-order levels of ecosystem processes, such as those linked to carbon and nutrient cycling, are affected by releases of chemicals (Cain et al., 2014; Gimme and Hengeveld, 2014). For example, the release of greenhouse gases and other atmospheric chemicals, such as particulate matter, ozone, NOx, and SO4, will inevitably be complicated because of air, land, and water-use policies and practices that potentially affect habitats as an outcome of exposures to multiple environmental stressors (eg, air pollution, climate change, land mass fragmentation) on ecosystem function. Even with integrated field and laboratory studies, there will be inherent complexity and uncertainty in understanding the relationships between chemical exposure and effects at the community or ecosystem levels (Luo and Magee, 2011). Therefore, investigators may have to opt for less-than-absolute certainty when it comes to characterizing cause and effect linkages between environmental chemicals and biological effects. Regardless of this complexity and uncertainty, ecotoxicologists and applied ecologists have tools and methods available to evaluate these higher levels of biological organization and develop an understanding, at some level, of the possible chemical effects encountered in field settings within the context of these larger spatial scales. Such tools and methods are frequently deployed under the auspices of biological assessment.
Scientific Support for Community Effects From Contaminants
There has been an extensive list of scientific studies showing that contaminants can affect entire communities. The greatest number of studies have occurred with aquatic micro- and macroinvertebrates because, compared with vertebrates, these organisms are easily maintained in the laboratory and can be tested in multiple-species combinations to derive some understanding of how a chemical may impact an entire community. Communities of fish and soil organisms have also been studied, but few if any studies have examined entire communities of birds or mammals. In this regard, scientists often find that some species are quite sensitive to the chemical of concern whereas others are less so and can tolerate higher concentrations before they show adverse effects, such as death.
To illustrate a scientific, nonregulatory investigation of how contaminants may affect communities, we turn to a study conducted by Schmidt et al. (2013) along streams in the Colorado Rockies. Their objective was to determine the relationship between metal contamination from mining activities and either insect larval abundance or the frequency of adults emerging from these streams. They identified linear gradients in total metal concentrations through the course of the streams and then sampled both larval abundance and adult emergence. The authors developed a complicated index based on the relative toxicity of metals and their concentration in the insects (see the study for more details) to assess a community-wide toxicity quotient. They found that larval abundance remained relatively constant and high until a threshold concentration of metal bioavailability was reached, and then it fell precipitously. In contrast, adult emergence fell drastically, even at very low metal concentrations and the authors concluded that adult emergence was a more sensitive marker of metal contamination than larval abundance. From an ecological perspective, a low frequency of adult emergence could result in fewer eggs laid for subsequent generations or a lower availability of food for terrestrial insectivores because these adults can be an important source of nutrition to animals living near or in the streams.
Biological Assessments
Biological assessments are evaluations of the condition of an area of interest using surveys of the structure and function of a community’s resident biota (eg, fish, benthic macroinvertebrates, periphyton, amphibians for aquatic habitats; see Karr and Chu, 1997; Barbour et al., 1999). As a long-used method, biological assessment has been routinely applied during the process of evaluating water quality. For example, water quality of streams or rivers flowing through a landscape may be affected by external conditions such as a leaky landfill or erosion because of connectivity among the different components (land, water, air) of a human-dominated landscape. Biological assessments provide resource managers with information on the condition of the biological community and help document the response of this community to environmental stressors. For surface waters, such as rivers, streams, wetlands, ponds, or lakes, biological assessments evaluate the condition of a waterbody by sampling species that spend all or part of their lives in that waterbody (Karr and Chu, 1997; Flotemersch et al., 2006; US EPA, 2004, 2007, 2011a). As such, field studies collect a snapshot biological survey of an area, wherein they collect a representative sample of the biological community found in a waterbody. A biological survey is a systematic method for collecting a consistent, reproducible, and reliable sample of the aquatic biological community in a waterbody. The same may be said of terrestrial and wetland habitats (Cooperrider et al., 1986; Pilz et al., 2006; US EPA, 2002, 2011b).
As an example of bioassessments, Martínez-Lladó et al. (2007) studied sediment-dwelling macroinvertebrates in Barcelona Harbor in the Port of Barcelona, Spain. Barcelona Harbor is one of the busiest harbors in the Mediterranean and the investigators wanted to see if either polycyclic aromatic hydrocarbons from oil or tributyltin, an antifouling biocide applied to ships’ hulls to ward off barnacles and the like, affected the macroinvertebrate communities. They collected chemical and biological samples throughout the harbor and found a significant trend in community characteristics from the inner harbor outward to reference areas outside of the harbor. They used species richness, total organism density, the Shannon-Weaver (H′) diversity index, and a biotic index that was a weighted composite of major invertebrate groups. Species richness was not always measurable, but species diversity increased from the inner harbor to the reference sites and the biotic index decreased in the same direction. This particular index gave higher values to taxa that were less sensitive to contamination and more likely to inhabit polluted sites. The species diversity and biotic indices correlated with tributyltin concentrations, but not polycyclic aromatic hydrocarbons, which led the authors to conclude that tributyltin exerted a toxic effect on invertebrate communities in the harbor sediments.
Recall the discussion of bioindicators in Chapter 3. These bioindicators are useful to determine if individual organisms are being affected by exposure to contaminants. There are also biological indicators to assess the heath of communities and ecosystems. When an ecotoxicologist is working with a new or poorly known species, he or she may compare the plasma characteristics of individuals thought to be exposed to those from reference sites and see if there is a difference, even when the appropriate values are unknown. Ecotoxicologists working at a community or ecosystem level will also compare community structure or species diversity between a reference site and one that is suspected or determined to be contaminated. Data collected at reference sites provide a basis or benchmark on the biological condition of a healthy site. Ideally, reference sites have not been perturbed, particularly within the context of anthropogenic stress. Unfortunately, human activities on aquatic systems have become increasingly widespread. Thus, reference sites likely display some degree of impact due to human activities, but they still represent a best approximation of natural conditions for comparison with other sites under investigation.
Although biological indicators were initially developed for assessments of surface water quality, other biological indicators have been developed to assess the condition of any environmental setting—freshwater, estuarine, or marine water bodies; wetlands of various types; or the wide range of terrestrial ecosystems. For example, if we look toward biological assessment of surface waters, an historic glimpse of biological indicators used in biological surveys includes:
 Fish (eg, trout, sunfish, perch, salmon)
Fish (eg, trout, sunfish, perch, salmon)
 Benthic macroinvertebrates (eg, insects, snails, crayfish, worms)
Benthic macroinvertebrates (eg, insects, snails, crayfish, worms)
 Amphibians (eg, frogs, salamanders)
Amphibians (eg, frogs, salamanders)
Biological assessments provide an empirical basis to evaluate the impacts of chemicals released to the environment, particularly those with no emission quality standards. In addition, the results of biological assessments address adverse effects potentially linked to exposures of chemical mixtures, conditions typically encountered in field settings. Biological assessments provide data to better characterize physical stressors, such as flow and siltation, or weather and indicate the extent to which biological stressors (eg, invasive species) may be contributing to reduced conditions of the community. In part, these biological indicators are evaluated in parallel with assessments of habitat condition; for example, for lotic habitats instream, riparian data, soil characteristics, or overall habitat quality are collected simultaneously with those gathered on biota in the system. Jointly, then, biological data and habitat data reflect the overall ecological integrity of an area under investigation, and provide a direct measure of both present and past effects of stressors on the biological integrity of an ecosystem. As such, the biological assessment process integrates cumulative effects of different stressors from multiple sources, providing an ecosystem-level measure of their aggregate effect, and contributes empirical data regarding biological responses that support development of stressor-response models (see Chapters 12 and 13).
As another example of bioassessments, this time a semiaquatic, semiterrestrial bioassessment, Cesar et al. (2014) evaluated a site that was used to dump dredged sediments from the Santos Bay in Brazil. In this case, the authors did not use a reference site, but employed an integrated field and laboratory approach. The sediments came from the marine environment and were now transposed to land so the assessment could be said to affect both land and water. The authors evaluated sediment quality by quantifying both the structure (eg, sand, silt, clay) and chemical composition, including polycyclic aromatic hydrocarbons, chlorinated hydrocarbons such as polychlorinated biphenyls, pesticides, phthalates, metals, and nutrients. By now you should have a pretty good idea of why these chemicals were chosen. The investigators also conducted acute and chronic laboratory toxicity tests using amphipods (Tiburonella viscana) and sea urchins (Lythechinus variegatus), both of which were found in the bay. They found evidence of toxicity through all the methods they used, which suggested that the area for disposal of the dredged material was significantly altered with respect to sediment quality and could probably generate harmful effects on the local biota. After further analysis of the contaminant data, the researchers determined that most contaminant concentrations were below the limits established by Brazilian regulation. Now several questions could be asked, such as:
(1) Were the Brazilian standards set too high?
(2) Was there something about the dredging and other handling of the sediments that increased their toxicity above what would occur with undisturbed sediments?
(3) Is it germane to use marine aquatic organisms to test the toxicity of what are now soil samples?
Overall, biological assessments contribute to technical findings regarding an area’s condition. If the area under investigation displays conditions reduced relative to those of the reference site, then the area is considered impaired. Yet, as critical as biological assessments are for detecting impacts on a system, such technical findings alone do not likely identify the cause or causes of impairment. Indeed, given the multiple stressors at play in these larger spatial scales and through time periods much greater than those achieved in traditional laboratory toxicity tests, the number of factors involved with exposure preclude such likelihoods. Take, for example, the study cited previously (Cesar et al., 2014). The authors did not really do an assessment of the macroinvertebrates on impacted site; that is, they did not sample the macroinvertebrate community in the sediment or soil to see if it showed signs of perturbation. Rather, they used organisms from the source of the sediment or soil. Did these organisms really reflect the multiple stressors in the impacted site?
Another study, conducted by Aazami et al. (2015), took a different approach in an aquatic environment. The authors wanted to assess the biological status of the Iranian Tajan River using a measurement (sometimes referred to as metric in these types of studies) on macroinvertebrates and a series of 28 physiochemical parameters and 10 habitat factors for 17 sites. The Shahid-Rajaee dam divides the Tajan River into upstream and downstream parts, with different land uses. A total of 18 metrics were used to represent four components of ecosystem quality: tolerance, diversity, abundance (total number of taxa, individuals, and members of the orders Ephemeroptera, Plecoptera, and Trichoptera), and composition of assemblages (percent of Ephemeroptera, Plecoptera, and Trichoptera). Results showed that the macroinvertebrate index decreased from upstream (with very good water quality) to downstream (with poorer water quality due to human activities). There were more industrial activities such as pulp mills and sand mining in the downstream part of the river. The authors admitted that, even with all of the variables measured, there was uncertainty in the relationships of habitat quality, pollution, and the physiochemical properties of highland versus lowland rivers.
There might be some differences in the concepts of exposures or effects compared with organisms or populations because levels of biological organization also capture differences in spatial and temporal scale. However, we can extend concepts of exposure and effects with only limited angst. Indeed, we need to only modify our expressions of exposure, less so effects on time and space, to begin integrating across levels of organization.
Communities and Ecosystems: Exposures and Effects
Although an oversimplification, conceptually, a community refers to the collection of species interacting in a particular area. Indeed, some community ecologists debate whether a community is nothing more than an aggregation of species co-occurring in one area, or whether a community exists as an integrated unit (Newman and Clements, 2007; Mittelbach, 2012). Yet from an analytical perspective, when considered within the context of communities or ecosystems, exposure and effects are characterized by endpoints that are principally derivatives of those we considered at the organism level of biological organization, and that we subsequently linked to endpoints commonly characterized at population level of organization (Newman, 2001; Newman and Clements, 2007). For example, toxicity tests measure outcomes such as survival and fecundity, which easily translate to inputs called life table analysis—deployed by ecotoxicologists interested in characterizing effects on populations exposed to chemicals released to the environment (Rowland, 2003; Skalski et al., 2005; Barnthouse et al., 2007; also see Chapter 10). Similarly, much of our focus related to endpoints from exposure and effects simply acknowledge the spatial and temporal attributes captured by communities or ecosystems (Gurney and Nisbet, 1998). These community or ecosystem endpoints, however, are likely to be confounded by factors other than exposure to environmental chemicals. Thus, outcomes of toxicity tests, based on their relatively simple experimental designs, yet prominent in characterizing exposure and effects relationships at the organism level, may reveal only a portion of the effects of contaminants on communities and ecosystems.
Exposure Within a Community and Ecosystem Context
When we discuss exposure at levels of biological organization that display larger spatial coverage than that experienced by biota exposed to chemicals in laboratory beakers and when we consider exposures that span lifetimes and multiple generations—well beyond most time periods examined during laboratory tests—we begin to see the complexity and difficulties in interpretations of how to discern the effects of contaminants on communities and ecosystems separately from all other stressors that might affect these levels of organization. However, such outcomes are critical to our understanding of effects that may very well be inconsistent with those effects observed in laboratory toxicity tests. Only the interpretative context has changed.
At community and ecosystem levels, exposure is commonly considered relative to load, a term that goes beyond the equally common terms of exposure that usually occur as a measure of concentration; that is, a mass of chemical in a given volume of exposure medium (eg, mg/L in water, mg/kg in soil). In contrast, load is a quantitative estimate of exposure to a chemical released to the environment whose volume is much greater than that encountered in laboratory toxicity tests and generally absent from the experimental controls in place. A critical load simply extends the term’s definition as a quantitative estimate of exposure to one or more chemicals below which significant harmful effects do not occur to sensitive biota in the environment. Thus, you can see how terms of concentration and load are linked and how distinctions between estimates of exposure follow from conditions specified by units of mass and volume applied in their derivation.
Although we will not cover the arithmetic for derivation of environmental loads, we need to briefly characterize environmental compartments frequently applied to the derivation of environmental loads—airshed and watershed—that identify frames of reference when considering exposure at the community or ecosystem levels of biological organization.
Airshed. Airshed finds much of its formal definition within regulatory contexts; however, that definition is consistent with underlying experimental and observational data applicable to the exposure considered by ecotoxicologists working with air quality. Simply stated, an airshed is the volume over an area of land in which airborne chemicals travel to reach a particular river, lake, bay, or other body of water given the area of the land surface. Not surprisingly, atmospheric loads of chemicals are usually expressed volumetrically. Airshed is roughly analogous to the concept of the watershed and also considers air basins as geographical areas that share the same air because of topography, meteorology, and climate. Ideally, an airshed is a portion of the atmosphere that behaves coherently with respect to dispersion of chemicals released to emissions. Most often, an airshed is considered to be a volume of air receiving emissions that affects a specific watershed or catchment. Thus, chemicals released into the air may be linked to their potential deposition to terrestrial ecosystems involving organisms dependent on soils and waterbodies for habitats (NRC/NAS, 2004; Sportisse, 2010). Although conceptually related, the definitions and characteristics of a watershed differ, sometimes subtly, other times markedly from those of the atmosphere.
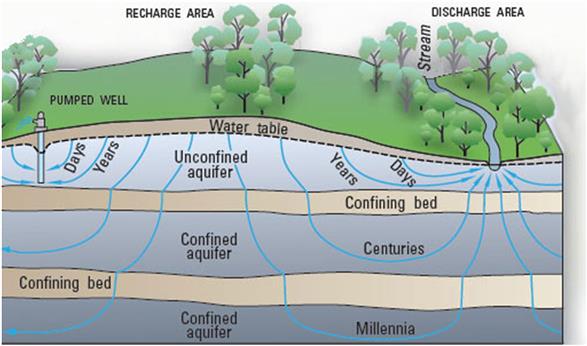
Watershed. Everyone knows what a watershed is, right? Local definitions of watershed, however, may or may not agree with a commonly applied definition. A watershed is an area from which all precipitation flows to a single stream or set of streams (Brooks et al., 2012). Depending on local terminology, individual users might use terms often considered as synonyms of watershed: catchment, catchment area, or drainage basin. However, watershed will be the term of choice in this discussion. Watersheds are bound by drainage divides or boundaries between drainage basins wherein all the precipitation on opposite sides of a drainage divide will flow into different drainage basins. For example, the total land area drained by the Mississippi River constitutes its drainage basin, whereas that part of the Mississippi River drained by the Ohio River is the Ohio River watershed, and that part drained by the Missouri River is the Missouri River watershed (Fig. 11.2). A watershed consists of surface water—lakes, streams, reservoirs, and wetlands—and all underlying groundwater. As in our example of the Mississippi River drainage basin, larger watersheds may consist of large subunits, which in turn contain many smaller watersheds. It all depends on the frame of reference, principally the point of outflow; all land that drains water to the outflow point is the watershed for that outflow location. There are subtle differences between the terms catchment and watershed; the latter is usually used in hydrologic applications and has a specific characterization that reflects physical attributes such as elevations, whereas the former simply refers to an amount of water caught in a given area of a watershed. Catchments occur as land features or built structures (eg, a dam) that catch and hold water. Some catchments are open, connected to adjacent geographic features that assure linkages and flows of surface water. Other catchments may be closed; that is, limited with respect to surface runoff as you would observe with depressional wetlands or with groundwater seepage lakes that have no inlets or outlets. As such, watersheds simply reflect topographically-limited surface areas that link physical attributes or processes to their hydrology.
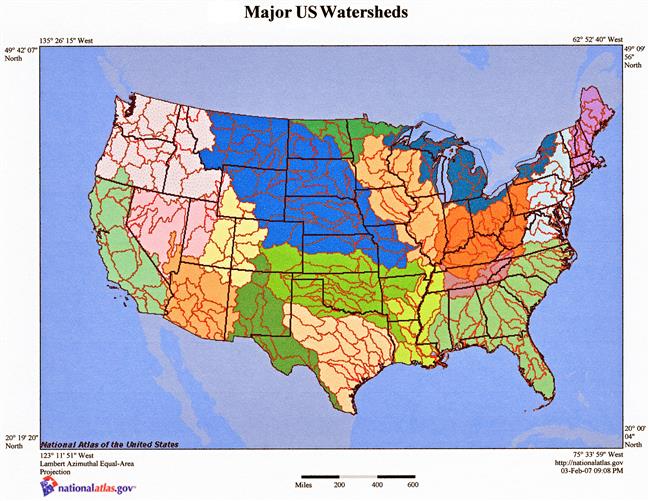
In the field, watersheds are significant geographic features; streamflow and water quality of waterbodies are affected by various factors, some human-induced, some not. Climatic variables, such as those related to precipitation, and variables describing water and sediment discharge, water storage, and evapotranspiration are commonly measured. Various hydrologic models may be used to characterize a watershed’s role within a larger landscape. Simply said, however, what happens on lands above a river-outflow point makes a difference in what we collect from discharge points at watershed boundaries. Rather like Las Vegas, don’t you think?
Loads. Recall our definition of load. Indeed, from an ecotoxicological perspective, critical loads might be considered a landscape-based value analogous to those measures derived from toxicity tests performed in laboratory exposures. For example, atmospheric critical loads as characterized through various models, all based on soil health, represent a quantitative estimate of exposure to environmental chemicals below which significant harmful effects on specified sensitive elements of the environment do not occur. Critical loads for particulates and chemicals—metals, volatile organic chemicals (VOCs), and semivolatile organic chemicals (SVOCs; eg, phenols and phthalates), and persistent organic pollutants (POPs)—released to the atmosphere have been developed by various regulatory agencies throughout the world. Although the concept of critical loads is becoming increasingly apparent in the environmental literature, much of the regulatory focus, particularly historic efforts to establish air quality criteria in the United States, have been expressed in terms of concentration per unit volume, not in loads. Regardless of the atmospheric chemicals, critical loads for chemical constituents of ambient air are usually derived from one of three general model systems—empirical, simple mass balance, or dynamic. Each is based to varying extents on observational studies or the compilation and assembly of existing data rather than on integrated field and laboratory studies. However, data from these studies have been used in the technical development of these model systems. Critical loads may be derived from observational data for a component of the landscape; for example, a surface water system such as a lake and its adjacent habitats. For such a system, physical habitat attributes such as surface water pH and soil physiochemical characteristics—say, nitrate and acid-neutralizing capacity—are measured along with vegetation biomass, cover, and species composition, and then observed vegetation responses are linked to habitat attributes. In this example, then, we might characterize a critical load for nitrate based on observed vegetative biomass. Regardless of methods deployed to estimate critical loads, each depends on available observational data. Empirical critical loads require observational data of known quality for both exposure (eg, deposition values for chemicals of concern) and effects (eg, spatially linked response data collected for vegetation during the period of deposition).
Similarly, chemical loads to surface waters are often managed through setting limits for an entire watershed. For example, within the United States, total maximum daily loads (TMDLs) are the daily load of a given contaminant that a given environment can be subjected to each day without showing any degradation (NRC/NAS, 2001). Groundwater is also regarded as an integral component of a watershed because watersheds are defined by their natural hydrology, which reflects the potential interconnectedness of groundwater and surface water, perhaps best displayed by riparian wetlands and groundwater recharge to rivers and streams.
There is a very complex relationship among the various components of water (Fig. 11.3). Water can evaporate from surface waters and land and enter the atmosphere. Alternatively, water from the atmosphere comes down as precipitation, initially striking land or immediately becoming part of the surface water found in rivers, streams, lakes, ponds, wetlands, etc. Much of this surface water enters larger bodies of water and eventually makes its way to oceans or seas. However, a good portion also percolates through the soil or sediments into deeper layers known as groundwater. Recharge areas are sufficiently elevated so that water tends to enter soil and recharge the aquifers. Some of this groundwater is close enough to the surface in unconfined aquifers that it can return to surface waters during drought or through tapping into the aquifers with wells. Discharge areas tend to be lower and promote the movement of water in unconfined aquifers back to the surface as seeps or upwellings. Deeper groundwater may be beyond the reach of all but the deepest wells and be stored for hundreds of thousands of years (USGS, 2015). All forms of water except perhaps those in very deep, confined aquifers are potentially open to contamination. Even this water might be contaminated through deep in-ground activities, such as mining.
Effects at Community and Ecosystem Levels
Results of any biological assessment provide descriptions—qualitative or quantitative—of relationships between environmental chemicals and biological or ecological endpoints. For our purposes, we are interested in endpoints as quantitative measures of an observed or measured effect; it is a measurable environmental characteristic that may be related to a characteristic interpretable within the context of resource management. As we have mentioned, work focused on communities and ecosystem endpoints may be measured in field, laboratory, or integrated studies. To ease the potentially long list of activities that could be pursued in field studies, ecotoxicologists have identified a menu of generic endpoints—ecological entities and attributes that have been shown to indicate stress or exposure at higher levels of ecological organization.
Community-Level Effects
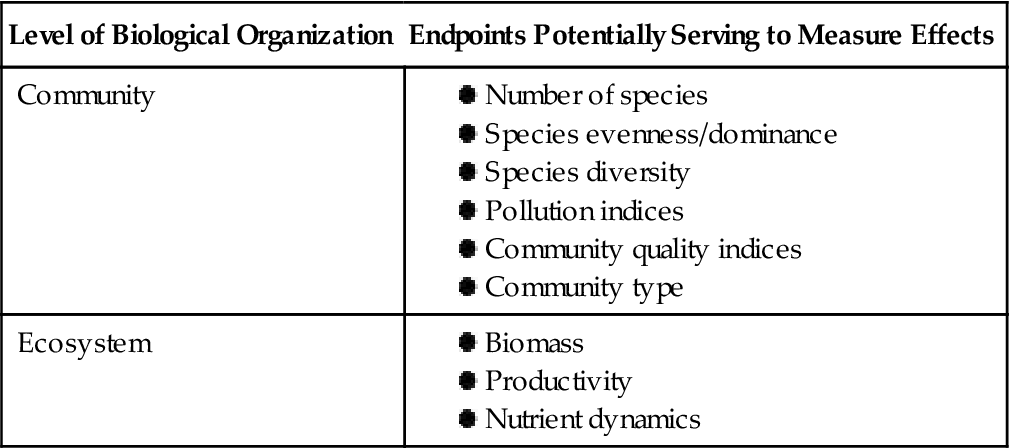
Examples of community-based endpoints that are routinely incorporated in biological assessments are listed in Table 11.1 as general classes that can be refined, depending on the community being surveyed. Among the most commonly used community characteristics are the number of species, species evenness, and species diversity. Sometimes key indicator species may receive particular attention. Indicator species are those known to live in a region and are particularly sensitive to perturbations. For instance, in streams and small rivers, insects, either larvae or adults, within the orders Ephemeroptera (mayflies), Trichoptera (caddisflies), and Plecoptera (stoneflies) are frequently used as bioindicators of good water quality (Fig. 11.4; see Smith, 2001 for life history backgrounds for these orders and their representative species). These and other parameters are convenient for summarizing data collected from biotic surveys, and are easily measured, appropriate for wider geographic scales typically linked to areas of interest, and serve to integrate outcomes from acute and chronic exposures to environmental chemicals. For most biota considered in designed field sampling efforts—vertebrates, invertebrates, or vegetation—sample statistics, particularly variance, benefit interpretation of effects on environmental health and possible contaminant exposure. The season may be very important in affecting abundance or life-stage dynamics; it would not be meaningful for investigators to survey woodland birds in one forest or macroinvertebrates in a wetland during May and another site in November in temperate areas, for example.
Table 11.1
Endpoints Commonly Evaluated in Field Surveys Completed as Part of Biological Assessments
| Level of Biological Organization | Endpoints Potentially Serving to Measure Effects |
| Community | |
| Ecosystem |

While these metrics are widely used for a variety of habitats, they may not be very diagnostic of specific problems nor well-standardized with respect to sampling methods. For instance, stoneflies, mayflies, and caddisflies are sensitive to many water-quality factors including pH, oxygen concentration, and turbidity in addition to contaminant exposures. Differences between two sample sites in indicator species may identify that something is happening, but not what that something may be. Thus, attempts to parallel reference and contaminated sites for as many factors as possible is often essential for meaningful comparisons. However, indicator species have been chosen for their ease of sampling as well as for their sensitivity to perturbations, so intensive sampling and data summarization will likely not be necessary to describe community changes.
Another type of community-level endpoint, generally termed an index of community quality, may be indicative of chemical effects or of habitat quality. For instance, observational studies may suggest that the replacement of one set of species with another was linked to conventional organic pollution (ie, sewage and similar effluents (Hynes, 1960)). The concept of indicator species is similar to community indices in that they are both intended to describe the state of communities relative to anthropogenic effects. The presence or abundance of a species considered to be either pollution-sensitive or pollution-tolerant has been used to indicate the status of a community. Other indices of generic community quality, such as the index of biological integrity (IBI) may serve as indicators of the state of communities because they are sensitive to physical habitat quality as well as to chemicals released to surface waters (see Karr, 1987; Barbour et al., 1999). All of these community-quality indices reduce to one number in the information obtained from a biotic survey. While this may sound simplistic, if an index is well characterized for a region, such as IBI, then it can be used to indicate how chemical effects compare with effects from other disturbances in similar communities. IBI is frequently implemented under the auspices of biological assessment and will be briefly considered as an example of a community-level evaluation procedure.
Index of Biological Integrity
In its simplest characterization, IBI is a scoring system that is often applied to biological assessments for rivers, streams, and wetlands. For example, in wetlands, depending on geographic location and wetland type, both plant and macroinvertebrate communities will be studied, wherein a selected number of metrics will be used to frame an empirical basis of wetland quality. There have been some efforts to develop IBIs for terrestrial environments (Diffendorfer et al., 2007). As such, each metric is evaluated based on specimens collected in the field, and then they are identified and their abundance is estimated. Subsequently, a score will be determined, based on the results of these metrics. Scores derived from multiple metrics are then combined to yield a total score—the IBI. The higher the score, the greater the likelihood an area is in excellent condition. Some would say, “the wetland or upland is healthy.” The level of analysis required in such an assessment depends on the extent to which a habitat was sampled as part of the biological assessment process; for example, an IBI score developed for macroinvertebrates and vegetation would require additional analysis than one based solely on macroinvertebrates, given the relationships between the IBI for macroinvertebrates and that IBI based on plants might also be considered jointly. Depending on the level of analysis—birds and amphibians may also be considered in completing a biological assessment for wetlands—ecotoxicologists and applied ecologists may characterize habitats as being in “excellent,” “moderate,” or “poor” condition, and the system’s health status is recorded so subsequent investigations could better gauge status and trends with respect to long-term outcomes potentially linked to environmental perturbations, such as a release of chemicals in the adjacent lands within the watershed. With our increased attention to an ecosystem level of biological organization, we begin to appreciate the complexity of the systems with which we work, as our next section suggests.
Glennon and Porter (2005) developed an IBI based on forest birds in Adirondack Park, New York, along a gradient of human impact. They created the IBI by placing birds into 12 different guild categories and scoring sample areas according to relative representation of specialist versus generalist guild types. The divisions were made along four categories: food, origin (native or exotic), nest placement, and primary habitat, with subdivisions within most categories. The authors found significant differences in total, functional, compositional, and structural integrity on five land-use types ranging from hamlet to wilderness. In all cases, integrity was lowest in towns and increased in wilderness areas. They also found that biotic integrity showed strong groupings amid five land-use classes. Biotic integrity was strongly related to roadlessness and level of human developments. Specific contaminants were not examined in this study, but it would not have been difficult to include a contaminant metric, which probably would have agreed with the human development versus wilderness impact.
However, before moving to our next section, we should briefly consider the analysis tools used to complete any biological assessment. Besides the metrics of IBI, many other tools of data analysis are available and may be applied to characterize communities in general, including those evaluated with respect to effects of occurring chemical exposures. For example, in any community, measures of species abundance, species richness, and species diversity may be observed. These are community-level endpoints commonly derived by ecologists in their studies of systems, be those perturbed or largely unaffected by anthropogenic effects. If an ecotoxicologist is interested in evaluating effects on species diversity that are potentially associated with chemical exposure, a commonly used diversity index may be applied in the community analysis, such as the Shannon-Weaver Index (H′), which provides an understanding of community structure. In general, a diversity index provides information about the number and distribution of species in a community. Various measures of community characteristics have been developed and can be applied to ecotoxicological investigations (Pielou, 1977).
In general, community ecologists interested in evaluating chemical effects focus on long-established endpoints, just as population ecologists do if chemical exposures are of interest for their sustainability of populations evaluations. Thus, population ecologists and community ecologists focus their ecological study on individual populations and interactions of species within natural communities, respectively. Ecotoxicologists focus on ecological patterns and dynamics at population, community, and ecosystem levels of organization, which enables them to link responses in populations, underwritten by organism-level observations for individual species, to communities and ecosystem responses linked to ecological patterns and dynamics of community assemblages that co-occur in the same geographic area.
Effects at the Ecosystem Level
Whereas biological communities are composed of species that occur together, ecosystems include biological communities and abiotic properties that are more generally related to the exchange of energy and nutrients among functionally defined groups of organisms and their environments. The most commonly measured ecosystem properties are the biomass of the system or its components (eg, trophic levels), productivity of the system or its components (eg, primary and secondary production), and nutrient dynamics (eg, nitrogen-mineralization rates).
In an extensive review of the effects of contaminants on ecosystem functioning in marine and estuarine habitats, Johnston et al. (2015) identified 264 relevant papers published across a wide range of contaminants. They found that toxic contaminants generally altered marine ecosystem functioning by reducing productivity and respiration. Effects varied, however, according to the type of contaminant and the component(s) of the system studied (eg, particular trophic levels, functional groups, or taxonomic groups). These studies were highly biased toward planktonic communities rather than overall biodiversity. The studies rarely included a measure of biodiversity and rarely interpreted their findings within an ecosystem function context. Many studies that included multiple communities within an ecosystem were more likely to find no effect of contamination, and the authors suggested that this might be due to ecological interactions among members of these communities. A strong majority of studies identified negative impacts of contaminants on primary production, which led the reviewers to believe that contaminants may greatly affect the ecosystem services and benefits provided by these systems. They recommended that future studies should focus on the relationships between biological diversity and ecosystem functioning. The understanding of chemical contaminant effects will remain incomplete until direct measures of both variables are undertaken within multiple ecosystems, and Johnston et al. (2015) suggested that productivity and respiration may serve as key endpoints.
We have spent a lot of this book discussing vertebrate and macroinvertebrate ecotoxicology. However, we have not spent much effort on the ecotoxicology of bacteria or microscopic algae. Yet both are incredibly important components of any ecosystem and lend themselves well to studies on respiration and productivity. They can also be strongly affected by contaminants such as pharmaceutical and personal care products (PPCP) and pesticides designed as microbiocides. In aquatic systems, a whole bacteria-driven ecosystem exists in a micrometer-thin film and is often surrounded by a self-produced matrix that keeps it together. Rosi-Marshall et al. (2013) looked at the effects of PPCP on the organisms living in these biofilms. They measured in situ responses of stream biofilms to six common pharmaceutical compounds: caffeine; cimetidine (a histamine treatment for gastrointestinal disorders such as ulcers); ciprofloxacin (a broad spectrum antibiotic); diphenhydramine (an antihistamine used to treat seasickness and some symptoms of Parkinson’s disease); metformin (used in treating diabetes); ranitidine (used for treating acid reflux and ulcers); and a mixture of each by deploying pharmaceutical-diffusing substrates in streams in Indiana, Maryland, and New York. They found fairly consistent results across seasons and regions. On average, algal biomass was suppressed from 4% to 22% relative to controls by caffeine, ciprofloxacin, diphenhydramine, and the mixed treatment. In addition, respiration of the biofilm was significantly suppressed by caffeine (53%), cimetidine (51%), ciprofloxacin (91%), diphenhydramine (63%), and the mixed treatment (40%). In autumn in New York, photosynthesis was also significantly suppressed by diphenhydramine (99%) and the mixed treatment (88%). Compared with controls, diphenhydramine exposure significantly altered bacterial community composition and resulted in significant increases in Pseudomonas sp. and decreases in Flavobacterium sp. in all three streams; both of these genera comprise hundreds of species and no attempt was made to identify them. From a purist attitude, the effects on respiration, biomass, and photosynthesis would be considered ecosystem effects, but the changes in bacterial species composition would be more appropriately classified as community effects and you can see how these measurements are related. The authors concluded that PPCP, alone or in combination, influenced stream biofilms, which could have consequences for higher trophic levels and important ecosystem processes.
Ecosystem properties can be difficult to measure within the time frame most often available for a biological assessment, and unless field-study design criteria allow sufficient time for implementation, empirical data will likely be highly variable and not particularly diagnostic. Interpretation will likely require a better grasp of uncertainties captured in a snapshot of a complex system at a single point in time, but may be broadly applicable and sufficient for informing resource management decisions.
Regardless of technical issues, largely dependent on time or, better said, the lack of time, evaluation of complex systems such as those observed at community and ecosystem levels of biological organization, resource managers in the regulatory arena frequently incorporate biological assessments in environmental quality programs. For example, as we noted previously in this chapter, water quality has a relatively well-established set of tools such as IBI for conducting biological assessments. Although the level of complexity likely precludes routine deployments at the ecosystem level of biological organization, such methods can be used to assess aquatic life and characterize surface waters as either impaired or not, provided sample designs are sufficient. Regardless of the scale, biological assessments provide empirical measures of stress and exposure by directly assessing the response of communities in the field. Applying tools such as IBI at large spatial scales, such as those typically encountered with ecosystems, likely require the development of study designs that are focused on community types across a series of ecosystems, wherein measurable changes in the biotic community—for example, the return of native species, decreases in anomalies and lesions in fish and amphibians, or decreases in pollution-tolerant species paired with an increase in pollution-sensitive species—can readily be communicated to resource managers and an interested public.
In addition, as response-stressor relationships are documented, biological assessments in combination with chemical stressor data can be used to help predict and track environmental outcomes of management actions. As such, a cause and effect analysis may be initiated, initially through a process targeted to identify a stressor or combination of stressors that cause biological impairment. The ability to identify stressors and evidence supporting those findings is a critical step in developing strategies that will improve the quality of resources vulnerable to those stressors, such as chemicals released to the environment. To identify stressors principally linked to adverse effects observed in biota across the range of exposures at play, the identification necessarily draws upon a wide variety of disciplines in a number of environmental areas: ecology, biology, geology, geomorphology, chemistry, statistics, and ecotoxicology. In completing such a stressor identification study, new avenues open that benefit our understanding of complex adaptive systems.
Communities and Ecosystems: Network Analysis and Modeling
Recent developments in the quantitative analysis of complex, nonbiological networks, based largely on the mathematics of graph theory, have been rapidly translated to studies of ecological networks. Many of the networks stem from conceptual food-web models and can be applied to exposure analysis. Studies of these exposures are often developed to evaluate the transfer of chemicals from foods to animals that consume those foods to deposition into the environment as waste products. Other conceptual models that cover atmospheric deposition or other networks are common. Ecological networks captured through conceptual models such as these consider the more complex structural and functional systems of communities and ecosystems as they demonstrate small-world topology, highly connected hubs, and modularity. Network analysis of biological systems, from subcellular to ecosystem levels of biological organization will only increase in their application to ecotoxicological studies.
Ecological networks are representations of the interactions that occur between species within a community or between communities within an ecosystem (Fig. 11.5). Traditionally, interactions encountered in such networks include symbiotic relationships such as competition, mutualism, and predation. Network properties of particular interest are generally focused on stability and structure. Yet, ecotoxicologists originally deployed such conceptual models to better understand how chemicals moved through the environment from abiotic compartments such as soils, sediments, and waters to biological compartments—terrestrial and aquatic plants and animals (eg, Cantwell and Forman, 1993). Such conceptual models as in Fig. 11.5 clearly suggest that tracking flows of chemicals between exposure matrices from abiotic compartments to biological components of the system will require forecasting models that might predict how chemicals would be “seen” by any lifeform during exposure. Such a goal has proven elusive, yet building from our increased understanding of ecotoxicology in general, and tracking chemicals as they move through the environment has benefited from developments in other fields. For an increasing number of ecotoxicologists, graph theory and network analysis have become integral tools in the analysis of a flow network, and because such analysis has benefited from empirical data derived from integrated field and laboratory studies and biological assessments in the past, present, and future. Ecotoxicology can also benefit from these recent advancements in ecological network research.
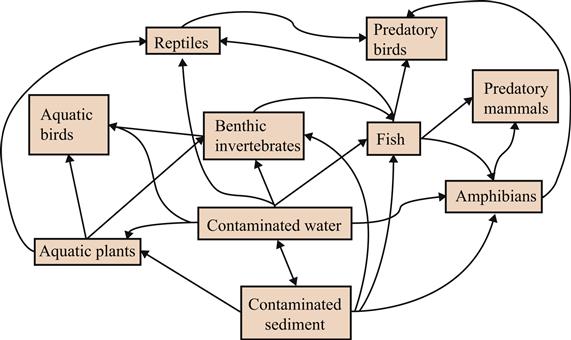
As such, ecological network analysis considers complexity, especially that observed in ecosystems and communities, and how that affects ecosystem functioning. Depending on the analyst’s perspective, ecological networks may be categorized and subdivided into various types. For ecotoxicologists, much of their work builds on traditional food webs, as illustrated in Fig. 11.5. Among a long list of items to pursue, future research in application of network analysis to ecotoxicological questions should focus on increasing our understanding of similarities and differences in chemical fate and effects among various communities that occur across ecosystems. Not surprisingly, based on what we already have discovered, these questions would inevitably require matching efforts in comparative ecotoxicology, so we can better address issues entangled with outcomes of organism- or population-centered studies. As such, ecotoxicologists would gain from developing an increased understanding of mechanisms underlying observations in the field. For example, analysis of compartments within network structures would allow ecotoxicologists to gain an increased understanding of food webs and mechanisms underlying chemical transfers between compartments within a network, or we could enhance our characterization of differences in chemical transfers linked to the underlying differences in abiotic factors that influence such transfers. Such refinements will enable us to improve our capability to forecast chemical transfers between compartments and reduce or mitigate impacts and consequences of increasing environmental perturbations, such as release of chemicals to the environment, and, of equal significance, improve our capability to discriminate among multiple stressors.
Biological assessment, particularly when completed within the context of integrated field and laboratory study, is essential to our understanding of chemical effects on biological systems at any level of organization. Of equal importance, biological assessment or monitoring provides critical empirical data that benefits management and conservation of ecosystems. However, current biological assessment and monitoring approaches are incompletely developed and tools, such as those of ecological network analysis, need to be developed and placed into service, particularly in fields of study focused on stressors—chemical, physical, or biological—and their interactions with biota when exposed in complex natural systems.
The analysis of ecological networks potentially offers insights into community and ecosystem responses to chemicals released to the environment; such network approaches likely yield capabilities for our interpretation of current ecological conditions. More importantly, if we augment biological assessment and monitoring activities by implementing those studies within a framework of ecological network analysis that relies on empirical data derived from direct observation and underwritten by designed laboratory studies targeted to data gaps identified in preliminary studies, this would potentially allow us to characterize ecological networks across environmental gradients encountered within various ecosystems. Future enhancements to the analysis of ecological networks may require our adopting emerging technologies and analytical approaches (eg, remote sensing capabilities to biosensors to nanosensors), enabling biological assessment, and monitoring to move beyond today’s implementation practices and address the many ecological responses that can only be understood from a network-based perspective.
Study Questions
1. What types of questions would ecotoxicologists interested in communities ask that would be different from those who are more interested in individual organisms?
2. True of False. In some ways, community ecotoxicology is an extension of ecotoxicology at the population level.
3. In general, what happens to the role of multiple stressors as we venture from population to community to ecosystem scales?
4. True or False. Lab studies no longer have a function in studying toxicity for scales larger than the population because we cannot manufacture anything as complex as an ecosystem in the laboratory.
5. If ecologists and ecotoxicologists are interested in such things as emigration, immigration, births, deaths, and age structures, what are comparable things that interest community-based scientists?
6. Pick a group of organisms (eg, macroinvertebrates, plants, fish, amphibians, frogs) and develop a design for a biological assessment of this group in an ecotoxicological context.
7. What is the difference between a bioindicator used for studying the responses of individual organisms to contaminant exposure and a biological indicator used for the same purpose but in a community context?
8. From a geographic perspective, is there a 100% overlap between airsheds and watersheds? Why or why not?
9. True or False. Some sources of groundwater may spend millions of years underground without ever surfacing.
10. Identify some of the environmental measures that would be of interest in the study of how contaminants affect ecosystems.